Abstract
Vitamin A, retinol, circulates in blood bound to retinol-binding protein (RBP) which, in turn, associates with transthyretin (TTR) to form a retinol-RBP-TTR ternary complex. At some tissues, retinol-bound (holo-) RBP is recognized by a membrane protein termed STRA6, which transports retinol from extracellular RBP into cells and, concomitantly, activates a JAK2/STAT3/5 signaling cascade that culminates in induction of STAT target genes. STRA6-mediated retinol transport and cell signaling are critically inter-dependent, and they both require the presence of cellular retinol-binding protein 1 (CRBP1), an intracellular retinol acceptor, as well as a retinol-metabolizing enzyme such as lecithin:retinol acyltransferase (LRAT). STRA6 thus functions as a “cytokine signaling transporter” which couples vitamin A homeostasis and metabolism to cell signaling, thereby regulating gene transcription. Recent studies provided molecular level insights into the mode of action of this unique protein.
Keywords: vitamin A, retinol-binding protein, STRA6, cytokine receptor, JAK/STAT
Introduction
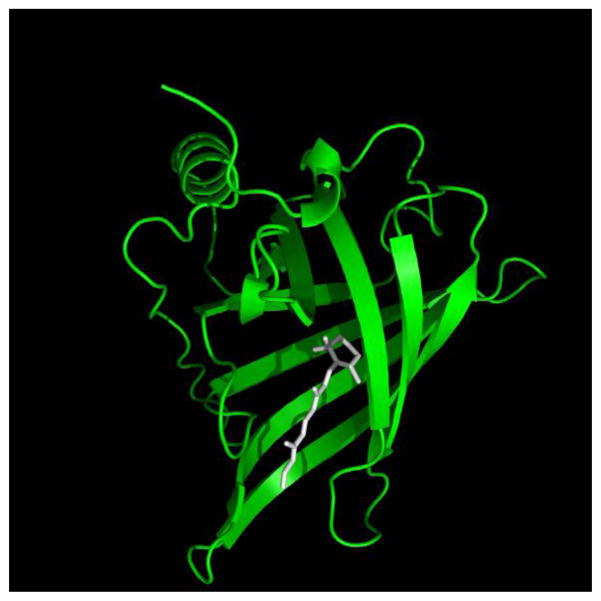
Vitamin A was recognized as an essential factor in foods about a century ago [1, 2] and a substantial body of knowledge on its mechanisms of action and biological functions has since accumulated [3]. It is usually believed that the parental vitamin A, retinol, is biologically inert and that it exerts its biological activities only by giving rise to active metabolites including 11-cis-retinal, critical for vision, and all-trans-retinoic acid (RA) (Fig. 1), which regulates gene transcription by activating several members of the nuclear receptor family of ligand-controlled transcription factors [4–7]. Vitamin A is stored in the body in the form of retinylesters (Fig. 1) and its major storage site is in stellate cells in the liver [8]. The vitamin is secreted from the hepatic pool into the circulation bound to retinol-binding protein (RBP), a member of the lipocalin family of proteins [9, 10]. Lipocalins share a low sequence identity but a highly conserved overall fold. They are comprised of an eight-stranded antiparallel β-sheet that is folded over itself to form a β-barrel which constitutes the ligand binding pocket. The amino termini of lipocalins wrap around the back of the barrel, ‘capping’ that side of the pocket. In contrast, the front of the β-barrel is open, providing an entryway for the ligand which is flanked by a single loop. Retinol binds to RBP with the β-ionone ring innermost and the hydroxyl head-group reaching to the protein surface where it is coordinated to a water molecule at the pocket entrance ([11, 12], Fig. 2).
Figure 1.
Chemical structures of vitamin A, retinol, and some of its metabolites. In cells, retinol can be metabolically converted to it storage species retinyesters. Retinol can also be metabolically transformed to active metabolites including all-trans-retinoic acid, which regulates gene transcription, and 11-cis-retinal, which serves as a cofactor for the visual chromophore rhodopsin and is critical for vision.
