Abstract
Objective:
Ossification of the posterior longitudinal ligament (OPLL) is a complex multi-factorial disease process having both metabolic and biomechanical factors. The role of surgical intervention as well as the choice of approach weather anterior or posterior is ambiguous. The objective of this study was to assess the surgical out come and post operative functional improvement in patients with cervical OPLL at a tertiary care centre.
Patients and Methods:
This prospective study included 63 patients of cervical OPLL who underwent either anterior and/or posterior surgeries in Department of Neurosurgery, Nizam's Institute of Medical Sciences, Hyderabad between June 2009 to May 2011. Patient's data including age, sex, pre and post operative functional status, radiographic findings and OPLL subtypes were recorded and analyzed over a follow up ranging up to minimum two years.
Results:
The mean age of the patients was 51.1 (range 30-80 years) involving 14 women and 49 men. Out of 63 patients, 14 patients underwent surgery by anterior approach (corpectomy and fusion) and all of them improved (P = 0.52). 49 patients underwent surgery by posterior approach where decompressive laminectomy was performed in 40, laminectomy with instrumentation was done in 5, laminoplasty was done in 3 and 1 patient underwent both anterior and posterior surgeries. Of those who underwent posterior surgery, 40 patients improved, 7 remained the same as their preoperative status (who were having signal intensity changes on T2W MRI) and 2 patients deteriorated in the immediate post operative period and then showed gradual improvement. All the patients were followed up for 24 months. The mean pre-operative Nurick grade was 2.82 which later on improved to 2.03 post surgery (P < 0.05). Minor complications included wound infections in two patients (1.26%).
Conclusions:
Anterior cervical decompression and reconstruction is a safe and appropriate treatment for cervical spondylitic myelopathy in the setting of single or two level OPLL. Laminectomy or laminoplasty is indicated in patients with preserved cervical lordosis having three or more levels of involvement. Younger patients with good pre operative functional status and less than 2 levels of involvement have better outcome following anterior surgery.
Keywords: Nurick grade, ossified posterior longitudinal ligament cervical spine, surgical outcome
Introduction
Ossification of the posterior longitudinal ligament (OPLL) is a disorder of progressive ectopic calcification and ossification of the cervical and thoracic segments of the posterior longitudinal ligament (PLL) that results in a compressive myelopathy and/or radiculopathy. Its prevalence in Japanese and East Asian countries has ranged from 1.9 to 4.3%, while in white populations it has ranged from 0.01 to 1.7%.[1,2] Although many clinical features of cervical OPLL are similar to those of cervical spondylotic myelopathy or cervical disc herniation, it also has several unique characteristics. Management of OPLL can involve either surgical or conservative treatments. Patients with severe progressive myelopathy due to OPLL have generally been considered definitive candidates for surgical treatment. We present a single institution experience in the surgical management of this complex entity where the overall functional outcome depends on variety of factors.
Patients and Methods
Study design
This prospective study included 63 patients with Cervical OPLL between June 2009 to May 2011 admitted in Department of Neurosurgery, Nizam's Institute of Medical Sciences, Hyderabad. Cervical OPLL was defined as abnormal radio-opacity along the posterior margins of the vertebral bodies on lateral radiographic views/magnetic resonance imaging (MRI)/computed tomography (CT) scans.
Inclusion criteria
Patients who fulfilled the following criteria were included in the study:
All patients with radiologically proven cervical OPLL along with clinical signs of myelopathy
All patients with minimum follow-up of 2 years.
Exclusion criteria
Patients with inadequate follow-up
Patients with spondylotic myelopathy not accounting to OPLL.
Clinical evalution
Apart from age, gender, occupation, socio-economic status, the duration of sensory and motor symptoms and the presence or absence of autonomic dysfunction was noted in all the patients. Neurological assessment included examination of tone and spasticity in upper and lower limbs (as per Modified Ashworth Scale). Functional disability was assessed as per the Nurick grade. All these grading systems were employed pre-operatively and post-operatively at the time of discharge and at all subsequent follow ups. The follow up period varied from 12 to 24 months.
