Abstract
Objective
Kidney involvement affects 40–60% of patients with lupus and is responsible for significant morbidity and mortality. Using depletion approaches, several studies have suggested that macrophages may play a key role in the pathogenesis of lupus nephritis. However, “off target” effects of macrophage depletion, such as altered hematopoiesis or enhanced autoantibody production, impeded the determination of a conclusive relationship.
Methods
In this study, we investigated the role of macrophages in mice receiving rabbit anti-glomerular antibodies, or nephrotoxic serum (NTS), an experimental model which closely mimics the immune complex mediated disease seen in murine and human lupus nephritis. GW2580, a selective inhibitor of the colony stimulating factor-1 (CSF-1) receptor kinase, was used for macrophage depletion.
Results
We found that GW2580-treated, NTS challenged mice did not develop the increased levels of proteinuria, serum creatinine, or serum urea seen in control-treated, NTS challenged mice. NTS challenged mice exhibited significantly increased kidney expression of inflammatory cytokines including RANTES, IP-10, VCAM-1 and iNOS, whereas GW2580-treated mice were protected from the robust expression of these inflammatory cytokines that are associated with LN. Quantification of macrophage related gene expression, flow cytometry analysis of kidney single cell suspensions, and immunofluorescence staining confirmed the depletion of macrophages in GW2580-treated mice, specifically within renal glomeruli.
Conclusions
Our results strongly implicate a specific and necessary role for macrophages in the development of immune glomerulonephritis mediated by pathogenic antibodies, and support the development of macrophage targeting approaches for the treatment of lupus nephritis.
Keywords: Macrophages, SLE, lupus nephritis, nephrotoxic serum nephritis (NTS)
1. Introduction
Systemic lupus erythematosus (SLE) is characterized by the production of autoantibodies and systemic inflammation, which collectively result in damage to various end organs. Kidney involvement, known as lupus nephritis (LN), is seen in up to 60% of SLE patients, and is associated with increased morbidity and mortality. Even with current immunosuppressive regimens, chronic renal insufficiency develops in about a quarter of patients. In a two year follow up of patients with LN, the complete remission rate with current treatment regimens was less than 50%, and the relapse rate was as high as 30% [1]. A better understanding of disease pathogenesis could lead to the discovery of more targeted therapies, which may prevent or suppress relapses and improve long term kidney prognosis.
Macrophages are innate immune system cells that are present in every tissue [2] where they represent 5–15% of the cells [3]. They have a very high degree of plasticity, and the ability to assume different functions ranging from trophic, to immune suppressive or tissue destructive phenotypes [2]. Macrophages derive from three known lineages within mice: the embryonic yolk sac, the fetal liver, and the adult bone marrow. Most tissue resident macrophages are thought to derive from the yolk sac, whereas macrophages originating from precursors in the bone marrow seed tissues mostly under inflammatory conditions [2, 4, 5].
Macrophages are believed to contribute to the pathogenesis of LN. In human lupus, renal mononuclear phagocyte infiltration is associated with poor outcomes [6]. Furthermore, studies have shown that unique, activated populations of macrophages are present during active murine LN [1, 7, 8]. Additionally, depletion studies have sought to demonstrate that macrophages are not just present during disease, but also actively contribute to pathogenesis. However, major limitations of the methods employed for macrophage depletion have seriously hindered a definitive determination of whether or not macrophages are truly involved in disease progression [8–14].
In this study, we sought to deplete macrophages in an inducible model of LN. Our aim was to minimize any potential confounding factors, so as to conclusively determine the role of macrophages in the pathogenesis of renal disease mediated by pathogenic antibodies. We utilized a classic model known as nephrotoxic serum transfer, or NTS, in which non-autoimmune mice are passively transferred with heterologous serum containing pre-formed, nephrotoxic antibodies. The immune response initiated by these antibodies mimics the glomerulonephritis seen in SLE patients, including immune complex deposition, complement activation, and inflammatory cell infiltration [15, 16].
GW2580 is a selective inhibitor of the CSF-1 receptor (CSF-1R) kinase [17], which is expressed on all macrophages [18–20]. GW2580's monospecificity for the CSF-1R has been validated against every kinase studied to date [17, 21]. By blocking signaling through CSF-1R, which is important for macrophage proliferation, survival, activation, and recruitment [20, 22, 23], GW2580 treatment selectively reduces macrophages in several tissues including the kidney [22, 24], and in response to inflammatory stimuli [17]. This particular kinase inhibitor is superior to other depletion methods due to its specificity for CSF-1R. Using GW2580, we determined whether macrophage depletion ameliorates disease within this inducible murine model of LN.
2. Methods
2.1. Mice
DBA/1J and B6 mice were purchased from The Jackson Laboratory and housed in the animal facility at the Albert Einstein College of Medicine (Bronx, NY). All animal studies were approved by the Institutional Animal Care Committee.
2.2. Nephrotoxic serum transfer
Nephrotoxic serum nephritis was induced as described [25]. In brief, on Day 0, 10 week old female DBA/J mice were immunized with rabbit IgG in CFA via intraperitoneal injection. On day 5, mice received either an intravenous injection of nephrotoxic serum (NTS) or PBS. Blood and urine were collected at baseline (day 0) and at subsequent time points throughout the experiment.
Three separate groups of mice were included in each experimental cohort. The first group (control mice) was immunized with rabbit IgG as described above on day 0, but not given the NTS transfer. The second group (NTS/GW2580 mice) was immunized with rabbit IgG on day 0, and injected with NTS on day 5 of the experiment. In addition, this group received a daily oral gavage, starting on day 0 and continuing until day 9, of 100 mg/kg of GW2580 (LC Laboratories, Woburn, MA) suspended in 0.2 ml of PBS. This dose was selected based upon the known pharmacokinetics of GW2580, and produces peak drug levels which effectively deplete macrophages [17]. The third group (NTS/ PBS mice) was similarly immunized with rabbit IgG and injected with NTS. In addition, mice in this group were orally gavaged with 0.2 ml of PBS alone as a control, using the same schedule. Two independent trials were performed, with trial #1 consisting of 5 control mice, 10 NTS/PBS mice, and 10 NTS/GW2580 mice, and trial #2 including 4 control mice, 8 NTS/PBS mice, and 8 NTS/GW2580 mice.
Separate experiments were performed that assessed the therapeutic potential of GW2580. In these studies GW2580 treatment was initiated later in the disease model (day 5), to insure that peak macrophage depletion would occur after the onset of proteinuria. GW2580 was given via oral gavage at the same dose as above, 100 mg/kg in 0.2 ml of PBS per day for 10 days. As before, three groups of mice were utilized for the study, including a total of 9, 18, and 18 mice in each of the control, NTS/PBS, and NTS/GW2580 groups, respectively.
2.3. Assessment of proteinuria and renal function
Levels of proteinuria were determined by Uristix test strips (Siemens Healthcare Diagnostic, Tarrytown, NY). Albumin levels were measured by the Mouse Albumin ELISA Quantitation Set (Bethyl Laboratories, Montgomery, TX). Serum blood urea nitrogen (BUN) was determined by the Quantichrom DIUR 500 kit (BioAssay Systems, Hayward, CA). Serum and urinary creatinine were measured by the QuantiChrom Creatinine Assay Kit (BioAssay Systems).
2.4. Renal histopathology
Paraffin kidney sections were deparaffinized and stained with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS). Kidney histology was analyzed and quantified by an experienced nephropathologist (L.H.), who was blinded to the treatment assignments. The slides were scored for the presence of endocapillary hypercellularity, extracapillary proliferation, immune deposits, tubular atrophy, tubular casts, tubular dilation and interstitial fibrosis and inflammation. A scale of 0–5 was used, where 0 is absent, 1 denotes involvement of 1–5% of glomeruli or cortical area, 2 is 6–10%, 3 is 11–20%, 4 is 21–50%, and 5 is greater than 50%.