Figure 2.
The three dimensional crystal structure of holo-RBP. The human holo-RBP structure (PDB ID 1BRP) was rendered using Pymol (http://www.pymol.org/). The structure shows the eight stranded antiparallel 3-sheet folded over itself to form a 3-barrel. Retinol (white) is encapsulated by the barrel with the 3-ionone ring buried in the binding pocket and the alcohol group is at the protein surface.
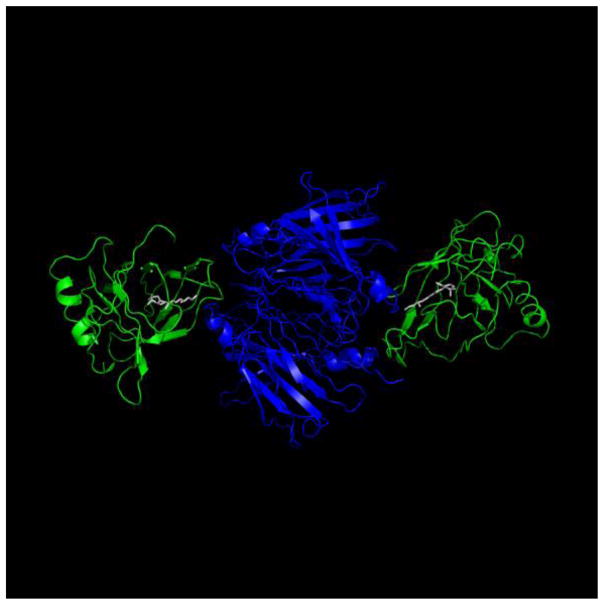
In blood, holo-RBP is associated with another protein termed transthyretin (transporter of thyroxin and retinol, TTR), a 56 KDa homotetramer which, in addition to binding RBP, also serves as a thyroid hormone carrier. The major sites of synthesis of TTR are the choroid plexus in the brain and the liver, and the protein is found in plasma and in cerebrospinal fluid [13]. Under normal physiological conditions, vitamin A circulates in plasma within a retinol:RBP:TTR ternary complex which forms at 1:1:1 molar ratio. It is believed that the association with TTR serves to prevent loss of the low molecular weight (21 KDa) RBP by glomerular filtration in the kidneys. Notably, association of RBP with TTR requires the presence of retinol, and the complex dissociates following loss of the ligand [14]. The reported 3-dimensional crystal structure of the complex of holo RBP with TTR [15] reveals that association with TTR blocks the entrance to the ligand-binding pocket of RBP (Fig. 3). Notably, although RBP can bind retinal and retinoic acid with an affinity similar to that displayed by retinol, it does not bind to TTR in the presence of these retinoids [16]. It seems that the larger head groups of retinal and retinoic acid interfere with binding of RBP to its serum partner protein.
Figure 3.
The three dimensional crystal structure of the retinol-RBP-TTR complex. The structure of the human retinol-RBP-TTR complex (PDB ID 1QAB) was rendered using Pymol (http://www.pymol.org/). The TTR tetramer (blue) is comprised of a dimer of dimers with two RBP molecules (green) bound to the opposite dimers. Interactions between RBP and TTR are mediated by residues at the entrance to the ligand binding pocket and span across the two TTR dimers.
Binding to RBP allows the poorly-soluble retinol to circulate in plasma, but the vitamin dissociates from the protein prior to uptake into cells. Due to its lipophilic nature, free retinol can readily enter cells by diffusion through the plasma membrane [17]. In addition, in some tissues, the vitamin can also be internalized by an integral plasma membrane protein termed stimulated by retinoid acid gene 6 (STRA6), a largely hydrophobic protein which is predicted by computer modeling to contain 11 trans-membrane helices, a number of loops, and a large cytosolic C-terminal tail (see Fig. 5). STRA6 binds extracellular RBP and facilitates transport of retinol from its plasma carrier into cells [18]. In the adult, STRA6 is expressed in blood-organ barriers, retinal pigment epithelial of the eye, brain, adipose tissue, spleen, kidney, testis, and female genital tract [19, 20].