Imageological assessment
X-ray
All patients underwent X-ray of cervical spine in AP and lateral view. Wherever indicated dynamic lateral radiograph with flexion and extension studies were obtained. OPLL was identified on plain X-ray as an abnormal radio opacity along the posterior margins of the vertebral bodies (not only involving the disc spaces).
MRI
All patients were advised MRI of cervical spine. MR imaging was conducted on a 1.5 T-superconducting unit. Imaging included T1-weighted, T2-weighted, and spin-echo sagittal sequences using a 22 cm field of view, a 4 mm thickness with a 1-mm gap, and a 192 × 256 matrix. Various patterns of OPLL including conical or mushrooming types were identified on axial cuts along with T2-weighted cord signal changes on sagittal images. CT cervical spine was done as and when necessary.
Characterization of OPLL
We used the classification of the Hirabayashi et al.[1] to characterize the varies sub-types of OPLL [Figure 1].
Figure 1.
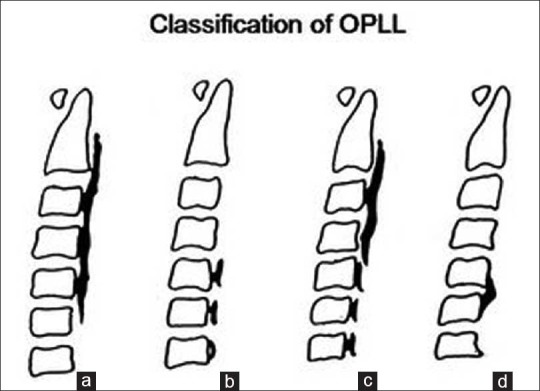
Types of OPLL: continous (a), semental (b), mixed (c), focal (d)
Diagnosis of OPLL
The diagnosis of cervical OPLL was made if following three conditions were met.
Clinical symptoms consistent with cervical cord compression were responsible for presentation.
MR imaging revealed evidence of cervical canal stenosis not predominantly due to any other degenerative spinal conditions.
X-ray/MRI/CT scan demonstrated that the cord compression was primarily due to a calcified mass consistent with the morphology of OPLL.
Management
Decisions regarding the type of surgery were based on clinical and established radiological findings.[3] Surgery was advised for patients with severe cervical myelopathy caused by OPLL. Cervical radiculopathy or neck pain alone was not considered to be surgical indications. In case of multi-segmental (>3 segments) involvement with preserved lordosis, posterior decompression (laminectomy) was performed. Patients with multilevel involvement and loss of lordosis or straightening were fused by lateral mass screw and rods after laminectomy, while two or lesser levels of involvement were approached anteriorly (corpectomy with fusion).[4,5]
Surgical procedure
Posterior approach
After induction of general anesthesia, the patient was intubated by the intubating laryngeal mask airway (ILMA) or awake bronchoscopic intubation technique. Later, the patient was placed in the prone position in a horse-shoe head rest with an indwelling bladder catheter. The abdomen was decompressed to avoid excessive venous congestion and epidural bleeding. After skin prepping and draping, a midline incision was given from the sub-occipital region to T1 level (depending on the extent of laminectomy). Sub-perioesteal dissection of the paraspinal muscles was performed. Laminectomy was performed by using rongeurs (or) a high-speed drill. Thecal sac was adequately decompressed. Instrumentation by lateral mass screws and rods was done wherever indicated by Magerl's technique under fluoroscopic guidance. Wound closure was done in layers. Post-operatively, patients were mobilized by physiotherapists with cervical orthoses at the earliest.
Anterior approach
After endotracheal intubation and induction of general anesthesia, all patients were placed in the supine position with the head slightly extended. A right-sided approach was mostly employed. A transverse skin-fold incision was made, beginning medially at the midline and extending up to the anterior border of the sternocleidomastoid muscle. Sometimes, a vertical incision medial to sternocleidomastoid was given when a larger exposure was warranted. The appropriate surgical level was confirmed by fluoroscopy and discectomy was performed. After the necessary level of discectomies, median part vertebral bodies along with the OPLL were drilled out (Midas-Rex drill, Fort Worth, Dallas, TX). The residual OPLL close to dura was drilled with a diamond burr till it got papery thin. If it was found tightly adherent to the dura, it was separated all around from the vertebral body and left as it was (floating OPLL). Once the dura was decompressed well, hemostasis was achieved. Later, the interbody cage with an internal diameter of 10-12 mm was filled with bony chips and was placed to maintain cervical alignment. Plating was done by locking screw and plates. Wound was closed over a drain. The patient was mobilized with hard cervical collar on the first post-operative day and a check X-ray was done to confirm the implant status before mobilization.
