Abstract
Cytokines such as IL-12p70 (“IL-12”) and IL-23 can influence tumor progression. We tested the hypothesis that blood levels of IL-12p40, the common subunit of both cytokines, are associated with melanoma progression. Blood from 2,048 white melanoma patients were collected at a single institution between March 1998 and March 2011. Plasma levels of IL-12p40 were determined for 573 patients (discovery), 249 patients (validation 1), and 244 patients (validation 2). Per 10-unit change of IL-12p40 level was used to investigate associations with melanoma patient outcome among all patients or among patients with early or advanced stage. Among stage I/II melanoma patients in the pooled data set, after adjustment for sex, age, stage and blood draw time from diagnosis, elevated IL-12p40 was associated with melanoma recurrence (hazard ratio[HR]=1.04 per 10-unit increase in IL-12p40, 95% CI 1.02–1.06, P=8.48×10−5); Elevated IL-12p40 was also associated with a poorer melanoma specific survival (HR=1.06, 95% CI 1.03–1.09, P=3.35×10−5) and overall survival (HR=1.05, 95% CI 1.03–1.08, P=8.78×10−7) in multivariate analysis. Among stage III/IV melanoma patients in the pooled data set, no significant association was detected between elevated IL-12p40 and overall survival, or with melanoma specific survival, with or without adjustment for the above covariates. Early-stage melanoma patients with elevated IL-12p40 levels are more likely to develop disease recurrence and have a poorer survival. Further investigation with a larger sample size will be needed to determine the role of IL-12p40 in advanced stage melanoma patients.
Keywords: cytokines, IL-12p40, melanoma, early-stage, progression
Introduction
Interleukin (IL)-12p40 (also called IL-12β) is a common subunit of both IL-12p70 (IL-12p35 plus IL-12p40, commonly referred to as “IL-12”) and IL-23 (IL-23α subunit plus IL-12p40). IL-12p70 plays a role in resistance and adaptive immunity1 and is also involved in antitumor responses2,3. In addition to associating with IL-12p35 and IL-23p19 to comprise the well-studied heterodimeric cytokines IL-12 and IL-23, IL-12p40 can exist in a monomeric form; the monomeric form may play an independent role in immune responses4. A recent investigation in mice has demonstrated that the free IL-12p40 monomer can combine with multiple distinct proteins to function as a pleiotropic adaptor5. A recently published immunization strategy using IL-12p70-producing dendritic cells in melanoma patients suggested a potential therapeutic role for this cytokine6. IL-23, on the other hand, contributes to autoimmune inflammation7 and stimulates tumor growth and progression by providing a tumor-promoting microenvironment8. Thus these cytokines can mediate host responses to tumors; however, their clinical importance in melanoma development and progression has not been determined. Standard assays for IL-12 measure the IL-12p40 subunit, and includes measurement of the free unit as well as the sum total of both IL-12p70 and IL-23 levels in blood. While assays for the IL-12p35 and IL-23α subunits are available, measured blood levels are typically very low relative to IL-12p40. We hypothesized that blood levels of IL-12p40 in melanoma patients might reflect an important aspect of patient immune and inflammatory response to melanoma, and therefore might be a predictor of disease susceptibility, progression or melanoma-related death.
Methods
Study populations
Our initial patient population was derived from a large hospital-based study of cutaneous melanoma, for which a total of 1,804 non-Hispanic white patients were recruited at The University of Texas MD Anderson Cancer Center (MD Anderson) between March 1998 and March 2011. Patients with all stages of invasive cutaneous melanoma were eligible for inclusion. All patients gave informed consent under an institutional review board-approved protocol. The study protocols have been described previously9. Patients with in situ disease or atypical melanocytic proliferation were excluded from the study.
Adequate plasma samples for IL-12p40 determination were available from 573 patients (discovery) and 249 patients (validation 1) from the initial cohort of 1,804 patients. Patient blood samples were drawn prior to receiving any treatment. In order to further validate the relationship between IL-12p40 and melanoma progression following initial analysis of our discovery and validation 1 patient cohorts, we evaluated an additional 244 patients with invasive melanoma (validation 2). To evaluate if IL-12p40 levels were different between cases and healthy controls, we tested IL-12p40 levels in a population of 299 healthy controls. Therefore in total we collected clinical and follow-up data from 2,048 patients, among whom 1,066 cases and 299 controls had adequate plasma stocks for IL-12p40 plasma determination. Sentinel lymph node biopsy was performed in 1,282 of 1,826 patients (70.2%) who presented with clinically localized disease, including 779 of 882 patients (88.3%) with tumors at least 1 mm in thickness. The overall rate of involved sentinel lymph nodes was 17.9% (230/1,282).