2.5. Mouse anti-rabbit IgG and rabbit anti-mouse glomerular basement membrane (GBM) ELISA
Serum titers of mouse anti-rabbit IgG and IgG rabbit anti-mouse GBM antibodies were measured by ELISA, as described in detail (25).
2.6. Renal IgG deposition
Glomerular immunoglobulin deposition was assessed by immunohistochemistry on paraffin embedded kidney sections, as described [26].
2.7. Flow cytometric analysis
Following systemic perfusion, kidneys and spleens were harvested for analysis. Single cell suspensions of kidneys were generated by a 30 min incubation of sliced kidney tissue in 2 mg/ml of collagenase IV (Worthington, Lakewood, NJ) at 37 °C, followed by serial pipetting. Spleens were mashed through a 70 mm filter with the back of a syringe in order to create a single cell suspension. Following red blood cell lysis, cells were Fc-blocked for 30 min on ice with anti-CD16/CD32 (BD Pharmingen) diluted 1:200 in 3% FBS in PBS. Cells were then washed three times with FBS/PBS and stained in the dark with the following antibodies for 30 min, on ice: APC-CD11c, PE-CD11b, PerCP-F480, and FITC-GR1 (all purchased from BioLegend, San Diego, CA). After gating out neutrophils (CD11b+GR-1hi), inflammatory macrophages were defined as CD11b+F4/80loGR-1+(Ly6C), whereas trophic, tissue resident macrophages were defined as CD11b+F4/80hi.
2.8. Immunofluorescence staining for IBA-1
Kidney sections were deparaffinized and rehydrated, followed by antigen retrieval in citrate buffer (pH 6) at 90–95 °C for two minutes. The slides were then washed 3 times in wash buffer (0.05% TWEEN20 in PBS) and blocked for 2 h at room temperature in 20% horse serum diluted in PBS. Slides were washed three times, and then incubated with the primary antibody, anti-IBA-1 (WAKO, Osaka, Japan) diluted 1:250 in 2% horse serum in PBS. After a 2 h incubation at room temperature the slides were washed 3 times, and incubated with FITC-conjugated anti-rabbit IgG antibody (Southern Biotech) diluted 1:250. After a one hour incubation at room temperature, the slides were washed and stained with DAPI, mounted in Fluoromount-G (Southern Biotech), and examined using a Zeiss AxioObserver CLEM.
2.9. Thioglycolate injection
Female B6 mice at 16 weeks of age were given either 100 mg/kg GW2580 suspended in 0.2 ml of PBS, or 0.2 ml of PBS alone, daily by oral gavage for 10 days starting on day 0. On day 8, mice were injected intraperitoneally with 1 ml of sterile 3.8% aged thioglycolate suspension. Mice were sacrificed 72 h later, and peritoneal macrophages were harvested by intraperitoneal lavage with ice cold 0.5% BSA/PBS/5 mM EDTA. The majority of cells collected were macrophages [27], which were counted using trypan blue exclusion.
2.10. CD3 staining
Kidney sections were deparaffinized and rehydrated, followed by antigen retrieval in citrate buffer (pH 6) at 90–95 °C for 10 min. The slides were then washed 3 times in wash buffer (0.05% TWEEN20 in PBS), and blocked with Dual Endogenous Enzyme Block (Dako, Carpinteria, CA) for 20 min at room temperature. Slides were then washed once and incubated with 5% goat serum +2% BSA in PBS for 30 min at 37 °C. After one wash, sections were incubated with anti-CD3 antibody (Seratec, Göttingen, Germany) diluted 1:500 overnight at 4 °C. The next day, slides were washed three times, and incubated with a biotinylated goat anti-rat IgG antibody (1:250) (Southern Biotech) for 1 h at 37 °C, followed by 3 washes and a 30 min incubation at room temperature in streptavidin-HRP (Thermo-scientific) diluted 1:5000. After three washes, slides were developed with DAB-chromagen (Dako), washed, counterstained in Mayer's hematoxylin, and mounted with permount.
2.11. RNA isolation, cDNA synthesis, and real time PCR
Total RNA was extracted from whole kidney tissue using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Synthesis of cDNA was performed using the Superscript III system (Invitrogen, Carlsbad, CA). Real time PCR was performed in triplicate, and the values normalized to GAPDH, as described [28].
2.12. Data analysis
Data was analyzed using GraphPad Prism 6, and presented as mean ± standard error. Student T-test was used to determine p-values between different treatment groups unless otherwise indicated, and values of <0.05 were considered significant.
3. Results
3.1. Treatment with GW2580 ameliorates proteinuria in nephrotoxic serum nephritis
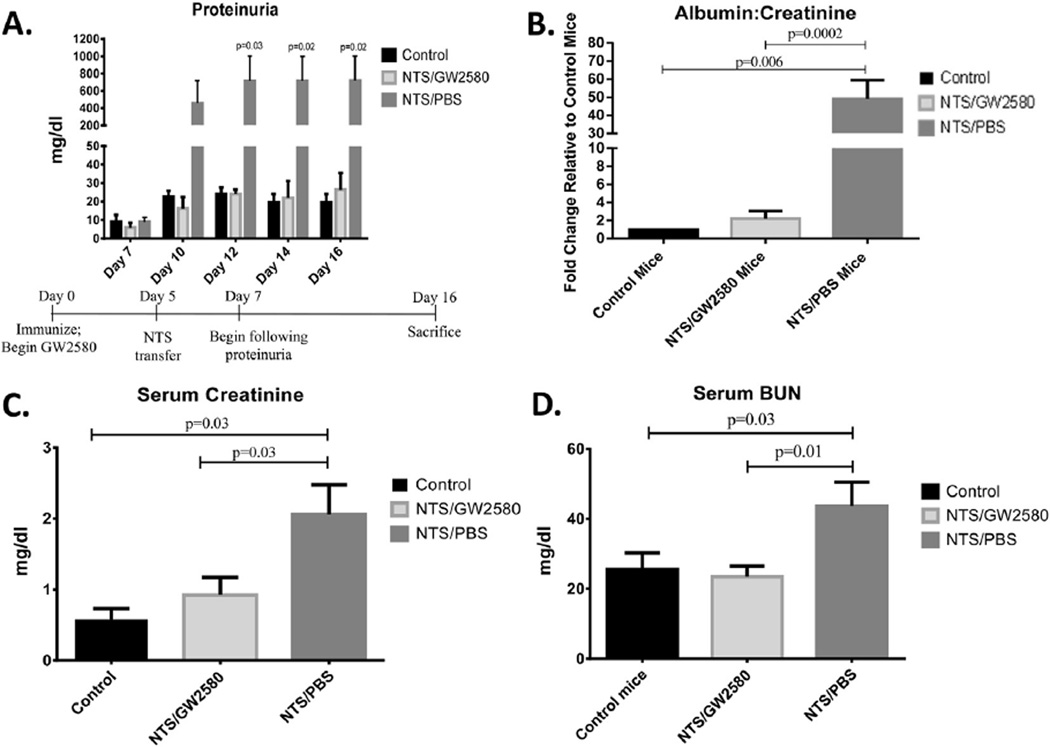
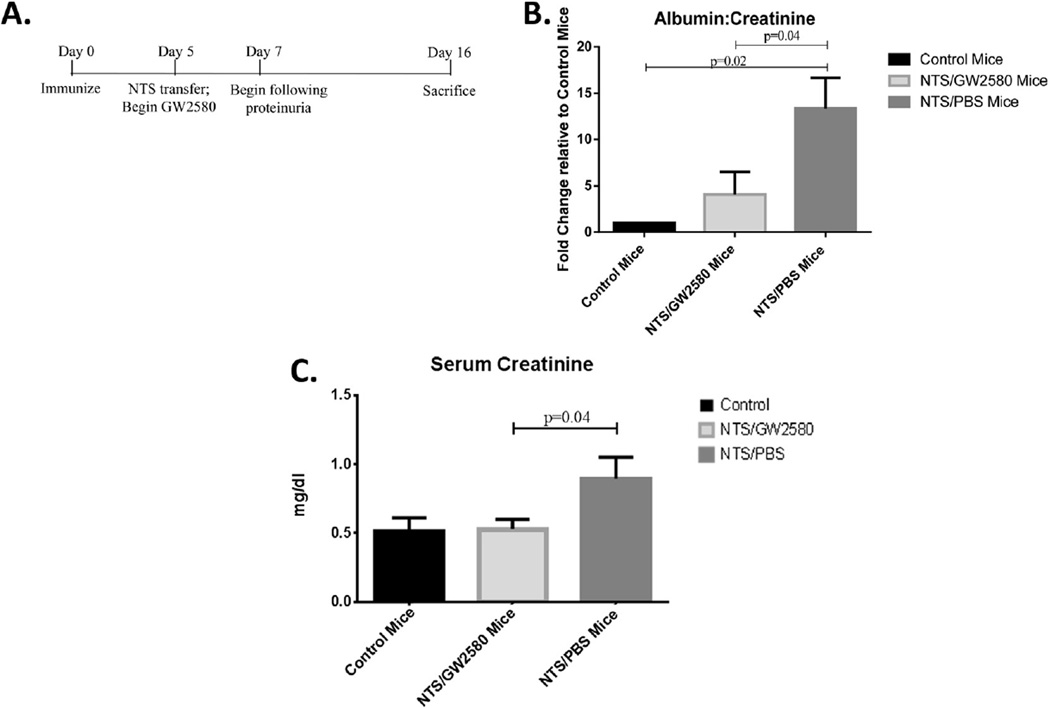
To analyze the role of macrophages in an inducible model of lupus nephritis, we examined the effect of the CSF-1R inhibitor, GW2580, in kidney disease mediated by nephrotoxic antibodies. NTS/PBS mice, which were immunized with rabbit IgG followed by the transfer of nephrotoxic serum (on day 5), developed significant proteinuria which peaked on day 12 of the experiment (p = 0.03) (Fig. 1A). The NTS/PBS mice continued to display significantly increased levels of proteinuria throughout the duration of the study. In contrast, NTS/GW2580 mice were protected from the development of proteinuria, as seen by both a semi-quantative assay (Fig. 1A) and urinary albumin concentrations normalized to urinary creatinine (Fig. 1B).