Outcome assessment
Before discharge, patient's functional outcome was assessed by means of Nurick grade and a follow-up was performed initially once in 3 months and later once in 6 months till the 2nd year. Patients were followed up with X-ray of cervical spine to check for the hardware status.
Results and Analysis
Patient demographics and presentation
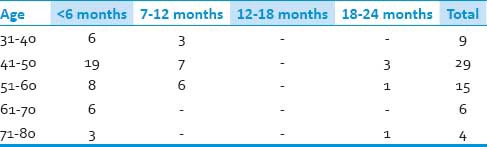
Out of these 63 patients, 49 were males and 14 females (M: F = 3.5:1) [Tables 1–3]. Age of all the patients ranged from 35 to 80 years (mean: 51.1 yrs). However, the maximum numbers of patients were between 41 and 50 years (n = 26), followed by 51 and 60 years (n = 20). All patients presented with sensory disturbances (mean duration: 9.6 months), while two patients had neck pain without motor and sensory complaints. Out of 63 patients, 61 patients presented with varying grades of upper and lower limb weakness (mean duration: 7.1 months) and 13 presented with sphincter disturbances. Only seven patients had signal intensity changes in T2W MRI suggestive of myelomalacia.
Table 1.
Patient demographics
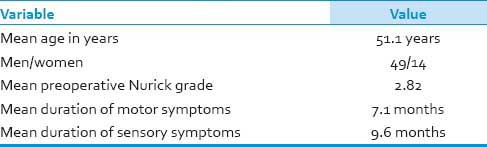
Table 3.
Duration of sensory disturbances
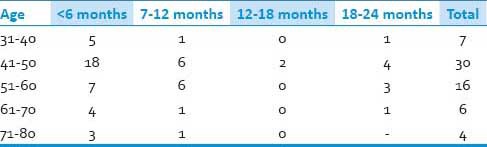
Table 2.
Duration of motor disturbances
Characteristics of OPLL
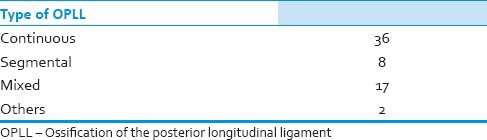
Ossification of the posterior longitudinal ligament morphology was determined based on X-ray and axial/sagittal MRI/CT images of cervical spine [Table 4]. The morphology of OPLL was continuous in 36 segmental in 17, mixed in 8, and others (focal) in 2 patients.
Table 4.
Characteristics of OPLL
Surgical intervention
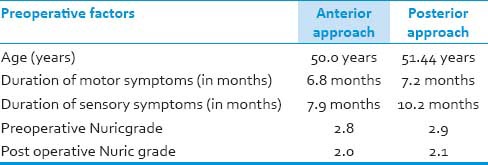
An anterior approach (corpectomy, fusion, and plating) was performed in 14 patients [Tables 5, 6], while the posterior approach was performed in 48 patients (decompressive laminectomy in 40, laminectomy with instrumented fusion in 5, laminoplasty in 3 patients). Both anterior and posterior approaches were done in one patient. Although the mean pos- operative Nurick grade was 2.03 (P < 0.05), the immediate post-operative Nurick grade in anterior or posterior approaches was 2.0 and 2.1, respectively. Two patients who underwent decompressive laminectomy with pre-operative Nurick grade of 4, deteriorated after surgery. Both of these patients had severe preoperative stenosis and MRI cord intensity changes. In seven patients there was no improvement in post operative Nurick grade and these patients were found to be having cord signal intensity changes on T2W images although this correlation was not found to be significant. Usually, the extent of laminectomy varied due to the extent of cord compression visualized on imageology. Surgical complications included wound infection or break down in two patients operated from posterior approach, dysphagia in five patients via anterior approach, whereas one patient required another surgery in the form of posterior decompression.