Data on demographic information and clinical prognostic factors (2009 American Joint Committee on Cancer stage, Breslow tumor thickness, ulceration, mitosis, and sentinel lymph node status) were investigated. Since analysis of patients who underwent multiple blood draws identified no significant change in IL-12p40 levels with length of patient follow-up (Supplementary Table 1), length of follow-up and survival duration was determined from the date of diagnosis until last contact date or death. All patients had follow-up data available. Patients were defined as having melanoma recurrence if they developed local, regional, in-transit or distant metastasis during the follow-up period.
Experiments
Plasma levels of IL-12p40 were determined in 1,066 patients. The 573 patients in the IL-12p40 discovery set represented patients who had undergone prior IL-12p40 determination for an earlier investigation. The 249 patients included in validation set 1 were those from the initial cohort of 1,804 patients who also had sufficient plasma available for IL-12p40 determination following analysis of the discovery set results; the 244 patients included in validation set 2 represent a more recently acquired cohort of samples. To evaluate if IL-12p40 levels were different between cases and healthy controls, we investigated IL-12p40 levels in a population of 299 healthy controls and compared these levels to those in melanoma patients. Samples were collected in heparinized tubes and subjected to centrifugation at 1500 rpm ×10 minutes. The plasma layer was stored at −80°C before analysis, and levels were determined from batch-thawed and processed samples. Plasma IL-12p40 levels were determined by an enzyme-linked immunosorbent assay (Invitrogen, Carlsbad, CA) using the sandwich technique with an antibody that recognizes IL-12p40 subunit (either in the form of free monomer or as part of a dimer). All measurements were performed according to the procedures recommended by the manufacturer. The minimum detectable level of IL-12p40 was <2pg/ml, and the upper limit of linearity was >500 pg/ml.
Statistical analysis
We evaluated the relationship between per 10-unit change of IL-12p40 levels and patient outcome measures (overall survival, melanoma-specific survival, and disease-free survival) by Cox regression in SAS Enterprise Guide 4.3 (SAS Institute, Cary, NC). Sex, age, stage and blood draw time from diagnosis were adjusted in the multivariate analysis. A P-value of 0.05 was considered significant. IL-12p40 levels were also dichotomized at 150 pg/ml, which represents the upper limit of normal according to the manufacturer, to evaluate the association of a binary IL-12p40 variable with melanoma patient outcome measures.
Results
The distribution of raw plasma IL-12p40 data in the discovery data set was skewed (Skewness=3.27, kurtosis=19.00; Supplementary Fig. 1a), but was symmetric following log transformation (Skewness=0.04, kurtosis=0.47; Supplementary Fig. 1b). We also performed log transformation of IL-12p40 data in the two case validation groups and the distribution of log-transformed data in the two validation groups was symmetric (Case validation set 1 and 2, Supplementary Figs. 2 and 3). Log transformation of IL-12p40 was used when we observed batch effect on change of IL-12p40 levels or compared IL-12p40 levels across different groups of a phenotypic factor.
There was no difference in IL-12p40 levels in validation case-sets compared with controls (P=0.9583; Supplementary Table 2). The mean plasma IL-12p40 level appeared lower in discovery cases (tested in 2007) compared to validation set cases (tested in 2011), although the difference was not significant; (discovery VS validation set 1, P=0.438; discovery VS validation set 2, P=0.200; Supplementary Table 2). We investigated the hypothesis that this potential difference was related to a batch effect. We re-tested 11 old samples that had been first tested in 2007 using the 2011 kit and compared the two test results. Results on re-testing in 2011 were slightly higher (log-transformed mean 4.5% higher) than results obtained in 2007, but had a high correlation with results from the old kit (r2=0.87, P=0.001; Supplementary Table 3), suggesting that there was in fact a batch effect due to a higher calibration of patient plasma IL-12p40 levels with the newer kit. A separate analysis of 11 samples for correlation of plasma IL-12p40 levels with sample storage time found no measurable change with storage over two years’ time (Supplementary Table 4, Wilcoxon matched-pairs signed-rank test P=0.1307; Pearson correlation r2=0.88, P<0.001), suggesting the change in measured IL-12p40 between batches was not due to differences in storage time. Finally, an analysis of 66 patients who underwent serial blood determinations found no measurable overall change between blood draws, with median time between blood draws of 6.3 months (Supplementary Table 1, P=0.1209; correlation between duration and change of log transformed IL-12p40 Pearson r2=0.14, P=0.1097). These findings taken together support a small batch effect due to differences in kit calibration. That is, any observed differences in IL-12p40 levels between the discovery and validation sets does not appear to have been due to differences in patient population or sample storage time, but rather due to differences in the kits themselves. In part because of this finding, the second validation set was obtained, and pooled data were evaluated via meta-analysis rather than by directly combining the data sets. The association of demographic and clinical factors with log(IL-12p40) in each data set is provided in Table 1. Females had significantly higher levels of IL-12p40 than did males in case validation set 1 (P=0.0002) and validation set 2 (P=0.0079); patients with stage III/IV melanoma had significantly higher levels of IL-12p40 than did those with stage I/II melanoma only in the discovery set (P=0.0171).