Fig. 1.
Renal function in NTS challenged mice. Proteinuria levels were analyzed by (A) Uristix at different time points throughout the experiment (experimental timeline provided at the bottom on the panel) or (B) Urine albumin ELISA, normalized to urine creatinine to adjust for variations in urine output, at terminal day 16. Results are shown normalized to control mice. NTS/PBS mice develop significantly higher levels of proteinuria by day 12, which remained elevated through the end of the experiment at day 16. Renal function was also analyzed by (C) serum creatinine and (D) serum blood urine nitrogen levels on terminal day 16. Shown here are results from one experiment (control mice, n = 5; NTS/GW2580 mice, n = 10; NTS/PBS mice n = 10), which is representative of the two independent studies performed.
3.2. GW2580 treated mice maintain normal kidney function
To establish whether GW2580-mediated macrophage depletion prevented the development of renal insufficiency, creatinine and BUN levels were measured in serum collected at the time of sacrifice (Day 16). GW2580 treated, NTS-challenged mice had significantly lower levels of both serum creatinine (p = 0.03) (Fig. 1C) and BUN (p = 0.01) (Fig. 1D) compared to NTS/PBS mice. Furthermore, there was no significant difference in renal function between NTS/ GW2580 and healthy control mice.
3.3. GW2580 treatment prevents renal histopathological injury
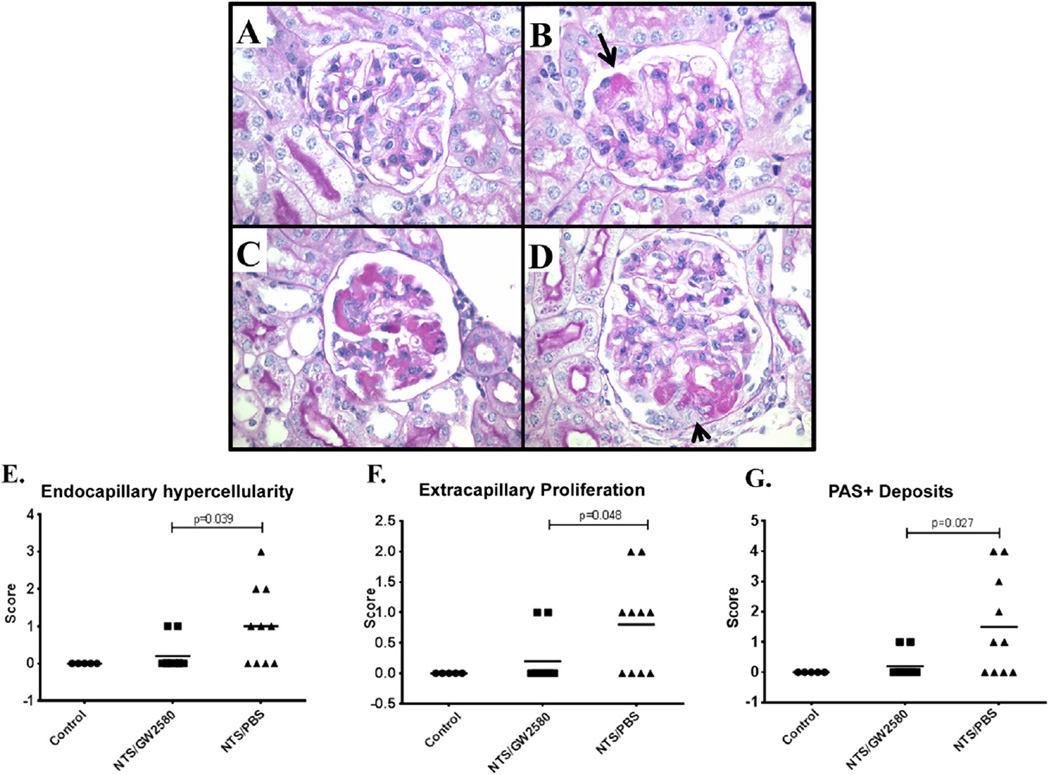
To determine if GW2580 treatment prevents the histopathological injury associated with NTS, histology of the kidney was analyzed by examining H&E and PAS-stained slides. As expected in this model, NTS/PBS mice displayed features of severe proliferative glomerulonephritis (Fig. 2C, D). NTS/GW2580 mice, however, had significantly less histopathological injury (Fig. 2A, B). Specifically, when compared to the NTS/PBS group, GW2580 treated mice exhibited significantly less endocapillary hyper-cellularity (p < 0.04), extracapillary proliferation (p < 0.05), and PAS + deposits (p < 0.03) (Fig. 2E–G).
Fig. 2.
Renal histopathology. Panels A & B are from GW2580 treated mice. A shows a normal appearing glomerulus; B shows a glomerulus with a focal deposit in a glomerular capillary (arrow) but no prominent glomerular inflammation. Panel C and D are from NTS/PBS mice. Panel C shows abundant intraluminal PAS+ deposits and focal intracapillary leukocyte infiltration. Panel D shows a segmental cellular crescent (arrow) overlying prominent intracapillary deposits. Panels A–D (600×, PAS stained) are representative of the mean severity of renal pathology seen in their respective groups. NTS/PBS mice had significantly worse endocapillary hypercellularity (E), extracapillary proliferation (F), and PAS+ deposits (G).