Table 5.
Type of surgery

Table 6.
Comparison of pre operative factors between two groups
Statistical analysis
Statistical analysis was performed using SPSS-13 [Table 7]. The data was categorized into two groups based on the post-operative change in the Nurick grade: Group 1: improvement in the Nurick grade 1 or more grades; Group 2: with no change or deterioration in the Nurick grade. Continuous data such as age, duration were compared using the independent sample t-test, categorical variables such as sex, type of surgery were compared using the Chi-square test and ordered categorical variables such as the Nurick grade and levels of involvement were compared using the Mann–Whitney U test. All tests were two-tailed and a P < 0.05 was considered as a significant difference between the two groups. There was no statistically significant difference in the age (P = 0.65), gender (P = 0.65), duration of motor (P = 0.88), sensory (P = 0.26), autonomic (P = 0.94) symptoms, preoperative Nurick grade (P = 0.35), number of levels of involvement (P = 0.82) type of surgery (anterior approach or posterior approach) (P = 0.52) between the two groups. The variables were compared between anterior and posterior approaches. Continuous data such as age, duration were compared using the independent sample t-test, categorical variables like sex, the presence of improvement were compared using the Chi-square test and ordered categorical variables such as the Nurick grade and levels of involvement were compared using the Mann–Whitney U test. All tests were two-tailed and a P < 0.05 was considered as a significant difference between the two groups. The number of levels of involvement of OPLL were significantly greater (P = 0.00) in those who underwent the posterior approach. However, there was no difference in the age (P = 0.38), gender (P = 0.01), duration of motor (P = 0.83), sensory (P = 0.56), autonomic (P = 0.94) preoperative (P = 0.48) or postoperative Nurick grade (P = 0.33) and improvement (P = 0.84) between the two approaches.
Table 7.
Statistical analysis
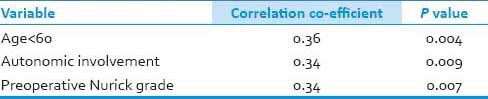
The data was categorized into two groups based on the post operative change in the Nurick grade: Group 1: Those with the Nurick grade 2 or less; Group 2: Those with the Nurick grade more than 2. The correlation between postoperative Nurick grade 2 or less and the noted variables was tested using Spearmans correlation. Age < 60, lower preoperative Nurick grade, the absence of autonomic involvement had significant correlation with improved outcome (post-operative Nurick grade 2 or less) and had no correlation with MRI signal intensity changes in our study.
Discussion
OPLL is an important cause of spinal cord compressive disease. Chronic compression of the spinal cord is believed to be the mechanism for myelopathy and may contribute to significant neurological disability. It has been estimated that 70% of OPLL cases involve the cervical spine, while 15% involve the thoracic and remaining 15% affect the upper lumbar spine (L1-3).[1,2,6] Recent studies indicate that single-nucleotide polymorphism in the collagen 11A2 gene (COL11A)[7,8,9,10,11] located within the Class II histocompatibility complex region on chromosome 6, which encodes the α2 chain of the Type XI collagen, could be responsible.[12,13]
Increased signal intensity of the spinal cord on T2-weighted MR imaging is often observed in patients with OPLL.[14,15,16] Takahashi et al. first reported the MR findings of intramedullary high signal intensity in patients with cervical spondylotic myelopathy.[17,18,19] We had seven patients with cord signal changes who did not show any significant improvement after surgery. Ischemia of the cord secondary to chronic compression has been postulated as one of the prime causes of deterioration not amenable to surgical decompression.
