Abstract
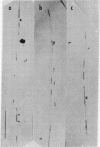
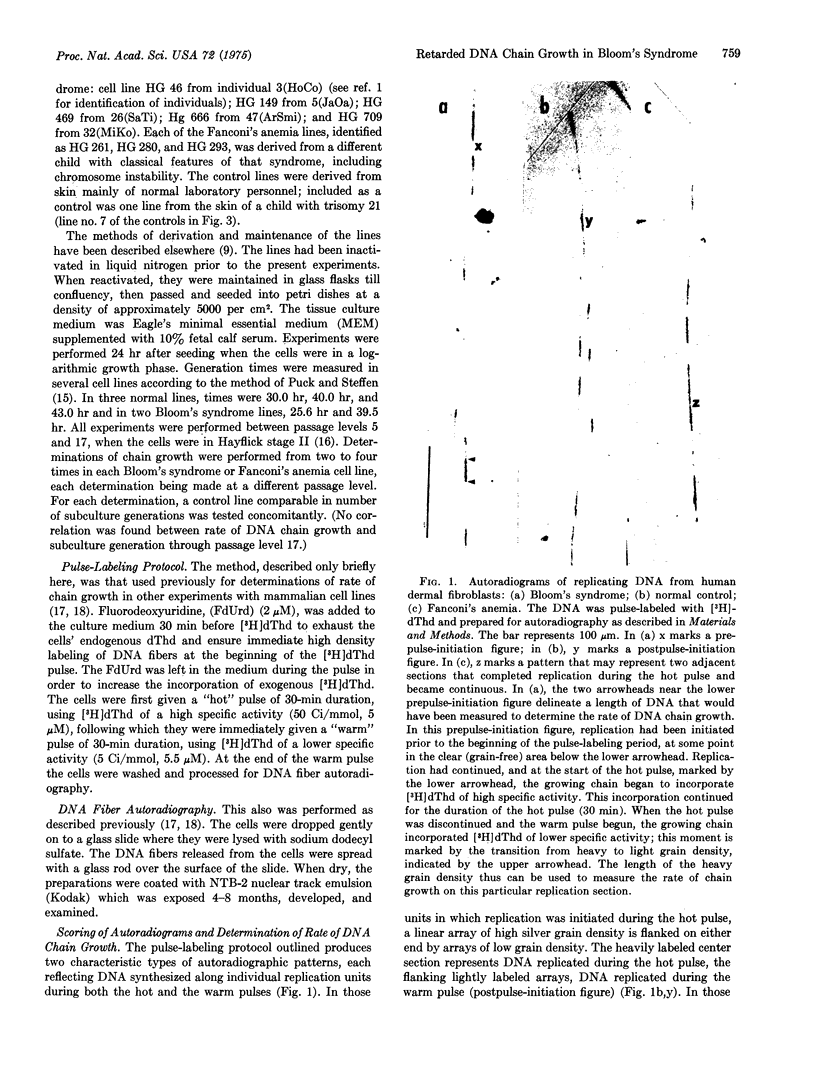
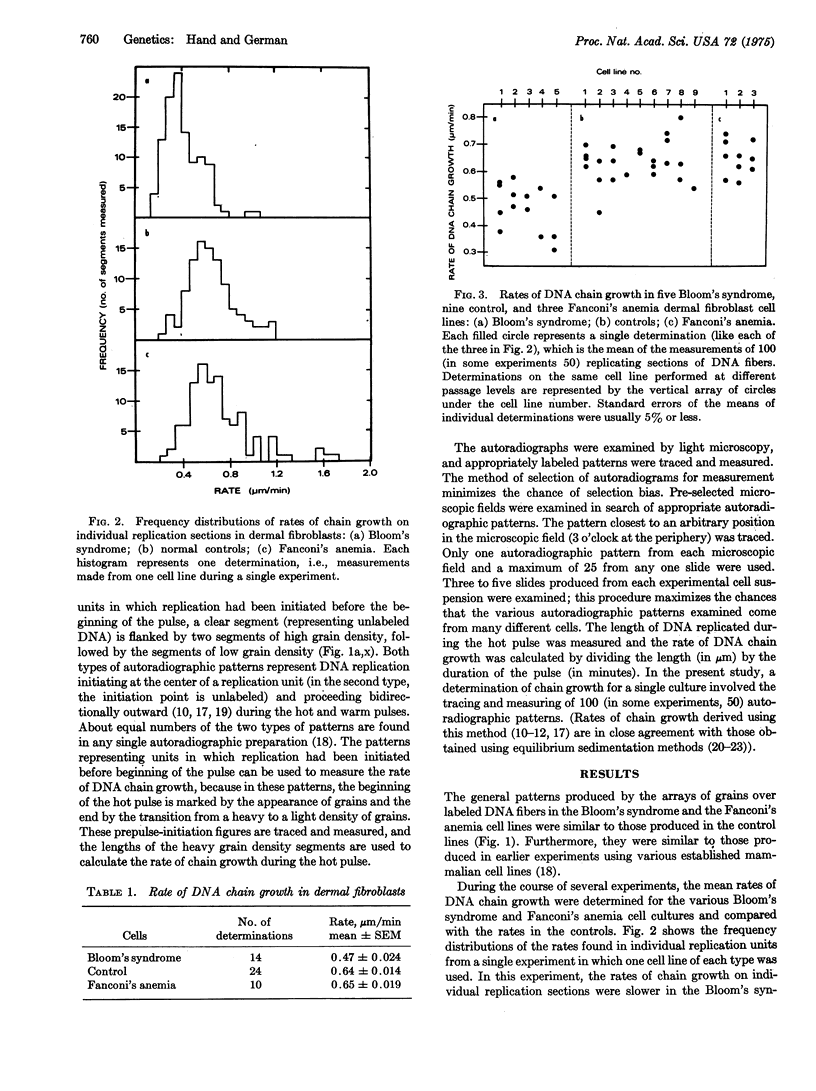
The cytogenetic observation that homologous chromatid interchange occurs in Bloom's syndrome more often than normal prompted an investigation of DNA replication in that rare genetic disorder. Using DNA fiber autoradiography, an estimation was made of the rate of one component of ongoing DNA replication, DNA chain growth. The rate in Bloom's syndrome dermal fibroblasts in tissue culture was found to be significantly slower than that in normal control cells. (The rate was found to be normal in Fanconi's anemia cells.) The explanation for the retarded chain growth may be either that an enzyme concerned directly with semiconservative DNA replication is defective or that a defective enzyme not itself concerned directly with replication results in disturbed cellular metabolism which in turn affects replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase in mammalian cells. J Biol Chem. 1971 Sep 25;246(18):5835–5837. [PubMed] [Google Scholar]
- Chang L. M., Brown M., Bollum F. J. Induction of DNA polymerase in mouse L cells. J Mol Biol. 1973 Feb 15;74(1):1–8. doi: 10.1016/0022-2836(73)90349-5. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Kowalski J., Yu K. K. Synthesis of high-molecular-weight cellular DNA in productive polyoma virus infection. J Mol Biol. 1972 Mar 28;65(2):347–364. doi: 10.1016/0022-2836(72)90286-0. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y. Effect of cycloheximide on regulatory protein for initiating mammalian DNA replication at the nuclear membrane. Cancer Res. 1972 Oct;32(10):2089–2095. [PubMed] [Google Scholar]