Figure 5.
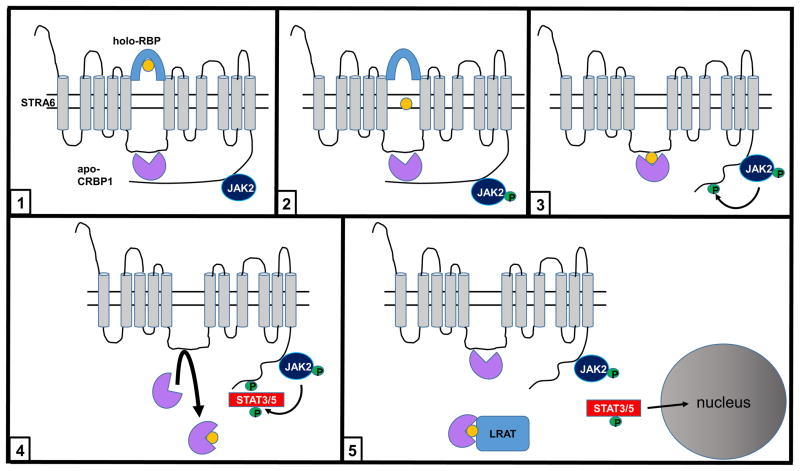
Model of the mechanism of action of STRA6 (see text).
STRA6 is both a retinol transporter and a cytokine signaling receptor
While STRA6 functions as a retinol transporter, studies of mice lacking the receptor established that it is not important for maintaining retinol availability in most tissues either during embryonal development or in the adult. The exception is the eye, where the expression level of the receptor exceptionally high [21]. Hence, it was reported that the retinoid content of many tissues is indistinguishable in Stra6-null and WT mice fed regular chow diet [22]. Moreover, while RBP is critical for development in mice fed vitamin A-deficient diet (VAD) [23], Stra6-null fetuses born from dams fed a VAD do not exhibit any hallmarks of fetal vitamin A deficiency syndrome [22]. It was further shown that the rate of uptake of retinol from circulating holo-RBP, and the retinoid content of STRA6-expressing tissues in adult mice fed a vitamin A deficient diet, when holo-RBP is the sole source of retinol, were only modestly reduced in Stra6-null mice. These observations strongly support the conclusion that the major fraction of cellular uptake of retinol from circulating holo-RBP occurs in a transporter-independent fashion, likely by diffusion across the plasma membrane, at rates that are sufficient for physiological needs. The exception is the eye where, due to a very high expression level of the receptor in retinal pigment epithelium, ablation of STRA6 leads to severe depletion of retinoid stores. Notably however, even in this organ, morphological changes and reduction in visual function in Stra6-null mice are mild, [21]. These observations suggest that STRA6 may have physiological function(s) other than serving as a retinol transporter.
Indeed, our recent studies revealed that, in addition to mediating retinol transport, STRA6 functions as signaling receptor that, upon binding holo-RBP, activates a JAK/STAT cascade [24]. Extracellular polypeptides such as cytokines, hormones, and growth factors often function by activating cognate cell surface receptors that transduce a signaling cascade mediated by tyrosine kinases called Janus kinases (JAK) and by their associated transcription factors Signal Transducers and Activators of Transcription (STAT). In turn, activated STATs reprogram gene expression and thus regulate multiple aspects of cellular behavior [25–27]. Binding of such extracellular ligands to their cognate cytokine receptors results in phosphorylation and activation of receptor-associated JAK which, in turn, phosphorylates a tyrosine residue in the cytosolic domain of the receptor. As STATs contain an SH2 domain that recognizes the resulting phosphotyrosine, these transcription factors are recruited to activated receptors where they are phosphorylated by JAK. Subsequently, STATs form dimers that translocate to the nucleus where they function as transcription factors. STATs thus regulate gene expression in response to a myriad of cytokines, hormones, and growth factors.