3.4. GW2580 does not interfere with the generation of mouse anti-rabbit antibodies
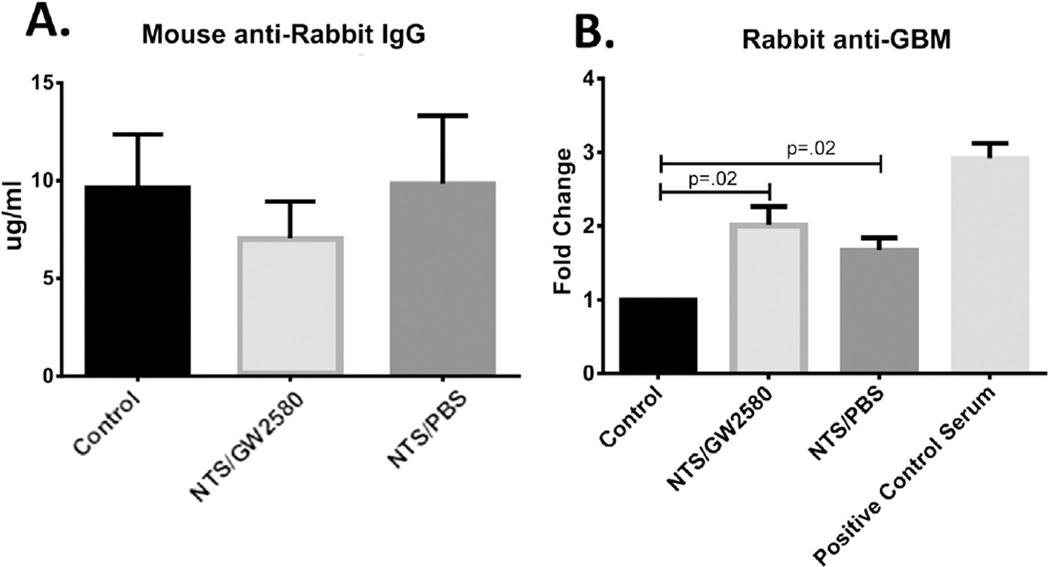
In the nephritis model utilized in these experiments, the initial immunization with rabbit IgG serves to worsen disease, since the anti-rabbit antibodies resulting from this immunization will crosslink the nephrotoxic rabbit anti-mouse GBM antibodies passively transferred on day 5. To exclude that the protection from nephritis seen with GW2580 treatment was a result of interference with the mouse anti-rabbit immune response rather than from the depletion of macrophages, we analyzed the serum levels of mouse anti-rabbit IgG antibodies. As seen in Fig. 3A, there were no significant differences in the levels of mouse anti-rabbit IgG antibodies between any of the three experimental groups (control, NTS/GW2580, and NTS/PBS mice), all which were immunized with rabbit IgG at baseline. Additionally, there were no differences in relative representation of the various IgG isotypes in the mouse anti-rabbit IgG response (data not shown). These data indicate that the mice were successfully immunized, and that simultaneous GW2580 treatment did not interfere with the mounted immune response.
Fig. 3.
NTS is successfully induced in GW2580 treated mice. (A) Levels of serum mouse anti-rabbit IgG antibodies were assessed by ELISA to determine if GW2580 treatment interfered with the initial immunization. (B) Rabbit anti-mouse GBM antibodies in serum were measured by ELISA to ascertain that NTS/PBS mice and NTS/GW2580 mice were exposed to comparable levels of nephrotoxic antibodies. Shown are results from one experiment (control mice, n = 5; NTS/GW2580 mice, n = 10; NTS/PBS mice, n = 10), which is representative of the two independent studies performed.
To confirm that the observed differences in the severity of nephritis did not result from different levels of circulating nephrotoxic antibodies, we examined the levels of rabbit-anti mouse GBM in the serum (Fig. 3B). Both the NTS/GW2580 mice and the NTS/PBS mice had significantly higher levels of these antibodies compared to the control mice, which did not receive the serum transfer (p = 0.02). Importantly, the two mouse groups that received nephrotoxic serum, NTS/PBS and NTS/GW2580, had comparable serum anti-GBM antibody levels.
3.5. GW2580 treatment does not affect glomerular IgG deposition
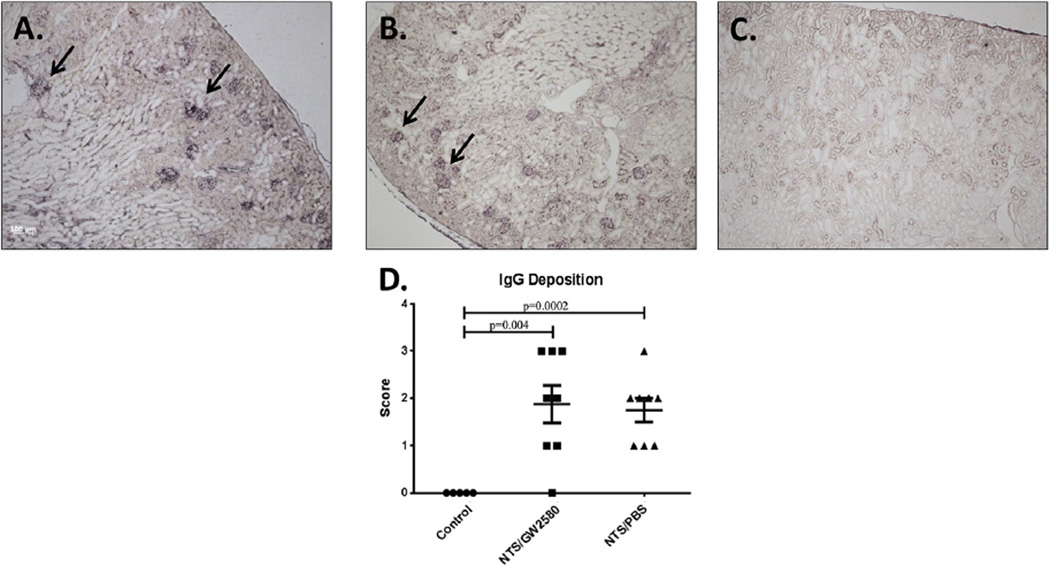
To determine if changes in renal function were contributed by differential immunoglobulin deposition, kidney sections were stained for the presence of glomerular IgG. As expected, the control mice did not have significant IgG deposition (Fig. 4C). In contrast, the NTS/PBS mice (Fig. 4B) and the NTS/GW2580 mice (Fig. 4A) both had significant glomerular deposition. Importantly, GW2580 treatment did not decrease kidney IgG deposition (Fig. 4D).
Fig. 4.
Glomerular IgG deposition. NTS/GW2580 mice (A) and NTS/PBS mice (B) have comparable levels of glomerular mouse IgG deposition. Arrows point to selected glomeruli exhibiting immunoglobulin deposition. Control mice (C), which did not receive the nephrotoxic antibodies, did not show significant glomerular mouse IgG deposition. Sections were scored (D), and significant differences were noted when comparing NTS/PBS and NTS/GW2580 mice to the control group.
3.6. GW2580 successfully depletes intraglomerular macrophages following NTS challenge
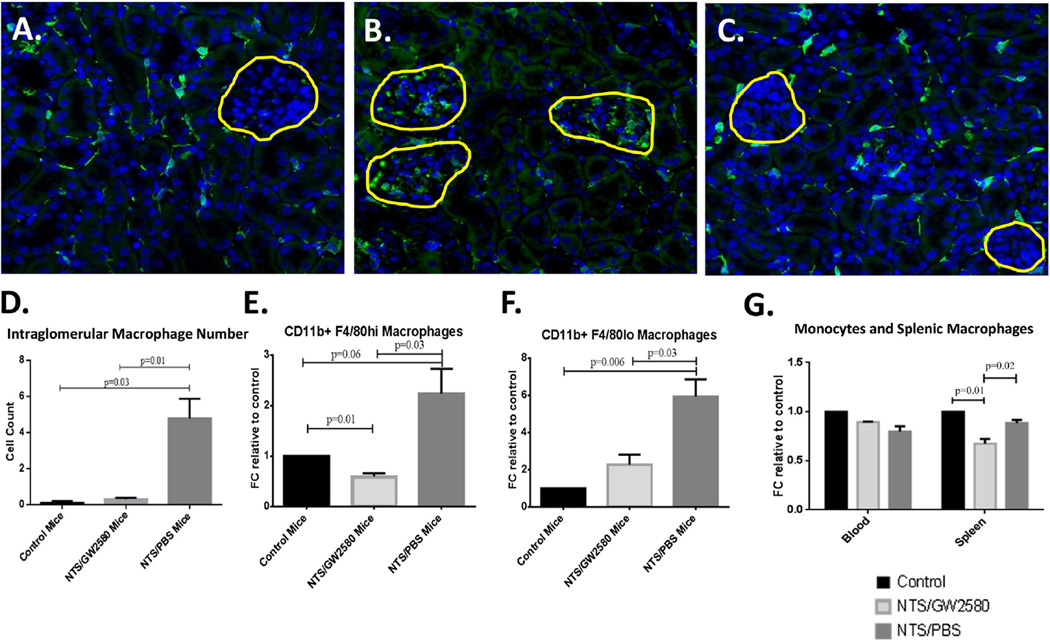
Kidney single cell suspensions and kidney sections were analyzed by flow cytometry and immunofluorescence staining, respectively, to assess the number of macrophages infiltrating the kidney following NTS transfer, and to study any effect of GW2580 treatment (Fig. 5).