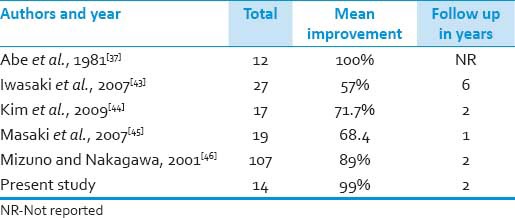
In our series, the clinical and radiographic presentation of OPLL was similar to the previously published data from East Asia. We found a varied distribution of OPLL morphologies, with a predominance of continuous and segmental forms, similar to that reported in the literature concerning the Japanese patients. In the present study, the sex ratio was 3.5:1 (male:female), which is in accordance with study of Matsunaga et al. where they found it be 2.4:1. Another study by Tsuyama et al. also registered a sex ratio of 2:1.[20] In Trojan et al.'s review of 73 cases of OPLL in non-Asians, several similarities with the Japanese literature were evident.[21] These findings included: (1) male predominance, (2) peak age of symptoms in the 5th-6th decade of life, (3) varied clinical presentations, and (4) predominance for the cervical spine. In the series of Jayakumar et al., 47 symptomatic Asian Indian patients of Caucasoid origin were studied.[22] Sixty-five percent of these patients were found to have continuous-type OPLL, and the disease predominantly affected the upper cervical spine. In our study, the majority was continuous type too. During the evaluation of patients with OPLL, preoperative planning is quintessential as this includes determining the extent of ossification and the direction of the surgical approach (anterior vs. posterior). Patient age and medical comorbidities also influence the decision to use either an anterior or posterior approach or whether to perform stabilization. The ideal surgical treatment option for multilevel ossification of posterior longitudinal ligament remains controversial and presents a significant surgical challenge.[23,24,25,26] Ventral ligaments should be removed to obtain ventral decompression, when the canal narrowing ratio exceeds 60%, or the ossification of posterior longitudinal ligament involves less than three segments. The advantages of anterior spinal fusion were as follows: (1) excision of the ossified mass enables complete decompression of the spinal cord; and (2) anterior spinal fluid creates a solid spinal fusion that can relieve pressure on the injured spinal cord (1). The disadvantages of anterior spinal approach were: (1) more techniques required, (2) more bone grafts for fusion, and (3) longer postoperative immobilization of the neck, etc., No statistical differences were noted between groups preoperatively in any of the studies, specifically looking at Nurick scores. Chen et al. found that anterior decompression had significantly better results than laminoplasty in postoperative JOA score, but the differences of postoperative JOA score did not reach statistical significance when comparing anterior corpectomy with posterior laminectomy or comparing laminectomy with laminoplasty.[27,25] Also, the neurological outcome of patients who underwent laminectomy and fusion was better than that of patients treated with laminoplasty. The existence of cervical kyphosis can result in tethering of the cord over ventral ossification of posterior longitudinal ligament protrusions. However, Vaccaro et al. noted 9% failure rate for two-level ACF with plating and a 50% failure rate for three-level ACF with plating.[28,29] Wang et al. concluded that a longer length graft was directly related to an increased incidence of graft displacement.[30] A longer length autograft or allograft increases the duration required for osteogenesis and creeping substitution along the graft. Hee et al. reported incidences of plate-related problems in multilevel corpectomies.[31] Sasso et al. reported a 6% failure rate after fixed-plated 2-level anterior corpectomy decompression and fusion reconstruction and a 71% failure rate after 3-level fixed-plated anterior corpectomy decompression and fusion reconstruction.[32,33] To avoid these complications, discontinuous corpectomy with adjacent-level diskectomy has been successfully performed with no plate loosening or graft migration.[34] The advantages of segmental anterior decompression and fusion, included significantly improved Nurick scores and JOA scores, restoration of cervical lordosis, and maintenance of height in fusion segments. Good to excellent clinical outcomes can be achieved in segmental anterior decompression and fusion if indications are well controlled, with thorough decompression, autografts from the vertebral body, and reconstruction of the lordotic cervical posture.[35] Posterior decompression is an alternative choice for multilevel ossification of the posterior longitudinal ligament when anterior corpectomy decompression and fusion threatens complications and poor fusion rate.[18] Lastly, the levels of corpectomies vary according to the surgeon's preference but the risks may outweigh the benefits in few. Our selection of surgical approach was based upon the guidelines of Hirabayashi et al., favouring posterior indirect decompressive approaches (laminectomy with instrumented fusion or laminoplasty) for longer segment and continuous-type OPLL, and anterior approaches for younger patients with more focal lesions because multi level cervical corpectomy has significant rate of instrument failure. Our results of the anterior approach are comparable to the study by Abe et al. where analysis of surgical results in OPLL patients was also done [Table 8].[36,37]
Table 8.