JAK/STAT signaling is “switched off” by several types of regulators. The signal can be dampened by dephosphorylation of activated receptors, JAKs, and STATs [26], and by inhibition of the transcriptional activity of STATs [28, 29]. Additional important negative regulators of these pathways are encoded by the direct STAT target genes called Suppressors of Cytokine Signaling (SOCS). Following their upregulation by STAT, SOCS function as components of negative feedback loops that inhibit JAK/STAT signaling by competing with STATs for binding to phosphotyrosines in activated receptors, by blocking the catalytic activity of JAK, and by recruiting ubiquitin ligases which facilitate the degradation of JAK by the proteasome [26, 30–32]. SOCS thus inhibit multiple cytokine-initiated responses. For example, insulin receptor (IR) signaling is potently and specifically inhibited by SOCS3 [33–37].
The cytosolic domain of STRA6 contains a stretch of residues that conform to a consensus phosphotyrosine motif [20, 24] and it was demonstrated that treatment of STRA6-expressing cells with holo-RBP triggers STRA6 phosphorylation and induces recruitment and activation of JAK2 and, in a cell-specific manner, STAT3 or STAT5. Holo-RBP thus upregulates the expression of STAT target genes, including SOCS3 [22, 24, 38–41]. Importantly, it was shown that neither RBP nor retinol triggers JAK/STAT signaling when administered alone, and that retinoic acid had no effect on this cascade. These observations established that holo-RBP functions like classical cytokines to activate a STRA6/JAK2/STAT3/5 pathway. Among cytokine receptors, STRA6 appears to be unique in that it functions both a transporter and as a signaling receptor. It is interesting to note that the two functions of this “cytokine signaling transporter” are strictly inter-dependent. It has thus been shown that STRA6 signaling critically depends on STRA6-mediated retinol transport and, vice versus, that retinol transport cannot proceed if STRA6 phosphorylation is impaired [22, 40].
STRA6 couples vitamin A status and metabolism to cell signaling
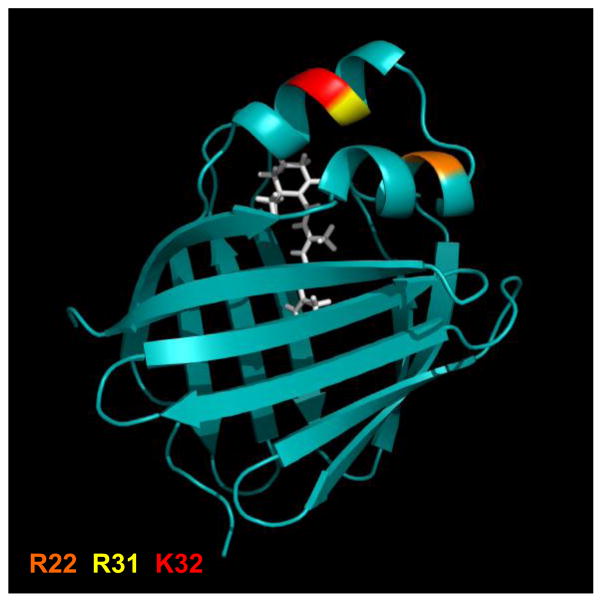
Holo-RBP-initiated retinol transport and cell signaling by STRA6 also requires expression of some intracellular accessory proteins. It was thus shown that cellular retinol-binding protein 1 (CRBP1), a member of the family of intracellular lipid-binding proteins (iLBP), binds to STRA6, functions as a direct intracellular acceptor for retinol, and is required for enabling STRA6 to function. Several structural features of CRBP1 and STRA6 that mediate their interactions and that link STRA6-mediated transport to STRA6 signaling have been identified. Apo-CRBP1 simultaneously interacts with an intracellular loop of STRA6, which was termed CRBP-binding loop (CBL), and with a region in the receptor’s cytoplasmic tail. The CBL contains a conserved LXXLL motif, often involved in mediating protein-protein interactions, and disrupting the motif by mutating even one of the Leu residues (L255) abolishes the interactions. It was shown further that CRBP1 dissociates from the CBL upon ligation [40]. These observations indicate that the interactions of CRBP1 with the STRA6 CBL must be mediated by a region that can “sense” retinol, i.e. whose conformation is altered upon retinol-binding. It was previously shown that ligand-binding by some iLBPs results in conformational changes in the helix-loop-helix region that flanks the entrance to their ligand binding pockets [42–44]. In some iLBPs (CRABP2, FABP4, FABP5) three basic residues in this region comprise a nuclear localization signal which mediates ligand-induced nuclear import of these proteins [43–45]. Interestingly, in CRBP1, the same residues, R22, R31 and K32 (Fig. 4), mediate the ligand-controlled interactions of the protein with STRA6 [40]. Interactions of the cytoplasmic tail of STRA6 with apo-CRBP1 are mediated by a region that contains the STRA6 phosphotyrosine motif. These interactions are disrupted upon phosphorylation of the signaling Y643 residue, indicating that the STRA6 tail dissociates from CRBP1 upon activation of the receptor [40].