Fig. 5.
Macrophage depletion in GW2580 treated mice. In (A)–(C), the yellow lines outline the glomeruli in each section. Control mice (A) stained positive for IBA-1 within the kidney interstitium (green stain), indicating the presence of normal tissue resident macrophages. No intra-glomerular staining was present, in contrast to what was seen in the NTS/PBS group (B). GW2580 treated mice (C) resembled the control mice, maintaining a population of interstitial macrophages, but without positive staining observed within the glomeruli. (D) Quantification of intraglomerular macrophages. Shown in (A)–(D) are results from one experiment (control mice, n = 5; NTS/GW2580 mice, n = 10; NTS/PBS mice, n = 10), which is representative of both early and late GW2580 treatment studies. (E, F) Flow cytometry analysis of kidney single cell suspensions confirmed decreased numbers of both CD11b+F4/80hi macrophages and CD11b+F4/80lo macrophages in GW2580 treated mice. (G) Flow cytometry analysis revealed no change in monocyte numbers in the blood in response to GW2580, but a significant decrease in macrophage numbers in the spleen. Shown in (E)–(G) are results from one experiment (control samples, n = 2; NTS/GW2580 samples, n = 4; and NTS/PBS samples, n = 4, with each cytometry sample containing cells from 2 randomly chosen mice of that group), which is representative of both the early and late GW2580 treatment studies.
NTS/PBS mice had significantly higher levels of CD11b+F4/80loGR1+ inflammatory macrophages (p < 0.006) (Fig. 5F), as well as a nearly significant expansion of the tissue resident CD11b+F4/80hi population (p = 0.06), compared to control (Fig. 5E). Furthermore, IBA-1 staining revealed increased infiltration of macrophages within the glomeruli of these control treated, NTS-challenged mice (Fig. 5B). In contrast, negative control mice not receiving nephrotoxic sera did not demonstrate glomerular macrophage infiltration, although interstitial macrophages that normally populate the tissue were present (Fig. 5A). Quantification of intraglomerular macrophages confirmed a significant increase of IBA-1+ macrophages in NTS/PBS (nephritic) mice compared to non-nephritic control mice (Fig. 5D).
GW2580 treatment successfully diminished macrophage numbers. Kidney inflammatory macrophages (CD11b+F4/80loGR1+) were significantly depleted compared to NTS/PBS mice, as assessed by flow cytometry (p = 0.03) (Fig. 5F). IBA-1 staining revealed depletion specifically within the glomeruli; the many glomerular infiltrating macrophages seen in the NTS/PBS mice were absent. GW2580 did not completely abolish tissue resident, interstitial macrophages (Fig. 5C); however, tissue resident CD11b+F4/80hi macrophage numbers were decreased as compared to negative control (p = 0.01) and NTS/PBS mice (p = 0.03) (Fig. 5E), suggesting that proliferation of tissue resident macrophages was inhibited by GW2580 treatment as well. No significant difference was seen in the kidney CD11b+F4/80loCD11chi population between any of the groups (data not shown).
Finally, the effect of GW2580 was not limited to the kidneys. Spleens of GW2580 treated mice contained significantly less CD11b + F4/80 + resident macrophages as compared to control and NTS challenged mice (p < 0.03; Fig. 5G). Circulating monocyte numbers were, however, unaffected by GW2580 treatment (Fig. 5G).
3.7. GW2580 decreases the number of macrophages recruited to the peritoneal cavity in sterile peritonitis
Intraperitoneal injection of thioglycolate is an inflammatory stimulus which results in an influx of a large number of macrophages into the peritoneal cavity [27]. We found that treatment with GW2580 administered using a similar schedule to that described above for NTS resulted in a ~40% reduction (p = 0.03) in the number of macrophages infiltrating into the peritoneal cavity after thioglycolate injection, when compared to control-treated mice (Supplemental Fig. 1). These data confirm that GW2580 depletes macrophages in another well-known, macrophage dependent model of inflammation, and supports a similar effect of the drug in the inducible nephritis model.
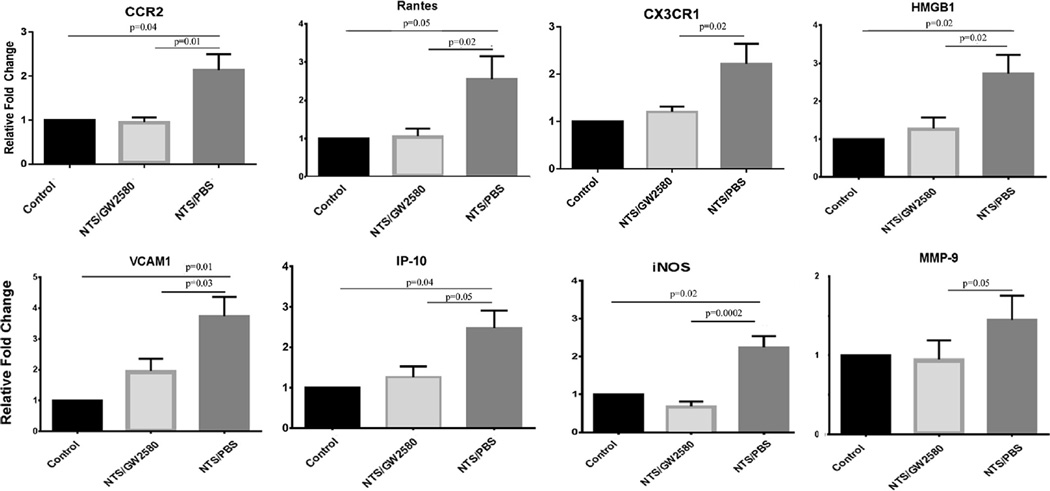
3.8. GW2580 treatment prevents the development of an inflammatory cytokine profile within the kidney and decreases the expression of macrophage specific markers
Since GW2580 treatment depleted macrophages and protected mice from developing kidney disease, we sought to determine the mechanism of protection. Macrophages are known to release numerous inflammatory mediators that contribute to tissue damage [29]. We investigated whether macrophage depletion with GW2580 affected the expression within the kidneys of a series of inflammatory cytokines and chemokines associated with the development of LN. Results showed a decrease in these inflammatory mediators, including RANTES, IP-10, VCAM-1, and iNOS (Fig. 6). For each cytokine, NTS/GW2580 mice displayed significantly lower levels of kidney expression compared to NTS/ PBS mice. Additionally, cytokine expression in NTS/GW2580 mice was not significantly different than that seen in the control mice (Fig. 6).
Fig. 6.
Kidney expression of inflammatory mediators. RT-PCR was performed on whole kidney mRNA to quantitate RANTES, IP-10, VCAM-1, iNOS, CCR2, CX3CR1, MMP-9, and HMGB1 gene expression. PCR reactions were done in triplicate, and the PCR for each cytokine repeated at least twice (FC, fold change compared to control). Shown are results from one experiment (control mice, n = 5; NTS/GW2580 mice, n = 10; NTS/PBS mice, n = 10), which is representative of the two independent studies performed.
