Abstract
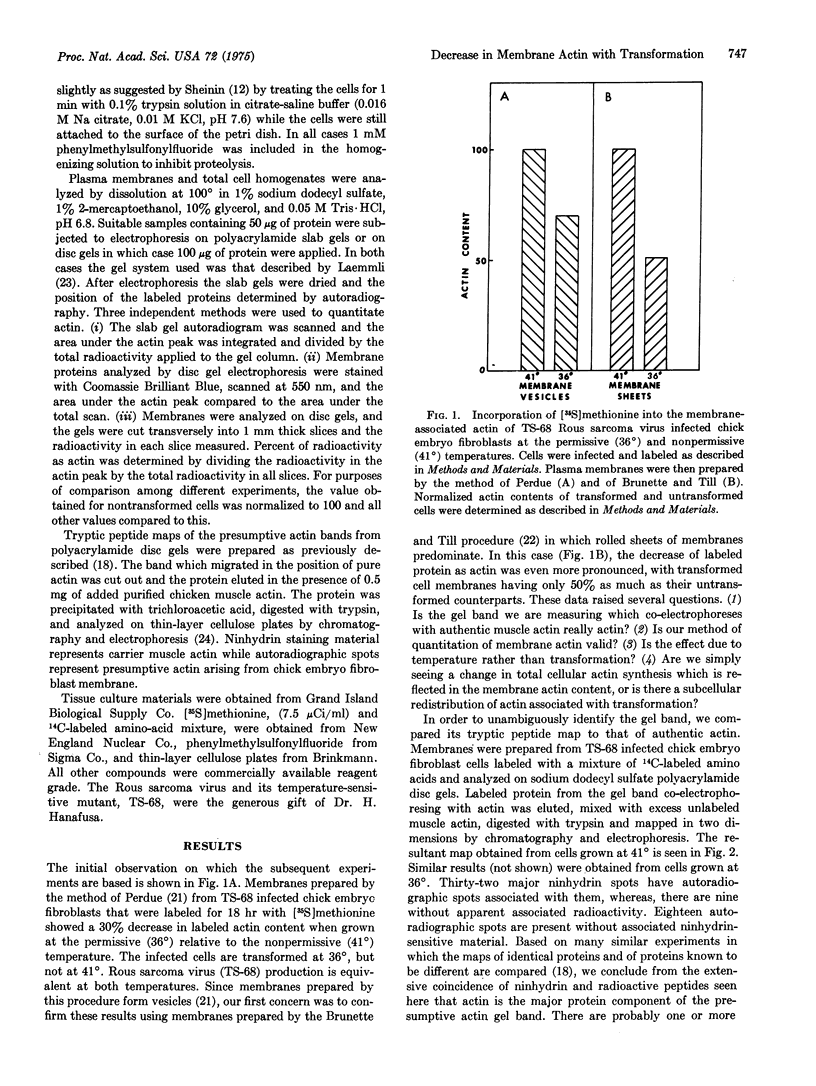
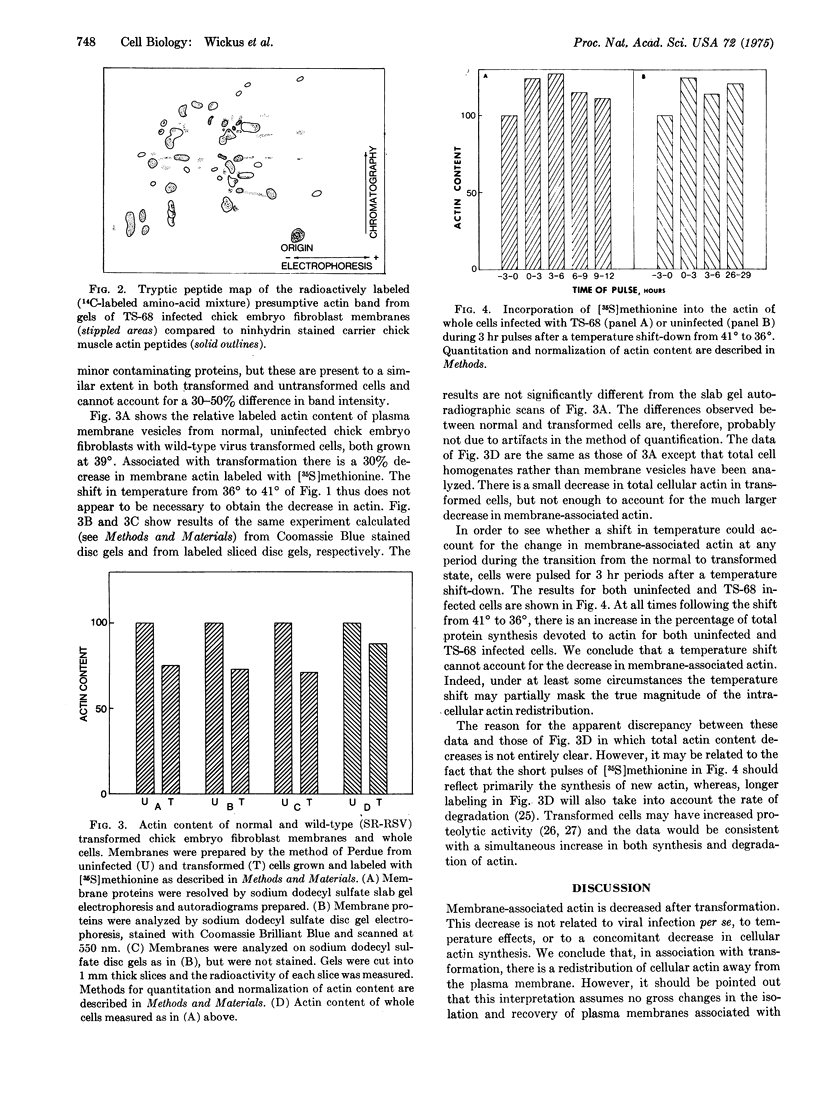
The actin content of membranes prepared from cultured chick embryo fibroblasts has been measured on polyacrylamide gels. The actin was identified by tryptic peptide mapping. After transformation of the cells by Rous sarcoma virus, the amount of actin associated with the membranes is decreased by 30-50%. This result is not due to infection per se, since infection by a temperature-sensitive strain of the virus decreases membrane-associated actin only at the permissive temperature. A shift from the nonpermissive (41 degrees) to the permissive (36 degrees) temperature results in an increase in the percentage of total cellular protein synthesis devoted to actin production, so that the decrease in membrane-associated actin appears to be a selective displacement from the membrane rather than a general decrease in total cellular actin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., HEAYSMAN J. E. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954 May;6(2):293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Bosmann H. B. Elevated glycosidases and proteolytic enzymes in cells transformed by RNA tumor virus. Biochim Biophys Acta. 1972 Apr 21;264(2):339–343. doi: 10.1016/0304-4165(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Brady R. O., Fishman P. H., Mora P. T. Membrane components and enzymes in virally transformed cells. Fed Proc. 1973 Jan;32(1):102–108. [PubMed] [Google Scholar]
- Gerday C., Robyns E., Gosselin-Rey C. High resolution techniques of peptide mapping. Separation of bovine carotid actin peptides on cellulose thin layers and of the corresponding dansyl-peptides on polyamide thin layers. J Chromatogr. 1968 Dec 3;38(3):408–411. doi: 10.1016/0021-9673(68)85069-1. [DOI] [PubMed] [Google Scholar]