Figure 4.
The three dimensional crystal structure of holo-CRBP1. The structure of CRBP-I in complex with ROH (PDB accession # 1KGL) was rendered using Pymol (http://www.pymol.org/). Residues R22, R31 and K32 which serve as the ligand-controlled switch that controls the association of CRBP1 with STRA6 are highlighted.
In addition to CRBP1, retinol transport and cell signaling by STRA6 also requires the expression of lecithin:retinol acyltransferase (LRAT), an enzyme that catalyzes the conversion of retinol to its storage species retinylesters (Fig. 1). It has long been established that CRBP1 shuttles retinol to some metabolizing enzymes, including LRAT [46]. Cooperation between the binding protein and the enzyme thus allows for direct movement of retinol to its sites of metabolism, bypassing the need for this lipophilic compound to traverse an aqueous milieu. LRAT fulfils two requirements that are critical for supporting STRA6 function: 1) it unloads retinol from CRBP1, enabling the binding protein to re-associate with STRA6 and accept additional incoming retinol, and 2) LRAT-catalyzed retinol esterification maintains an inward-directed retinol gradient, necessary for cellular uptake of the vitamin, and, in turn, for STRA6 signaling.
Taken together, available data suggest the following model for understanding the mechanism of action of STRA6 (Fig. 5): 1. In the quiescent state, STRA6 is “primed” with basal levels of bound apo-CRBP1 and JAK2. CRBP1 associates with STRA6 through interactions with residues LRNLL in an intracellular loop, and by binding to a region that contains the phosphotyrosine motif of the cytoplasmic tail. 2. Retinol transfers through STRA6 from extracellular holo-RBP to apo-CRBP1, a process that triggers phosphorylation of JAK2. 3. JAK2 phosphorylates STRA6-Y643, leading to release of the C-terminus tail from CRBP1. 4. Holo-CRBP1 dissociates from STRA6, and is replaced by apo-CRBP1 freshly arriving from the cytosol. STAT3/5 is recruited and activated. 5. Activated STAT3/5 moves to the nucleus to induce the expression of target genes. Holo-RBP delivers retinol to LRAT to regenerate apo-CRBP1 which re-associates with STRA6, allowing STRA6-mediated retinol transport and cell signaling to proceed. STRA6 thus couples “sensing” of serum holo-RBP levels and intracellular retinol metabolism to a signaling cascade, and regulates expression of multiple STAT target genes.
Transthyretin inhibits association of holo-RBP with STRA6
Holo-RBP circulates in blood in complex with its serum binding partner TTR and it has been reported that the association effectively blocks the ability of holo-RBP with STRA6 [38]. Consequently, STRA6 mediates retinol uptake only from free and not from TTR-bound holo-RBP. As TTR does not block the ability of retinol to move into cells by diffusion through the plasma membranes, the data further support the conclusion that supply of retinol to cells occurs primarily by a receptor-independent process [14, 17, 47, 48]. Moreover, the observations that TTR effectively blocks the association of RBP with STRA6 indicate that, in addition to preventing filtration of the 21 KDa RBP in the kidney, TTR plays an important role in protecting cells from excessive holo-RBP-induced, STRA6-mediated signaling.