The RT-PCR studies using kidney mRNA provided additional evidence confirming the depletion of macrophages with treatment. Specifically, GW2580 treated mice exhibited significantly reduced kidney expression of CCR2 and CX3CR1 (Fig. 6), two receptors expressed on macrophages.
3.9. GW2580 treatment inhibits kidney MMP-9 and HMGB1 expression
Besides cytokines and chemokines, macrophages can produce several other molecules which majorly contribute to local inflammatory disease. Matrix Metalloproteinase 9 (MMP-9) and High Mobility Group Box 1 (HMGB1) are expressed by macrophages and are thought to be important mediators of disease in LN [30–32]. Therefore, we studied whether depletion of macrophages with GW2580 affected the expression of these two molecules within the kidney. As shown in Fig. 6, diseased kidneys in NTS/PBS mice expressed significantly upregulated levels of both HMGB1 and MMP-9. In contrast, in GW2580-treated mice, kidney expression of these molecules was not significantly different than the control mice.
3.10. GW2580 treatment prevents the accumulation of kidney T cells
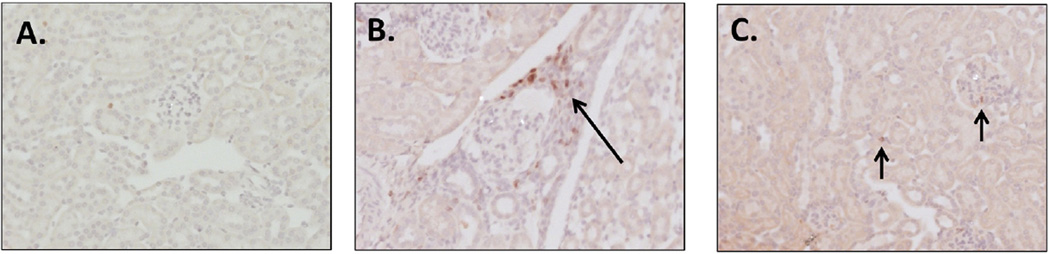
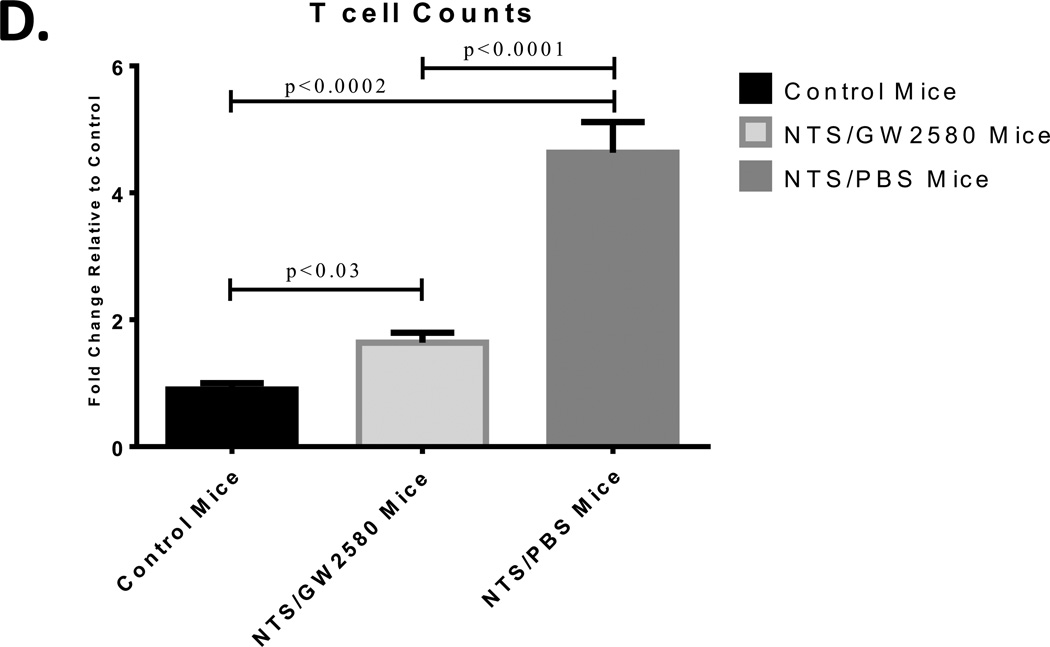
To assess the effect of GW2580 treatment on T cells, we stained kidney sections for CD3. Kidneys from NTS/PBS mice had significant tissue infiltration of CD3 cells, which were absent from normal healthy kidneys (Fig. 7A, B). In contrast, kidney accumulation of CD3+ T cells following the nephritic challenge was abrogated in the NTS/GW2580 group (Fig. 7C, D).
Fig. 7.
Quantification of kidney infiltrating T cells. Control mice (A) had minimal CD3+ cells, while NTS/PBS mice (B) had significant T cell infiltrates (arrow). GW2580 treated mice (C) had significantly less CD3+ cells (short arrows) than NTS/PBS mice. CD3+ cells were quantified (D) in representative images taken at 20× magnification, and values normalized to control mice. Shown are results from one experiment (control mice, n = 5; NTS/GW2580 mice, n = 10; NTS/PBS mice, n = 10), which is representative of the two separate experiments performed. Depicted images are at 20× for A and C, while B is at 40× for enhanced viewing of the infiltrating population. Images are representative of their respective groups.
3.11. Delay in initiation of GW2580 treatment does not diminish treatment effectiveness
Initiating treatment with GW2580 at a later time point in the course of disease in this model was also effective at ameliorating disease. For these experiments, GW2580 treatment began on day 5 (immediately after the NTS transfer) as opposed to day 0 (see timeline, Fig. 8A). Day 5 was chosen so that peak macrophage depletion would occur after the onset of proteinuria, allowing for an assessment of the therapeutic potential of macrophage depletion. GW2580 treated mice developed significantly lower levels of proteinuria (p = 0.04) (Fig. 8B) and serum creatinine (p = 0.04) (Fig. 8C) compared to NTS/PBS mice. As with the experiments described above with earlier initiation of treatment, GW2580 did not interfere with the induction of the disease model (data not shown). Furthermore, as assessed by flow cytometry, significant macrophage depletion was achieved also with later treatment (data not shown).
Fig. 8.
Delayed treatment with GW2580. Treatment with GW2580 starting on day 5, rather than day 0, remains effective in attenuating the development of kidney dysfunction in NTS challenged mice (A) Experimental time line. (B) Proteinuria was significantly reduced in GW2580 treated mice, as determined by the urinary albumin to creatinine ratio. Results are shown as the values normalized to control mice. (C) Serum creatinine does not rise in response to the nephrotoxic challenge in GW2580 treated mice. Results shown are from one experiment (control mice, n = 4; NTS/GW2580 mice, n = 8; NTS/PBS mice, n = 8), which is representative of the two experiments performed.
4. Discussion
LN is seen in a significant proportion of SLE patients, and is associated with a poor prognosis. Further understanding of disease pathogenesis is required in order to uncover more targeted and effective therapies [33]. We have shown in this study that macrophages are an important driver of disease in an inducible murine model of LN that relies upon passive transfer of pre-formed, nephrotoxic antibodies. Macrophage depletion by a selective CSF-1R kinase inhibitor in this model protected mice from developing proteinuria, renal dysfunction, and abnormal renal histology. The experimental system we used here is unique since: a) it does not involve congenital dysregulation of the CSF-1R pathway; and b) administering GW2580 before and with disease induction allowed a more complete assessment of the therapeutic benefit of inhibiting CSF-1R in antibody mediated nephritis.
All mice developed a comparable response to the initial rabbit IgG immunization, regardless of treatment assignment. Furthermore, both NTS/GW2580 mice and NTS/PBS mice were exposed to similar levels of the nephrotoxic antibodies, and had comparable amounts of glomerular deposited IgG. The difference in PAS+ deposits observed between mice receiving NTS with or without GW2580 is therefore likely to be due to other components of these deposits, including non-IgG antibodies and/or complement. Overall, these data suggest that the difference in kidney pathology seen between the groups was not due to the GW2580 drug interfering with the NTS protocol.