Table 1.
Association of clinical factors with log(IL-12p40) among 1066 patients
| Discovery set(=573) | Case validation set 1(=249) | Case validation set 2(=244) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | N | Mean(SD) | P-value | N | Mean(SD) | P-value | N | Mean(SD) | P-value |
| Age at diagnosis | 573 | 53.15(15.09) | - | 249 | 52.79(13.64) | - | 244 | 46.06(14.98) | - |
|
| |||||||||
| Sex | |||||||||
| Male | 321 | 4.28(0.59) | 0.2657 | 159 | 4.47(0.58) | 0.0002 | 118 | 4.14(0.56) | 0.0079 |
| Female | 252 | 4.34(0.61) | 90 | 4.75(0.51) | 126 | 4.35(0.64) | |||
|
| |||||||||
| Stage at diagnosis | |||||||||
| I, II | 453 | 4.28(0.58) | 0.0171 | 106 | 4.55(0.56) | 0.5903 | 192 | 4.26(0.64) | 0.6467 |
| III, IV | 120 | 4.42(0.64) | 143 | 4.59(0.57) | 52 | 4.21(0.48) | |||
|
| |||||||||
| Tumor thickness | |||||||||
| 0–1 | 222 | 4.23(0.51) | 0.3046 | 68 | 4.52(0.62) | 0.7973 | 116 | 4.27(0.64) | 0.8501 |
| 1–2 | 150 | 4.33(0.64) | 51 | 4.58(0.56) | 47 | 4.27(0.66) | |||
| 2–4 | 89 | 4.33(0.61) | 66 | 4.61(0.56) | 40 | 4.19(0.52) | |||
| 4- | 53 | 4.36(0.68) | 23 | 4.53(0.53) | 23 | 4.18(0.56) | |||
|
| |||||||||
| Ulceration | |||||||||
| Not present | 396 | 4.29(0.58) | 0.6541 | 155 | 4.57(0.57) | 0.5076 | 179 | 4.27(0.63) | 0.2380 |
| Present | 92 | 4.32(0.64) | 47 | 4.51(0.61) | 41 | 4.18(0.54) | |||
|
| |||||||||
| Mitosis | |||||||||
| 0–1 | 124 | 4.27(0.57) | 0.7849 | 51 | 4.52(0.53) | 0.4165 | 1 | 3.58(-) | - |
| 1- | 291 | 4.29(0.60) | 141 | 4.60(0.60) | 206 | 4.26(0.62) | |||
|
| |||||||||
| Sentinel lymph node | |||||||||
| Negative | 339 | 4.26(0.59) | 0.1147 | 70 | 4.53(0.59) | 0.7592 | 137 | 4.31(0.63) | 0.3331 |
| Positive | 70 | 4.38(0.63) | 67 | 4.57(0.61) | 40 | 4.20(0.50) | |||
Standard clinical predictors of outcome including AJCC stage at presentation (all patients), and tumor thickness, ulceration, mitotic rate and AJCC stage at presentation (stage I/II patients) were significantly associated with relevant patient outcome measures, including disease recurrence, melanoma-specific survival, and overall survival (Supplementary Table 5).