Taken together, the observations indicate that STRA6 functions only under circumstances in which its expression level is very high or when serum RBP level exceeds that of TTR. These findings raise the question of when such physiological states may occur. With the exception of the observations that STRA6 transcription is regulated by retinoic acid [49], the factors that control the expression of the receptor remain to be elucidated. The RBP/TTR ratio in blood may be altered by changes in the expression level of RBP, or TTR, or both. TTR is expressed in the central nervous system and in the liver with the latter serving as the main source for the protein in serum [50]. Expression of hepatic TTR is downregulated and, consequently, serum TTR level dramatically decreases during acute phase response (APR) which in response to pronounced inflammation [51, 52]. The low serum level of TTR associated with APR may release holo-RBP thereby activating STRA6. Hence, STRA6 signaling may play a role in APR. It has also been reported that hepatic TTR expression is regulated by sex hormones [53]. The expression of RBP in brown adipose tissue and liver was reported to be regulated by cAMP-mediated pathways and by the nuclear receptors PPARγ and PPARγ [54, 55]. Whether, by controlling TTR or RBP expression, these factors regulate the RBP-TTR ratio in blood and thus modulate STRA6 signaling remains to be clarified.
Acknowledgments
Work from the author’s laboratory was supported by NIH grants DK060684 and DK088669.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCollum EV, Davis M. The necessity of certain lipins in the diet during growth. The Journal of biological chemistry. 1913;15:167–175. doi: 10.1111/j.1753-4887.1973.tb07065.x. [DOI] [PubMed] [Google Scholar]
- 2.Osborne TB, Mendel LB. The vitamins in green foods. The Journal of biological chemistry. 1919;37:187–200. [Google Scholar]
- 3.Noy N. Vitamin A. In: Stipanuk MH, Caudill MA, editors. Biochemical, Physiological, and Molecular Aspects of Human Nutrition. 3. Elsevier Saundres; St. Louis, Missouri: 2012. pp. 683–702. [Google Scholar]
- 4.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1996;10:940–954. [PubMed] [Google Scholar]
- 5.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, Gronemeyer H. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 6.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. The Journal of biological chemistry. 2003;278:41589–41592. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 7.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- 9.Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochimica et biophysica acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 10.Salier JP, Akerstrom B, Borregaard N, Flower DR. Lipocalins in bioscience: the first family gathering. BioEssays : news and reviews in molecular, cellular and developmental biology. 2004;26:456–458. doi: 10.1002/bies.20013. [DOI] [PubMed] [Google Scholar]
- 11.Zanotti G, Berni R, Monaco HL. Crystal structure of liganded and unliganded forms of bovine plasma retinol-binding protein. The Journal of biological chemistry. 1993;268:10728–10738. [PubMed] [Google Scholar]
- 12.Newcomer ME, Jones TA, Aqvist J, Sundelin J, Eriksson U, Rask L, Peterson PA. The three-dimensional structure of retinol-binding protein. The EMBO journal. 1984;3:1451–1454. doi: 10.1002/j.1460-2075.1984.tb01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber G. The evolutionary and integrative roles of transthyretin in thyroid hormone homeostasis. J Endocrinol. 2002;175:61–73. doi: 10.1677/joe.0.1750061. [DOI] [PubMed] [Google Scholar]
- 14.Noy N, Xu ZJ. Interactions of retinol with binding proteins: implications for the mechanism of uptake by cells. Biochemistry. 1990;29:3878–3883. doi: 10.1021/bi00468a012. [DOI] [PubMed] [Google Scholar]