Review of literature (anterior approach)
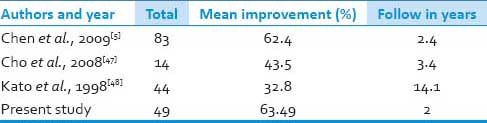
In our patients, out of 63 patients, 49 patients were underwent decompressive laminectomy ranging from C2 to C7 depending on extent of OPLL.[37,38,35] Five patients underwent laminoplasty. Although laminoplasty has advantages of widening the spinal canal with preservation of posterior elements for the sake of stability after decompression (i.e., to prevent the development of kyphosis) our institutional preference was more toward laminectomy than just laminoplasty. But improvement after laminectomy or laminoplasty remains the similar as shown by Kaminsky et al., where he compared two similar groups of patients treated with laminoplasty or laminectomy for CSM.[39,40] Results of our posterior approach were comparable to the study by Chen et al. [Table 9].[41,42] After comparing the outcome in both anterior and posterior approaches, anterior approaches were found to have better outcome than posterior (P = 0.84). Also surgery at younger age had better outcome than older age patients (P = 0.65). Also patients with less preoperative Nurick grade had better chances of improvement (P = 0.33). The overall improvement from the study was 85% (54/63), while 11.1% (7/63) remained static and remaining 3.1% (2/63) deteriorated. Mizuno and Nakagawa reported their experience in treating 107 patients with OPLL who underwent anterior cervical corpectomy and direct removal of the ossified mass. Surgery-related outcomes were excellent or good in 89% and fair in 11%. Patients underwent follow-up for 6 months, and the overall fusion rate was 97%, with three patients requiring additional surgery for pseudoarthrosis. Kato et al. reported the largest series of patients treated with cervical laminectomy for OPLL. In their series, patients underwent cervical laminectomy with a mean follow-up of 14.1 years. The neurological recovery rate of 44.2% at 1 year after laminectomy was maintained at 5 years.
Table 9.
Review of literature (laminectomy)
In the present study, the factors affecting the better outcome were: (1) age of the patient at the time of surgery, (2) pre-operative Nurick grade, (3) The presence of autonomic symptoms. The duration of motor and sensory symptoms, history of trauma, number of vertebral level involvement, and type of OPLL weres found to be adversely affecting the outcome although it never reached the level of significance. Our study had some limitations also. These included a follow up of only 1 year duration and inter-surgeon variability in terms of the extent of laminectomy. Also, due to financial reasons, all patients could not undergo CT cervical spine to diagnose OPLL conclusively.
Conclusions
Anterior cervical decompression and reconstruction is a safe and appropriate treatment for cervical spondylotic myelopathy in the setting of single or two level OPLL. Younger patients with good pre-operative functional status and less than two levels of involvement have better outcome following anterior surgery as more than two level corpectomy is associated with very high instrumentation failure rate hence posterior decompression has remained good option. Posterior decompressive surgery with/without maintained lordosis can be in more than three segments OPLL and preferably instrumentation can be done to obviate the sequelae. Overall, this complex entity needs careful assessment of the pre-operative imaging so that tailor-made surgeries for individual patients could be offered with due prognostification.