- GERMAN J., ARCHIBALD R., BLOOM D. CHROMOSOMAL BREAKAGE IN A RARE AND PROBABLY GENETICALLY DETERMINED SYNDROME OF MAN. Science. 1965 Apr 23;148(3669):506–507. doi: 10.1126/science.148.3669.506. [DOI] [PubMed] [Google Scholar]
- GERMAN J. CYTOLOGICAL EVIDENCE FOR CROSSING-OVER IN VITRO IN HUMAN LYMPHOID CELLS. Science. 1964 Apr 17;144(3616):298–301. doi: 10.1126/science.144.3616.298. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R., Kern R. M. DNA replication in mammalian cells in the presence of cycloheximide. Exp Cell Res. 1973 Jul;80(1):15–26. doi: 10.1016/0014-4827(73)90270-x. [DOI] [PubMed] [Google Scholar]
- German J. Bloom's syndrome. I. Genetical and clinical observations in the first twenty-seven patients. Am J Hum Genet. 1969 Mar;21(2):196–227. [PMC free article] [PubMed] [Google Scholar]
- German J., Crippa L. P., Bloom D. Bloom's syndrome. III. Analysis of the chromosome aberration characteristic of this disorder. Chromosoma. 1974;48(4):361–366. doi: 10.1007/BF00290993. [DOI] [PubMed] [Google Scholar]
- German J., La Rock J. Chromosomal effects of mitomycin, a potential recombinogen in mammalian cell genetics. Tex Rep Biol Med. 1969 Summer;27(2):409–418. [PubMed] [Google Scholar]
- Hand R., Tamm I. DNA replication: direction and rate of chain growth in mammalian cells. J Cell Biol. 1973 Aug;58(2):410–418. doi: 10.1083/jcb.58.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R., Tamm I. Initiation of DNA replication in mammalian cells and its inhibition by reovirus infection. J Mol Biol. 1974 Jan 15;82(2):175–183. doi: 10.1016/0022-2836(74)90339-8. [DOI] [PubMed] [Google Scholar]
- Hand R., Tamm I. Rate of DNA chain growth in mammalian cells infected with cytocidal RNA viruses. Virology. 1972 Feb;47(2):331–337. doi: 10.1016/0042-6822(72)90268-1. [DOI] [PubMed] [Google Scholar]