- Gruenstein E., Rich A., Weihing R. R. Actin associated with membranes from 3T3 mouse fibroblast and HeLa cells. J Cell Biol. 1975 Jan;64(1):223–234. doi: 10.1083/jcb.64.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Morgan W. D., Pastan I. Regulation of cell motility by cyclic AMP. Nature. 1972 Jan 7;235(5332):54–56. doi: 10.1038/235054a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund R. E., Pastan I., Adelstein R. S. Myosin in cultured fibroblasts. J Biol Chem. 1974 Jun 25;249(12):3903–3907. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Perdue J. F. The distribution, ultrastructure, and chemistry of microfilaments in cultured chick embryo fibroblasts. J Cell Biol. 1973 Aug;58(2):265–283. doi: 10.1083/jcb.58.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Korn E. D. Electron microscopic identification of actin associated with isolated amoeba plasma membranes. J Biol Chem. 1973 Jan 25;248(2):448–450. [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Sheinin R., Onodera K. Studies of the plasma membrane of normal and virus-transformed 3 T 3 mouse cells. Biochim Biophys Acta. 1972 Jul 3;274(1):49–63. doi: 10.1016/0005-2736(72)90279-9. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H., GOLDBERG B. D. TRANSFORMATION OF PROPERTIES OF AN ESTABLISHED CELL LINE BY SV40 AND POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of cells before and after transformation by oncogenic viruses. Fed Proc. 1973 Jan;32(1):80–85. [PubMed] [Google Scholar]
- Weber K., Lazarides E., Goldman R. D., Vogel A., Pollack R. Localization and distribution of actin fibers in normal transformed and revertant cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):363–369. doi: 10.1101/sqb.1974.039.01.047. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. II. Glycosyltransferases and endogenous acceptors of the undifferentiated cell surface membrane. J Biol Chem. 1973 Apr 10;248(7):2542–2548. [PubMed] [Google Scholar]
- Wickus G. G., Robbins P. W. Plasma membrane proteins of normal and Rous sarcoma virus-transformed chick-embryo fibroblasts. Nat New Biol. 1973 Sep 19;245(142):65–67. doi: 10.1038/newbio245065a0. [DOI] [PubMed] [Google Scholar]