GW2580 treatment successfully depleted kidney macrophages, as evidenced by flow cytometry and immunofluorescent staining. These results were further confirmed by RT-PCR, which showed a significant decrease in CCR2 and CX3CR1 expression within the kidney. IBA-1 staining showed macrophage depletion specifically within the glomeruli of treated mice. In addition to this direct evidence, GW2580 treatment significantly reduced the number of intraperitoneal macrophages after thioglycollate challenge, confirming the previous observation by Conway et al. [17]. Therefore, we conclude that kidney macrophages within NTS/GW2580 mice were reduced, and that this depletion is likely responsible for amelioration of disease.
Our results help to clarify past studies which looked at the potential relationship between macrophages and LN, but which were marred by confounding factors and conflicting results. Studies by Davidson et al. [1, 7] described distinct macrophage populations present within murine LN kidneys. These studies, while suggestive, do not necessarily distinguish whether macrophages are the primary cause of disease, or whether macrophage infiltration is a secondary phenomenon. MCP-1 is a chemokine important for attracting macrophages to sites of inflammation. Several studies have targeted MCP-1, either through a specific antagonist [9] or anti-MCP-1 gene therapy [10]. By targeting MCP-1, macrophage recruitment to the kidney was decreased, and kidney disease was attenuated. However, MCP-1 also plays a role in activating kidney tubular epithelial cells [11, 34]. Consequently, any beneficial effect seen in MCP-1 targeting studies may not have necessarily been solely specific to macrophages.
Denny et al. [12], used clodronate liposomes as a means of macrophage depletion in NZBxNZW F1 lupus mice. Contrary to what was expected, diminishing macrophage numbers actually led to a worsening of disease in this strain. However, clodronate depletes macrophages by forcing them to undergo apoptosis. Within the context of spontaneous lupus, the increased apoptotic load was thought to have led to the increase in circulating autoantibody titers which was seen in clodronate treated mice, consequently worsening disease which may have been independent of macrophage depletion. The disease model we employed is not dependent upon generation of anti-nuclear antibodies de-novo for disease induction.
Several other studies examined macrophage depletion within the MRL-lpr/lpr spontaneous murine lupus model. Lenda et al. generated MRL/lpr mice in which the genes for CSF-1 or CSF-1R were knocked out, depleting macrophages from birth. CSF-1 or CSF-1R deficient MRL-lpr/lpr mice did have significantly less severe nephritis [13, 14]. However, CSF-1R regulated macrophages play an important trophic role in normal development [35]. Indeed, congenital CSF-1 or CSF-1R deficiency leads to pronounced tissue abnormalities, including osteopetrosis, altered hematopoiesis, and neurologic deficits [22, 23, 36], which are potentially critical confounding factors. Additionally, Lenda et al. [14] noted that CSF-1 deficiency in these models interfered with autoantibody production and renal deposition. As mentioned above, we validated that neither disease-inducing antibody levels, nor IgG deposition in the kidneys, were affected by GW2580.
Depletion of macrophages from birth (i.e. in the op/op strain (13)) has an additional limitation in that the monocyte pool is significantly depleted, while with GW2580 monocytes were unaffected. Furthermore, the congenital absence of CSF-1 is not equivalent to inhibition of the CSF-1R. The CSF-1R has a second ligand, IL-34, which has unique roles in the regulation of monocyte/macrophage populations [37]. Thus, in the CSF-1 or CSF-1R knockout strains reported previously, nephritis may have been attenuated because it developed on an altered background, preventing the conclusive determination of the therapeutic efficacy of suppressing CSF-1R in lupus.
Several studies addressed role of macrophages within a parallel disease model in rats [38–40]. The most relevant, by Han et al. [38], analyzed the effect of fms-I, a kinase inhibitor which also depletes macrophages, on the progression of nephrotoxic nephritis. While treatment with fms-I did improve renal function, fms-I is not solely specific for the CSF-1R kinase. Rather, this kinase inhibitor also affects c-kit and flt [38], thus introducing off target effects. Moreover, since a well-established spontaneous lupus model does not exist in rats, murine models are preferable. Our studies here can be validated within a wide array of LN models, which makes a murine system more ideal for modeling of human disease. Therefore, either because of the model or the method of depletion, previous rodent studies were not definitive.
Our use of GW2580 represents a depletion method which minimizes confounding factors, and allows us to isolate the role macrophages play in renal disease mediated by nephrotoxic antibodies. GW2580 selectively inhibits the signaling of CSF-1 through the CSF-1R [17, 21, 41]. This signaling is important in several areas of macrophage function, particularly proliferation and survival [20]. It is also important in aiding with macrophage recruitment to inflammatory sites [23]. CSF-1 signaling can affect macrophage membrane ruffling, making macrophages more mobile, and therefore more readily recruited to sites of inflammation within tissues [42]. Additionally, CSF-1 signaling can stimulate monocyte precursors in the bone marrow to differentiate into inflammatory monocytes [20]. The salutatory effect of GW2580 may have been due to the effects of the drug on minimizing kidney macrophage infiltration by inhibiting the recruitment of inflammatory monocytes from the circulation, by negatively affecting proliferation and survival of tissue resident macrophages, or by some combination of these effects.
Our studies strongly indicate that macrophages are instrumental in the pathogenesis of kidney disease in this inducible model of LN. One mechanism by which macrophages can be contributing to disease is through the release of inflammatory mediators including multiple cytokines and chemokines. We found that depleting macrophages significantly downregulates the kidney expression of cytokines known to contribute to disease pathogenesis. Additionally, the decreased expression of certain chemokines, such as RANTES, would explain the decreased number of T cells seen in kidneys of GW2580 treated mice. Furthermore, we were able to show that macrophage depletion prevented the increased expression of both MMP-9 and HMGB1, both macrophage secreted mediators believed to be important in LN progression.
MMP-9 is involved in LN pathogenesis by contributing to extracellular matrix damage and podocyte injury [43]; indeed, CD11B+F4/80high tissue resident macrophages, in the context of nephritis, increase their MMP expression [44]. The proinflammatory activity of HMGB1 is mediated via TLR2, TLR4 and RAGE receptor signaling on both local and infiltrating cells. Both MMP-9 [45] and HMGB1 [46] are increased within diseased kidney tissue and in lupus patient serum, while urinary HMGB1 levels correlate with disease activity scores [47]. HMGB1 stimulated macrophages have been shown to release proinflammatory cytokines, including MCP-1 [31]. MCP-1 can then attract more macrophages into the kidneys, thereby creating a positive feedback loop that amplifies disease. Finally, we had previously demonstrated that anti-DNA antibodies, acting via TLR2 and 4 signaling on mesangial cells, can cause the release of HMGB1 [48]. Therefore, any treatment that decreases HMGB1 expression, such as GW2580, can be beneficial for nephritis mediated by pathogenic antibodies. In fact, GW2580 has translational application to patients. The related CSF-1R inhibitors PLX 3397 and 5622 are in clinical trials for rheumatoid arthritis and cancer, showing that this family of drugs can be adapted for treatment of human disease. This potential approach is further validated by the fact that GW2580 ameliorated disease even when beginning treatment later in the disease model.
5. Conclusions
Our results point toward an important role of macrophages in disease development in immune glomerulonephritis mediated by nephritogenic antibodies, providing strong support for the pivotal role of macrophages in spontaneous lupus nephritis as well. Macrophages seem to be important in translating immune complex deposition into active disease by contributing to the inflammatory environment through the release of cytokines, as well MMP-9 and HMGB1. Overall, this study highlights the important role of the innate immune system, and specifically macrophages, within the context of disease development, and encourages further development of macrophage targeting approaches for the treatment of lupus nephritis.
Supplementary Material
Acknowledgments
These studies were supported by research grants from the National Institutes of Health (DK090319 and AR065594) to C. Putterman. Dr. Putterman is currently a Weston Visiting Professor at the Weizmann Institute of Science.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaut.2014.11.007.