- 15.Naylor HM, Newcomer ME. The structure of human retinol-binding protein (RBP) with its carrier protein transthyretin reveals an interaction with the carboxy terminus of RBP. Biochemistry. 1999;38:2647–2653. doi: 10.1021/bi982291i. [DOI] [PubMed] [Google Scholar]
- 16.Noy N, Slosberg E, Scarlata S. Interactions of retinol with binding proteins: studies with retinol-binding protein and with transthyretin. Biochemistry. 1992;31:11118–11124. doi: 10.1021/bi00160a023. [DOI] [PubMed] [Google Scholar]
- 17.Noy N, Xu ZJ. Kinetic parameters of the interactions of retinol with lipid bilayers. Biochemistry. 1990;29:3883–3888. doi: 10.1021/bi00468a013. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 19.Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, Dugger DL, Pham T, Yansura DG, Wong TA, Grimaldi JC, Corpuz RT, Singh JS, Frantz GD, Devaux B, Crowley CW, Schwall RH, Eberhard DA, Rastelli L, Polakis P, Pennica D. Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer research. 2001;61:4197–4205. [PubMed] [Google Scholar]
- 20.Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, Habib S, Tosha C, Radu RA, Ghyselinck NB, Nusinowitz S, Bok D. Retinoid content, visual responses and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci. 2012 doi: 10.1167/iovs.11-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry DC, Jacobs H, Marwarha G, Gely-Pernot A, O’Byrne SM, DeSantis D, Klopfenstein M, Feret B, Dennefeld C, Blaner WS, Croniger CM, Mark M, Noy N, Ghyselinck NB. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. The Journal of biological chemistry. 2013;288:24528–24539. doi: 10.1074/jbc.M113.484014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology. 2005;146:4479–4490. doi: 10.1210/en.2005-0158. [DOI] [PubMed] [Google Scholar]
- 24.Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4340–4345. doi: 10.1073/pnas.1011115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 26.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):S121–131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 27.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Yang Y, Chernishof V, Loo RR, Jang H, Tahk S, Yang R, Mink S, Shultz D, Bellone CJ, Loo JA, Shuai K. Proinflammatory stimuli induce IKKalpha-mediated phosphorylation of PIAS1 to restrict inflammation and immunity. Cell. 2007;129:903–914. doi: 10.1016/j.cell.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 29.Rytinki MM, Kaikkonen S, Pehkonen P, Jaaskelainen T, Palvimo JJ. PIAS proteins: pleiotropic interactors associated with SUMO. Cellular and molecular life sciences : CMLS. 2009;66:3029–3041. doi: 10.1007/s00018-009-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 31.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 32.Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol. 2008;19:414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. The Journal of biological chemistry. 2000;275:15985–15991. doi: 10.1074/jbc.275.21.15985. [DOI] [PubMed] [Google Scholar]
- 34.Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. The Journal of biological chemistry. 2001;276:47944–47949. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- 35.Krebs DL, Hilton DJ. A new role for SOCS in insulin action. Suppressor of cytokine signaling. Sci STKE. 2003;2003:PE6. doi: 10.1126/stke.2003.169.pe6. [DOI] [PubMed] [Google Scholar]
- 36.Pirola L, Johnston AM, Van Obberghen E. Modulation of insulin action. Diabetologia. 2004;47:170–184. doi: 10.1007/s00125-003-1313-3. [DOI] [PubMed] [Google Scholar]
- 37.Dong F, Ren J. Fitness or fatness--the debate continues for the role of leptin in obesity-associated heart dysfunction. Curr Diabetes Rev. 2007;3:159–164. doi: 10.2174/157339907781368959. [DOI] [PubMed] [Google Scholar]