Because the IL-12p40 heterodimers IL-12 and IL-23 represent the best-studied IL-12p40 cytokines and could play opposing roles in melanoma tumor formation and progression, we preliminarily evaluated relative levels of IL-12p70 and IL-23 compared to IL-12p40 in plasma from a limited number of melanoma patients using high-sensitivity Elisa assays (R&D Systems). Among 53 melanoma patient samples tested for IL-12p70, 75% of samples had levels below the detectable limit, with a median level of 0.43 pg/ml. Among 17 melanoma patient samples tested for IL-23, 90% of samples had levels below the detectable limit, with a median level of 3.83 pg/ml. In contrast, among all 493 melanoma patients from validation 1 and 2 cohorts analyzed using a current-generation assay for IL-12p40, the median measured level was 79.85 pg/ml. While additional investigation of IL-12p70 and IL-23 levels in melanoma patients is warranted, it appears plausible that major amounts of measured IL-12p40 in the blood of melanoma patients commonly exists in forms other than as components of IL-12p70 or IL-23.
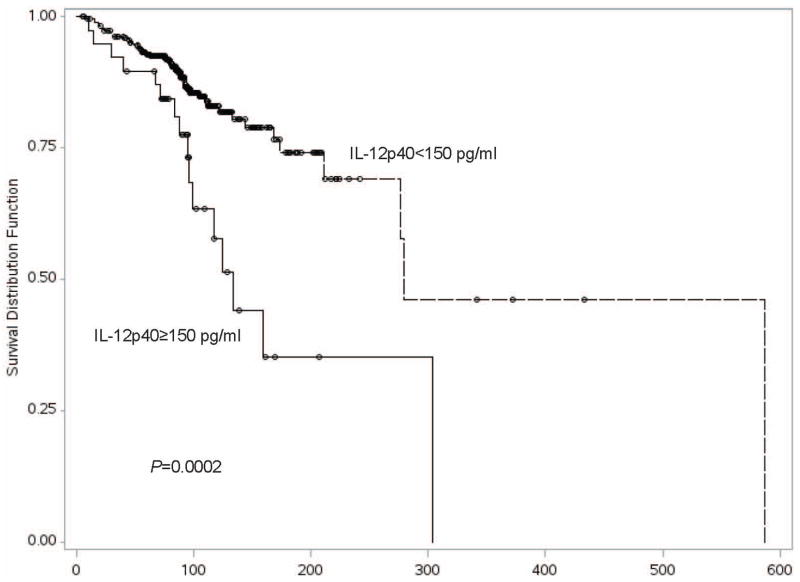
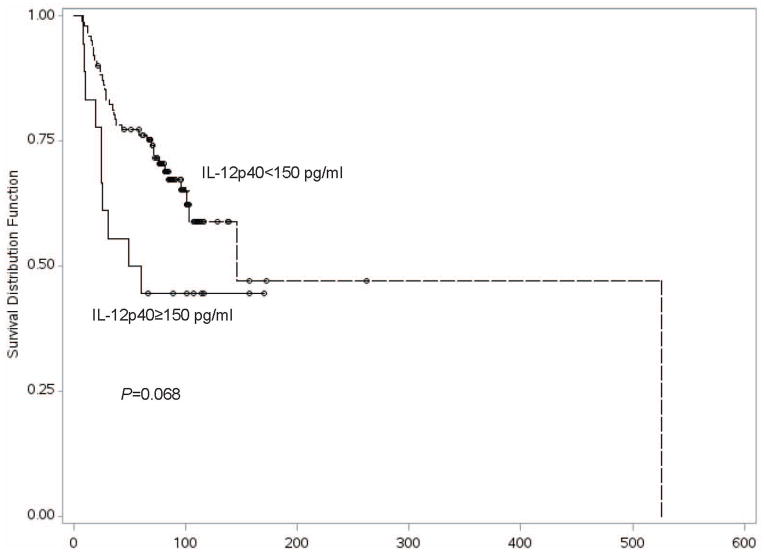
We evaluated the relationship between IL-12p40 blood levels and melanoma patient outcomes. The median follow-up time from the date of diagnosis was 87.5 months in the discovery set, 64.5 months in case validation set 1, and 66.7 in case validation set 2. Univariate analysis of blood levels in the discovery set demonstrated that elevation in IL-12p40 was significantly associated with overall survival among all-stage patients [HR=1.04 per 10 unit increase in IL-12p40, 95% CI 1.02–1.06, P<0.0001] and among the subset of patients with stage I or II melanoma [HR=1.04 per 10 unit increase in IL-12p40, 95% CI 1.02–1.06, P=0.0003] (Table 2) [Log-rank test to compare overall survival between IL-12p40≥150 pg/ml group and IL-12p40 <150 pg/ml group, P=0.0002] (Figure 1). Multivariate analysis of blood levels in the discovery set further demonstrated the association of elevated IL-12p40 with overall survival among all-stage patients [HR=1.04 per 10 unit increase in IL-12p40, 95% CI 1.02–1.06, P=0.0003] and among the subset of patients with stage I or II melanoma [HR=1.04 per 10 unit increase in IL-12p40, 95% CI 1.02–1.07, P=0.0008]. The association of elevated plasma IL-12p40 level with poorer OS in early-stage melanoma was replicated in both case validation sets 1 and 2, and in the pooled data set using meta-analysis [HR=1.05 per 10 unit increase in IL-12p40 in the pooled data set, 95% CI 1.03–1.08, P=8.78×10−7] (Table 2, Supplementary Table 6). In contrast, an elevated IL-12p40 level was neither significantly associated with poorer overall survival among stage III or IV patients in the pooled data set [HR=1.02, 95% CI 0.99–1.04, P=0.200], nor in each separate data set (Table 2) [Log-rank test to compare overall survival between IL-12p40≥150 pg/ml group and IL-12p40 <150 pg/ml group, P=0.068] (Figure 2).
Table 2.
Association of IL-12p40 with overall survival among 1066 patientsd
| Variable | Discovery set(N=573) | Case validation set 1(N=249) | Case validation set 2(N=244) | Pooled results in a meta-analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| N/Events | HR(95%CI) | P-value | N/Events | HR(95%CI) | P-value | N/Events | HR(95%CI) | P-value | HR(95%CI) | P-value | |
| All patients | |||||||||||
| Univariate | 573/121 | 1.04(1.02–1.06) | <0.0001 | 249/68 | 1.03(1.00–1.05) | 0.0552 | 244/43 | 1.03(0.99–1.08) | 0.1835 | 1.04(1.02–1.05) | 6.44×10−7 |
| Multivariatea | 573/121 | 1.04(1.02–1.06) | 0.0003 | 249/68 | 1.02(1.00–1.04) | 0.2178 | 244/43 | 1.07(1.02–1.13) | 0.0135 | 1.03(1.02–1.05) | 3.01×10−5 |
| Stage I/II | |||||||||||
| Univariate | 453/75 | 1.04(1.02–1.06) | 0.0003 | 106/11 | 1.06(1.01–1.12) | 0.0197 | 192/20 | 1.08(1.03–1.15) | 0.0033 | 1.05(1.03–1.07) | 8.02×10−7 |
| Multivariateb | 453/75 | 1.04(1.02–1.07) | 0.0008 | 106/11 | 1.08(1.02–1.16) | 0.0157 | 192/20 | 1.12(1.05–1.19) | 0.0005 | 1.05(1.03–1.08) | 8.78×10−7 |
| Stage III/IV | |||||||||||
| Univariate | 120/46 | 1.04(1.00–1.09) | 0.0570 | 143/57 | 1.01(1.00–1.05) | 0.4391 | 52/23 | 0.96(0.83–1.12) | 0.6237 | 1.02(1.00–1.05) | 1.04×10−1 |
| Multivariatec | 120/46 | 1.05(1.00–1.09) | 0.0628 | 143/57 | 1.01(0.98–1.04) | 0.6206 | 52/23 | 0.95(0.83–1.08) | 0.4300 | 1.02(0.99–1.04) | 2.00×10−1 |
Adjusted for sex, age, stage(I–IV) and blood draw time from diagnosis;
Adjusted for sex, age, stage(I, I/II, II) and blood draw time from diagnosis;
Adjusted for sex, age, stage(III, IV) and blood draw time from diagnosis;
Follow-up months:
| Discovery set | All: 93.8±56.0, median 87.5; | I–II: 98.0±54.6, median 88.9; | III–IV:78.0±58.5, median 77.8 |
| Validation set 1 | All: 74.4±57.4, median 64.5; | I–II: 90.2±78.9, median 65.3; | III–IV:62.6±28.5, median 63.1 |
| Validation set 2 | All: 64.1±31.1, median 66.7; | I–II: 69.3±29.7, median 66.9; | III–IV:36.9±21.8, median 32.8 |
Figure 1.
Figure 2.