References
- 1.Bethunaickan R, Sahu R, Davidson A. Analysis of renal mononuclear phagocytes in murine models of SLE. Methods Mol Biol. 2012;900:207–232. doi: 10.1007/978-1-60761-720-4_10. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181:5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 4.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 6.Hill GS, Delahousse M, Nochy D, Rémy P, Mignon F, Méry JP, et al. Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages. Kidney Int. 2001;59:304–316. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180:1938–1947. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahu R, Bethunaickan R, Singh S, Davidson A. Structure and function of renal macrophages and dendritic cells from lupus-prone mice. Arthritis Rheumatol. 2014;66:1596–1607. doi: 10.1002/art.38410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa H, Kohno M, Sasaki M, Inoue A, Ito MR, Terada M, et al. Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum. 48:2555–2566. doi: 10.1002/art.11231. 200. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu S, Nakashima H, Masutani K, Inoue Y, Miyake K, Akahoshi M, et al. Anti-monocyte chemoattractant protein-1 gene therapy attenuates nephritis in MRL/lpr mice. Rheumatology (Oxford) 2004;43:1121–1128. doi: 10.1093/rheumatology/keh277. [DOI] [PubMed] [Google Scholar]
- 11.Viedt C, Dechend R, Fei J, Hänsch GM, Kreuzer J, Orth SR. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J Am Soc Nephrol. 2002;13:1534–1547. doi: 10.1097/01.asn.0000015609.31253.7f. [DOI] [PubMed] [Google Scholar]
- 12.Denny MF, Chandaroy P, Killen PD, Caricchio R, Lewis EE, Richardson BC, et al. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J Immunol. 2006;176:2095–2104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
- 13.Lenda DM, Kikawada E, Stanley ER, Kelley VR. Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1-deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol. 2003;170:3254–3262. doi: 10.4049/jimmunol.170.6.3254. [DOI] [PubMed] [Google Scholar]
- 14.Lenda DM, Stanley ER, Kelley VR. Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J Immunol. 2004;173:4744–4754. doi: 10.4049/jimmunol.173.7.4744. [DOI] [PubMed] [Google Scholar]
- 15.Du Y, Fu Y, Mohan C. Experimental anti-GBM nephritis as an analytical tool for studying spontaneous lupus nephritis. Arch Immunol Ther Exp (Warsz) 2008;56:31–40. doi: 10.1007/s00005-008-0007-4. [DOI] [PubMed] [Google Scholar]
- 16.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A. 2005;102:16078–16083. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilbert LJ, Stanley ER. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980;85:153–159. doi: 10.1083/jcb.85.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne PV, Guilbert LJ, Stanley ER. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981;91:848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanley ER, Chitu V. CSF-1 Receptor Signaling in Myeloid Cells. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 22.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 23.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Wei S, Lightwood D, Ladyman H, Cross S, Neale H, Griffiths M, et al. Modulation of CSF-1-regulated post-natal development with anti-CSF-1 antibody. Immunobiology. 2005;210:109–119. doi: 10.1016/j.imbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Xia Y, Campbell SR, Broder A, Herlitz L, Abadi M, Wu P, et al. Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin Immunol. 2012;145:108–121. doi: 10.1016/j.clim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff MD, Diamond B. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc Natl Acad Sci USA. 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chitu V, Ferguson PJ, de Bruijn R, Schlueter AJ, Ochoa LA, Waldschmidt TJ, et al. Primed innate immunity leads to autoinflammatory disease in PSTPIP2-deficient cmo mice. Blood. 2009;114:2497–2505. doi: 10.1182/blood-2009-02-204925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen J, Xia Y, Stock A, Michaelson JS, Burkly LC, Gulinello M, et al. Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. J Autoimmun. 2013;43:44–54. doi: 10.1016/j.jaut.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 31.Moreno JA, Sastre C, Madrigal-Matute J, Muñoz-García B, Ortega L, Burkly LC, et al. HMGB1 expression and secretion are increased via TWEAK-Fn14 interaction in atherosclerotic plaques and cultured monocytes. Arterioscler Thromb Vasc Biol. 2013;33:612–620. doi: 10.1161/ATVBAHA.112.300874. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, et al. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J. 2004;68:396–399. doi: 10.1253/circj.68.396. [DOI] [PubMed] [Google Scholar]
- 33.Bomback AS, Appel GB. Updates on the treatment of lupus nephritis. J Am Soc Nephrol. 2010;21:2028–2035. doi: 10.1681/ASN.2010050472. [DOI] [PubMed] [Google Scholar]
- 34.Viedt C, Orth SR. Monocyte chemoattractant protein-1 (MCP-1) in the kidney: does it more than simply attract monocytes? Nephrol Dial Transplant. 2002;17:2043–2047. doi: 10.1093/ndt/17.12.2043. [DOI] [PubMed] [Google Scholar]
- 35.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamane F, Nishikawa Y, Matsui K, Asakura M, Iwasaki E, Watanabe K, et al. CSF-1 receptor-mediated differentiation of a new type of monocytic cell with B cell-stimulating activity: its selective dependence on IL-34. J Leukoc Biol. 2014;95:19–31. doi: 10.1189/jlb.0613311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Ma FY, Tesch GH, Manthey CL, Nikolic-Paterson DJ. c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat. Lab Invest. 91:978–991. doi: 10.1038/labinvest.2011.61. 201. [DOI] [PubMed] [Google Scholar]
- 39.Ikezumi Y, Hurst LA, Masaki T, Atkins RC, Nikolic-Paterson DJ. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int. 2003;63:83–95. doi: 10.1046/j.1523-1755.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 40.Eddy AA, McCulloch LM, Adams JA. Intraglomerular leukocyte recruitment during nephrotoxic serum nephritis in rats. Clin Immunol Immunopathol. 1990;57:441–458. doi: 10.1016/0090-1229(90)90118-a. [DOI] [PubMed] [Google Scholar]
- 41.Conway JG, Pink H, Bergquist ML, Han B, Depee S, Tadepalli S, et al. Effects of the cFMS kinase inhibitor 5-(3-methoxy-4-((4-methoxybenzyl)oxy)benzyl)pyrimidine-2,4-diamine (GW2580) in normal and arthritic rats. J Pharmacol Exp Ther. 2008;326:41–50. doi: 10.1124/jpet.107.129429. [DOI] [PubMed] [Google Scholar]
- 42.Pixley FJ. Macrophage migration and its regulation by CSF-1. Int J Cell Biol. 2012;2012:501962. doi: 10.1155/2012/501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tveita AA, Rekvig OP, Zykova SN. Increased glomerular matrix metalloproteinase activity in murine lupus nephritis. Kidney Int. 2008;74:1150–1158. doi: 10.1038/ki.2008.308. [DOI] [PubMed] [Google Scholar]
- 44.Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, et al. A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis. J Immunol. 2011;186:4994–5003. doi: 10.4049/jimmunol.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang YH, Lin IL, Tsay GJ, Yang SC, Yang TP, Ho KT, et al. Elevated circulatory MMP-2 and MMP-9 levels and activities in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Biochem. 2008;41:955–959. doi: 10.1016/j.clinbiochem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Pisetsky DS. HMGB1: a smoking gun in lupus nephritis? Arthritis Res Ther. 2012;14:112. doi: 10.1186/ar3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdulahad DA, Westra J, Bijzet J, Dolff S, van Dijk MC, Limburg PC, et al. Urine levels of HMGB1 in Systemic Lupus Erythematosus patients with and without renal manifestations. Arthritis Res Ther. 2012;14:R184. doi: 10.1186/ar4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qing X, Pitashny M, Thomas DB, Barrat FJ, Hogarth MP, Putterman C. Pathogenic anti-DNA antibodies modulate gene expression in mesangial cells: involvement of HMGB1 in anti-DNA antibody-induced renal injury. Immunol Lett. 2008;121:61–73. doi: 10.1016/j.imlet.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.