- 38.Berry DC, Croniger CM, Ghyselinck NB, Noy N. Transthyretin blocks retinol uptake and cell signalling by the holo-retinol-binding protein receptor STRA6. Molecular and cellular biology. 2012;32:3851–3859. doi: 10.1128/MCB.00775-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berry DC, Noy N. Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochimica et biophysica acta. 2012;1821:168–176. doi: 10.1016/j.bbalip.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry DC, O’Byrne SM, Vreeland AC, Blaner WS, Noy N. Cross Talk between Signaling and Vitamin A Transport by the Retinol-Binding Protein Receptor STRA6. Molecular and cellular biology. 2012;32:3164–3175. doi: 10.1128/MCB.00505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marwarha G, Berry DC, Croniger CM, Noy N. The retinol esterifying enzyme LRAT supports cell signaling by retinol-binding protein and its receptor STRA6. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:26–34. doi: 10.1096/fj.13-234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reese-Wagoner A, Thompson J, Banaszak L. Structural properties of the adipocyte lipid binding protein. Biochimica et biophysica acta. 1999;1441:106–116. doi: 10.1016/s1388-1981(99)00154-7. [DOI] [PubMed] [Google Scholar]
- 43.Gillilan RE, Ayers SD, Noy N. Structural basis for activation of fatty acid-binding protein 4. J Mol Biol. 2007;372:1246–1260. doi: 10.1016/j.jmb.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sessler RJ, Noy N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Molecular cell. 2005;18:343–353. doi: 10.1016/j.molcel.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong EH, Goswami D, Griffin PR, Noy N, Ortlund EA. Structural basis for ligand regulation of the Fatty Acid Binding Protein 5, Peroxisome Proliferator Activated Receptor beta/delta (FABP5-PPARbeta/delta) signaling pathway. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M113.514646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herr FM, Ong DE. Differential interaction of lecithin-retinol acyltransferase with cellular retinol binding proteins. Biochemistry. 1992;31:6748–6755. doi: 10.1021/bi00144a014. [DOI] [PubMed] [Google Scholar]
- 47.Hodam JR, Creek KE. Comparison of the metabolism of retinol delivered to human keratinocytes either bound to serum retinol-binding protein or added directly to the culture medium. Exp Cell Res. 1998;238:257–264. doi: 10.1006/excr.1997.3857. [DOI] [PubMed] [Google Scholar]
- 48.Fex G, Johannesson G. Retinol transfer across and between phospholipid bilayer membranes. Biochimica et biophysica acta. 1988;944:249–255. doi: 10.1016/0005-2736(88)90438-5. [DOI] [PubMed] [Google Scholar]
- 49.Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mechanisms of development. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 50.Felding P, Fex G. Cellular origin of prealbumin in the rat. Biochimica et biophysica acta. 1982;716:446–449. doi: 10.1016/0304-4165(82)90040-x. [DOI] [PubMed] [Google Scholar]
- 51.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 52.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 53.Goncalves I, Alves CH, Quintela T, Baltazar G, Socorro S, Saraiva MJ, Abreu R, Santos CR. Transthyretin is up-regulated by sex hormones in mice liver. Molecular and cellular biochemistry. 2008;317:137–142. doi: 10.1007/s11010-008-9841-2. [DOI] [PubMed] [Google Scholar]
- 54.Bianconcini A, Lupo A, Capone S, Quadro L, Monti M, Zurlo D, Fucci A, Sabatino L, Brunetti A, Chiefari E, Gottesman ME, Blaner WS, Colantuoni V. Transcriptional activity of the murine retinol-binding protein gene is regulated by a multiprotein complex containing HMGA1, p54 nrb/NonO, protein-associated splicing factor (PSF) and steroidogenic factor 1 (SF1)/liver receptor homologue 1 (LRH-1) Int J Biochem Cell Biol. 2009;41:2189–2203. doi: 10.1016/j.biocel.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosell M, Hondares E, Iwamoto S, Gonzalez FJ, Wabitsch M, Staels B, Olmos Y, Monsalve M, Giralt M, Iglesias R, Villarroya F. Peroxisome proliferator-activated receptors-alpha and -gamma, and cAMP-mediated pathways, control retinol-binding protein-4 gene expression in brown adipose tissue. Endocrinology. 2012;153:1162–1173. doi: 10.1210/en.2011-1367. [DOI] [PubMed] [Google Scholar]