In order to further evaluate the association of plasma IL-12p40 level with early-stage melanoma patient outcome, we investigated potential associations of plasma log(IL-12p40) with melanoma recurrence (disease-free survival, DFS) and melanoma-related death (melanoma-specific survival, MSS). An elevated IL-12p40 level was significantly associated with a poorer DFS in stage I/II melanoma patients in the pooled data set [Univariate analysis HR=1.03, 95% CI=1.02–1.05, P=4.21×10−4; multivariate analysis HR=1.04, 95% CI=1.02–1.06, P=8.48×10−5], as well as in the discovery and validation 1 data sets (Table 3 and Supplementary Table 5), and with a poorer MSS in stage I/II melanoma patients in the pooled data set [Univariate analysis, HR=1.05, 95% CI=1.02–1.07, P=3.04×10−4; multivariate analysis, HR=1.06, 95% CI=1.03–1.09, P=3.35×10−5], as well as in the discovery and validation 2 data sets (Table 4 and Supplementary Table 6).
Table 3.
Association of IL-12p40 with disease-free survival among 743 patients with stage I/II
| Variable | Discovery setb | Case validation set 1b | Case validation set 2b | Pooled results in a meta-analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| N/Events | HR(95%CI) | P-value | N/Events | HR(95%CI) | P-value | N/Events | HR(95%CI) | P-value | HR(95%CI) | P-value | |
| Univariate | 453/86 | 1.04(1.01–1.06) | 0.0018 | 111/26 | 1.03(0.99–1.08) | 0.1398 | 179/23 | 1.03(0.96–1.09) | 0.4389 | 1.03(1.02–1.05) | 4.21×10−4 |
| Multivariatea | 453/86 | 1.04(1.02–1.06) | 0.0016 | 111/26 | 1.05(1.00–1.10) | 0.0410 | 179/23 | 1.04(0.97–1.12) | 0.2339 | 1.04(1.02–1.06) | 8.48×10−5 |
Adjusted for sex, age, stage(I, I/II, II) and blood draw time from diagnosis;
Mean(± standard deviation) and median follow-up months: in the discovery set: 63.4(±46.7) and 61.0; in the case validation set 1: 79.2(±74.3) and 64.9; in the case validation set 2: 71.3(±32.0) and 72.1
Table 4.
Association of IL-12p40 with melanoma-specific survival
| Variable | Discovery set(N=573) | Case validation set 1(N=249) | Case validation set 2(N=244) | Pooled results using meta-analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| N/Events | HR(95%CI) | P-value | N/Events | HR(95%CI) | P-value | N/Events | HR(95%CI) | P-value | HR(95%CI) | P-value | |
| All patients | |||||||||||
| Univariate | 573/91 | 1.04(1.01–1.06) | 0.0012 | 249/59 | 1.03(1.00–1.06) | 0.0233 | 244/36 | 1.02(0.96–1.08) | 0.5325 | 1.03(1.02–1.05) | 7.59×10−5 |
| Multivariatea | 573/91 | 1.04(1.01–1.07) | 0.0053 | 249/59 | 1.02(1.00–1.05) | 0.0914 | 244/36 | 1.07(1.00–1.15) | 0.0672 | 1.03(1.02–1.05) | 3.97×10−4 |
|
| |||||||||||
| Stage I/II | |||||||||||
| Univariate | 453/48 | 1.04(1.01–1.07) | 0.0134 | 106/8 | 1.05(0.99–1.12) | 0.0944 | 192/13 | 1.09(1.02–1.17) | 0.0148 | 1.05(1.02–1.07) | 3.04×10−4 |
| Multivariateb | 453/48 | 1.05(1.01–1.08) | 0.0059 | 106/8 | 1.07(1.00–1.15) | 0.0557 | 192/13 | 1.15(1.06–1.25) | 0.0013 | 1.06(1.03–1.09) | 3.35×10−5 |
|
| |||||||||||
| Stage III/IV | |||||||||||
| Univariate | 120/43 | 1.03(0.99–1.08) | 0.1889 | 143/51 | 1.02(0.99–1.06) | 0.1429 | 52/23 | 0.96(0.83–1.12) | 0.6237 | 1.02(1.00–1.05) | 6.69×10−2 |
| Multivariatec | 120/43 | 1.03(0.98–1.09) | 0.1844 | 143/51 | 1.02(0.99–1.05) | 0.2321 | 52/23 | 0.95(0.83–1.08) | 0.4300 | 1.02(1.00–1.05) | 1.25×10−1 |
Adjusted for sex, age, stage(I–IV) and blood draw time from diagnosis;
Adjusted for sex, age, stage(I, I/II, II) and blood draw time from diagnosis;
Adjusted for sex, age, stage(III, IV) and blood draw time from diagnosis;
Discussion
Melanoma is a relatively immunogenic tumor; recent reports have demonstrated greatly increased efficacy of immunotherapies in the treatment of advanced-stage melanoma patients10–13. Immunotherapeutic approaches to the treatment of melanoma have included IL-2, IFN-α, vaccines, adoptive immunotherapy, anti-CTLA4, anti-PD-1 and other biologic response modifiers. Importantly, dual checkpoint blockade with combinations of both anti-CTLA-4 and anti-PD-1 appears to be significantly more effective and probably no more toxic than monotherapy with either agent alone10,12,13. While these are encouraging results, not all patients benefit, long-term outcomes are still unknown, and treatment-related toxicities can be high. Therefore, it remains important to investigate the role of immune and inflammatory mechanisms in melanoma prognosis, in part through continued biomarker discovery. The cytokine subunit IL-12p40 is the form of IL-12 most readily measurable in blood and plays an important role in inflammation and tumor growth1. IL-12p40 is a subunit of IL-12p70, which may have therapeutic value in treating advanced-stage melanoma patients6. We therefore sought to investigate the role of IL-12p40 blood levels in melanoma patient prognosis.