Acknowledgment
I thank Dr. Vijayasaradhi, Praveen A, Suchanda B, Vamsikrishna Y, Rajesh A, Ashish K, Dinesh S, and Dr. Padmaja Durga for the help in statistical analysis, Vamshidhar A, Kranthi S for their help in graphs and tables.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kalb S, Martirosya NL, Perez-Orribo L, Kalani MYS, Theodore N. Analysis of demographics, risk factors, clinical presentation, and surgical treatment modalities for the ossified posterior longitudinal ligament. Neurosurg Focus. 2011;30:E11:1–9. doi: 10.3171/2010.12.FOCUS10265. [DOI] [PubMed] [Google Scholar]
- 2.Wang MY, Thambuswamy M. Ossification of the posterior longitudinal ligament in non-Asians: Demographic, clinical, and radiographic findings in 43 patients. Neurosurg Focus. 2011;30(E4):1–5. doi: 10.3171/2010.12.FOCUS10277. [DOI] [PubMed] [Google Scholar]
- 3.Sait Naderi, Edward C. Benzel, Nevan G. Baldwin. Cervical spondylotic myelopathy: Surgical decision making. Neurosurgical Focus. 1996;1:13–7. doi: 10.3171/foc.1996.1.6.1. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chen D, Wang X, Lu X, Guo Y, He Z, Tian H. Anterior corpectomy and fusion for severe ossification of posterior longitudinal ligament in the cervical spine. International Orthopaedics (SICOT) 2009;33:477–82. doi: 10.1007/s00264-008-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso MJ, Koski TR, Liu AGJC. Approach-related complications after decompression for cervical ossification of the posterior longitudinal ligament. Neurosurg Focus. 2011;30(E12):1–5. doi: 10.3171/2011.1.FOCUS10278. [DOI] [PubMed] [Google Scholar]
- 6.Saetia K, Cho D, Lee S, Kim DH, Kim SD. Ossification of the posterior longitudinal ligament: A review. Neurosurg Focus. 2011;30:E1:1–16. doi: 10.3171/2010.11.focus10276. [DOI] [PubMed] [Google Scholar]
- 7.Won-Sang Cho, Chung CK, Tae-Ahn Jahng, Kim HJ. Post-laminectomy kyphosis in patients with cervical ossification of the posterior longitudinal ligament, does it cause neurological deterioration. J Korean Neurosurg Soc. 2008;43:259–64. doi: 10.3340/jkns.2008.43.6.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayakumar PN, Kolluri VR, Vasudev MK, Srikanth SG. Ossification of the posterior longitudinal ligament of the cervical spine in Asian Indians a multiracial comparison. Clin Neurol Neurosurg. 1996;98:142–48. doi: 10.1016/0303-8467(96)00004-2. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga S, Kukita M, Hayashi K, Shinkura R, Koriyama C, Sakou T, et al. Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;96:168–72. doi: 10.3171/spi.2002.96.2.0168. [DOI] [PubMed] [Google Scholar]
- 10.Smith ZA, Buchanan CC, Raphael D, Khoo LT. Ossification of the posterior longitudinal ligament: pathogenesis, management and current surgical approaches. Neurosurg Focus. 2011;30(3) E10:1–10. doi: 10.3171/2011.1.FOCUS10256. [DOI] [PubMed] [Google Scholar]
- 11.Shunjimatsunaga, Sakou T, Hayashi K, Ishidou Y, Hirotsu M, Komiya S. Trauma-induced myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg: Spine. 2002;97:172–75. doi: 10.3171/spi.2002.97.2.0172. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton CJ, Pham MH, Attenello FJ, Hsieh PC. Ossification of the posterior longitudinal ligament: genetics and pathophysiology. Neurosurg Focus. 2011;30:1–5. doi: 10.3171/2010.12.FOCUS10271. [DOI] [PubMed] [Google Scholar]
- 13.Stetler WR, La Marca F, Park P. The genetics of ossification of the posterior longitudinal ligament. Neurosurg Focus. 2011;30:E7:1–6. doi: 10.3171/2010.12.FOCUS10275. [DOI] [PubMed] [Google Scholar]
- 14.Fernández de Rota JJ, Meschian S, Fernández de Rota A, Urbano V, Baron M. Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. J Neurosurg Spine. 2007;6:17–22. doi: 10.3171/spi.2007.6.1.4. [DOI] [PubMed] [Google Scholar]