- Hecht N. B. Interconvertibility of mouse DNA polymerase activities derived from the nucleus and cytoplasm. Biochim Biophys Acta. 1973 Jul 13;312(3):471–483. doi: 10.1016/0005-2787(73)90445-0. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Tsai A. Direction of DNA replication in mammalian cells. J Mol Biol. 1973 Mar 25;75(1):5–12. doi: 10.1016/0022-2836(73)90525-1. [DOI] [PubMed] [Google Scholar]
- Lark C. G., Consigli R., Toliver A. DNA replication in Chinese hamster cells: evidence for a single replication fork per replicon. J Mol Biol. 1971 Jun 28;58(3):873–875. doi: 10.1016/0022-2836(71)90046-5. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Ormerod M. G. The replication of DNA in murine lymphoma cells (L5178Y). I. Rate of replication. Biochim Biophys Acta. 1970 Mar 19;204(1):128–143. doi: 10.1016/0005-2787(70)90496-x. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., STEFFEN J. LIFE CYCLE ANALYSIS OF MAMMALIAN CELLS. I. A METHOD FOR LOCALIZING METABOLIC EVENTS WITHIN THE LIFE CYCLE, AND ITS APPLICATION TO THE ACTION OF COLCEMIDE AND SUBLETHAL DOSES OF X-IRRADIATION. Biophys J. 1963 Sep;3:379–397. doi: 10.1016/s0006-3495(63)86828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Schaefer A. W. Rate of synthesis along replicons of different kinds of mammalian cells. J Mol Biol. 1969 Nov 14;45(3):467–479. doi: 10.1016/0022-2836(69)90306-4. [DOI] [PubMed] [Google Scholar]
- SHAW M. W., COHEN M. M. CHROMOSOME EXCHANGES IN HUMAN LEUKOCYTES INDUCED BY MITOMYCIN C. Genetics. 1965 Feb;51:181–190. doi: 10.1093/genetics/51.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. H. Rates of chain growth and units of replication in DNA of mammalian chromosomes. J Mol Biol. 1968 Feb 14;31(3):579–594. doi: 10.1016/0022-2836(68)90429-4. [DOI] [PubMed] [Google Scholar]