We measured IL-12p40 levels in plasma using an assay that recognizes IL-12p40 as a component of the heterodimers IL-12p70 and IL-23, as well as the free IL-12p40 subunit. While coordinated, direct determination of IL-12p35 and IL-23α subunit levels in blood could assist in understanding the relative roles of IL-12p70, IL-23 and free IL-12p40 in the observed associations, measured levels of these subunits in blood using currently available assay systems are typically very low; we confirmed this by testing a limited number of melanoma patient samples. We have not yet formally investigated levels of these subunits in a large number of melanoma patients. Such an investigation could be informative but is beyond the scope of the current report, as it would ideally be performed following development of more sensitive assays to more accurately identify the often very low amounts of heterodimers present, as well as techniques to identify both potentially novel subunits and measure the free IL-12p40 subunit directly. The discrepancy between measured IL-12p40 levels compared to IL-12p70 and IL-23 levels do suggest, however, that the observed association between IL-12p40 levels and melanoma patient outcome could be more dependent on levels of free IL-12p40 subunit, rather than levels of the intact heterodimers.
Somewhat higher levels of IL-12p40 were identified among cases from the validation group compared to cases from the discovery group; this discrepancy appears to have been the result of a small batch effect (differences in the assay system used in the discovery and validation groups). For studies with multiple batches, mixed effect models can address inter-assay variability and provide a measure of quality assurance14. One can also use meta-analysis to address heterogeneity across different groups. We therefore analyzed IL-12p40 data from different assays separately, obtained a second validation set, and used meta-analysis to combine results from each group to control for the batch effect. We also found a strong correlation among sample IL-12p40 levels for samples tested in batches 1 and 2 even though the mean levels varied among batches by about 5%. We assumed that batch effect of IL-12p40 among different case groups would not affect IL-12 within the same assay batch and therefore we could evaluate the effect of IL-12p40 levels on patient outcome within each group; our results support this assumption insofar as fundamental findings in the two validation data sets mirror those in the discovery data set.
Elevated levels of IL-12p40 in plasma were associated with a higher risk of all-cause death among stage I and II melanoma patients in our discovery set and both validation sets. Identified associations of elevated IL-12p40 with higher risks of melanoma recurrence and melanoma-specific mortality suggest that most of the excess mortality is melanoma-related. Although our pooled meta-analysis suggests a possible association of elevated plasma IL-12p40 with poorer OS and MSS in advanced-stage melanoma patients, we regard these associations as unconfirmed and requiring further investigation. The lack of a consistent association of IL-12p40 level with stage of disease at presentation combined with data suggesting IL-12p40 levels remain quite stable in individual patients over time suggests that measured IL-12p40 levels in blood are not a reflection of underlying tumor burden, but instead are more representative of a relatively fixed immunologic “set point” that could possibly be controlled at least in part by genetic factors. Furthermore, in contrast to our reproducible findings regarding disease progression in early-stage melanoma patients, the absence of an identifiable difference in IL-12p40 plasma levels between melanoma cases and controls may indicate that blood levels of IL-12p40 may not be an etiological risk factor for the development of melanoma.
In summary, the data presented provide evidence linking elevated IL-12p40 blood levels with a poorer outcome in early-stage melanoma patients. This finding was replicated twice within this investigation but awaits additional confirmation in external studies. In order to evaluate potential mechanisms associating IL-12p40 levels with melanoma patient outcomes, we believe it will be important to investigate genetic regulation of IL-12p40 blood levels in melanoma patients and controls. To better understand the molecular basis of melanoma susceptibility and disease progression, the function of a variety of cytokines should also be explored and incorporated into a systematic network. These data, taken together, suggest that strategies directed towards IL-12p40 and related cytokines could ultimately have therapeutic value in melanoma patients.
Supplementary Material
What’s new?
As a common subunit of both IL-12p70 (“IL-12”) and IL-23, IL-12p40 is believed to play a role in inflammation, epithelial tumorigenesis, and control of tumor growth and metastasis formation. The authors performed a hospital-based study and identified the role of IL-12p40 in melanoma recurrence, melanoma-specific survival and overall survival. The results suggest that IL-12p40 represents an independent marker of disease progression in early-stage melanoma patients, and could represent a promising target for therapeutic intervention.
Acknowledgments
FUNDING
This work was supported by the National Cancer Institute SPORE grant P50 CA093459, the Miriam and Jim Mulva Melanoma Research Fund and the Marit Peterson Fund for Melanoma Research.
Footnotes
Conflict of interest statement: Merrick I. Ross declared a potential financial conflict of interest as follows: GSK, Amgen, Merck. The other coauthors declared no financial conflicts of interest.
Reference List
- 1.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature Reviews Immunology. 2003 Feb;3(2):133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 2.Fallarino F, Uyttenhove C, Boon T, et al. Endogenous IL-12 is necessary for rejection of P815 tumor variants in vivo. Journal of Immunology. 1996 Feb 1;156(3):1095–100. [PubMed] [Google Scholar]
- 3.Quaglino E, Rovero S, Cavallo F, et al. Immunological prevention of spontaneous tumors: a new prospect? Immunology Letters. 2002 Feb 1;80(2):75–9. doi: 10.1016/s0165-2478(01)00323-6. [DOI] [PubMed] [Google Scholar]
- 4.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends in Immunology. 2007 Jan;28(1):33–8. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Abdi K, Singh NJ, Spooner E, et al. Free IL-12p40 Monomer Is a Polyfunctional Adaptor for Generating Novel IL-12-like Heterodimers Extracellularly. The Journal of Immunology. 2014 Jun 15;192(12):6028–36. doi: 10.4049/jimmunol.1400159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreno BM, Becker-Hapak M, Huang A, et al. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J Clin Invest. 2013 Jul 11;123(8):0. doi: 10.1172/JCI68395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langrish CL, McKenzie BS, Wilson NJ, et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunological Reviews. 2004 Dec;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 8.Langowski JL, Zhang XQ, Wu LL, et al. IL-23 promotes tumour incidence and growth. Nature. 2006 Jul 27;442(7101):461–5. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 9.Amos CI, Wang LE, Lee JE, et al. Genome-wide association study identifies novel loci predisposing to cutaneous melanoma. Human Molecular Genetics. 2011 Dec 15;20(24):5012–23. doi: 10.1093/hmg/ddr415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamid O, Robert C, Daud A, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013 Jun 2;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagai H, Oniki S, Fujiwara S, et al. Antimelanoma immunotherapy: clinical and preclinical applications of IL-12 family members. Immunotherapy. 2010 Sep;2(5):697–709. doi: 10.2217/imt.10.46. [DOI] [PubMed] [Google Scholar]
- 12.Riley JL. Combination Checkpoint Blockade-Taking Melanoma Immunotherapy to the Next Level. N Engl J Med. 2013 Jun 2;369(2):187–9. doi: 10.1056/NEJMe1305484. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013 Jun 2;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitcomb BW, Perkins NJ, Albert PS, et al. Treatment of Batch in the Detection, Calibration, and Quantification of Immunoassays in Large-scale Epidemiologic Studies. Epidemiology. 2010 Jul;21:S44–S50. doi: 10.1097/EDE.0b013e3181dceac2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.