- 15.Lin-Feng Wang, Ying-Ze Zhang, Yong Shen, Yan-Ling Su, Jia-Xin Xu, Wen-Yuan Ding, Ying-Hua Zhang. Using the T2-weighted magnetic resonance imaging signal intensity ratio and clinical manifestations to assess the prognosis of patients with cervical ossification of the posterior longitudinal ligament. J Neurosurg: Spine. 2010;13:319–23. doi: 10.3171/2010.3.SPINE09887. [DOI] [PubMed] [Google Scholar]
- 16.Izumikoyanagi, Iwasaki Y, Kazutoshihida, Iiamura H, Hiroshiabe Magnetic resonance imaging findings in ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg. 1998;88:247–54. doi: 10.3171/jns.1998.88.2.0247. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Jiang LS, Dai LY. A review of prognostic factors for surgical outcome of ossification of the posterior longitudinal ligament of cervical spine. Eur Spine J. 2008;17:1277–88. doi: 10.1007/s00586-008-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi M, Sakamoto Y, Miyawaki M, Bussaka H. Increased MR signal intensity secondary to chronic cervical cord compression. Neuroradiology. 1987;29:550–6. doi: 10.1007/BF00350439. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi M, Yamashita Y, Sakamoto Y, Kojima R. Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology. 1989;173:219–24. doi: 10.1148/radiology.173.1.2781011. [DOI] [PubMed] [Google Scholar]
- 20.Tsuyama N. Ossification of the posterior longitudinal ligament of the spine. Clin Orthop Relat Res. 1984;184:71–84. [PubMed] [Google Scholar]
- 21.Trojan DA, Pouchot J, Pokrupa R, Ford RM, Adamsbaum C, Hill RO, et al. Diagnosis and treatment of ossification of the posterior longitudinal ligament of the spine: report of eight cases and literature review. Am J Med. 1992;92:296–306. doi: 10.1016/0002-9343(92)90080-u. [DOI] [PubMed] [Google Scholar]
- 22.Pham MH, Attenello FJ, Lucas J, Shuhan He BS, Stapleton CJ, Hsieh PC. Conservative management of ossification of the posterior longitudinal ligament. Neurosurg Focus. 2011;30(E2):1–6. doi: 10.3171/2011.1.FOCUS10273. [DOI] [PubMed] [Google Scholar]
- 23.Junjiexu, Kaizhang, Xiangyang MA, Qingshuiyin, Zenghuiwu, Hongxia Systematic Review of Cohort Studies Comparing Surgical Treatment for Multilevel Ossification of Posterior Longitudinal Ligament: Anterior vs Posterior Approach. Spot light on spine. 2011;34:e397–e402. doi: 10.3928/01477447-20110627-15. [DOI] [PubMed] [Google Scholar]
- 24.Qizhi S, Xuelei W, Lili Y, Lei L, Linwei C, Lang Y, et al. Segmental anterior decompression and fusion for multilevel ossification of the posterior longitudinal ligament. Orthopedics OrthoSuperSite.com. 2012;35:e403–07. doi: 10.3928/01477447-20120222-38. [DOI] [PubMed] [Google Scholar]
- 25.Chen, Liu X, Chen D, Wang X, Yuan W. Surgical strategy for ossification of the posterior longitudinal ligament in the cervical Spine. Orthopedics/Healio.com/Orthopedics. 2012;35:e1231–7. doi: 10.3928/01477447-20120725-25. [DOI] [PubMed] [Google Scholar]
- 26.Hai-song Yang, De-yu Chen, Xu-hua Lu, Li-li Yang, Wang-jun Yan, Wen Yuan, Yu Chen. Choice of surgical approach for ossification of the posteriorlongitudinal ligament in combination with cervical disc hernia. Eur Spine Journal. 2010;19:494–501. doi: 10.1007/s00586-009-1239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe H, Tsuru M, Ito T, Iwasaki Y, Koiwa M. Anterior decompression for ossification of the posterior longitudinal ligament of the cervical spine. Journal of Neurosurgery. 1981;55:108–16. doi: 10.3171/jns.1981.55.1.0108. [DOI] [PubMed] [Google Scholar]
- 28.Rick C. Sasso, Robert A. Ruggiero, Jr, Thomas M. Reilly, Peter V. Hall. Early Reconstruction Failures After Multilevel Cervical Corpectomy. SPINE. 2003;28:140–42. doi: 10.1097/00007632-200301150-00009. [DOI] [PubMed] [Google Scholar]
- 29.Okawa A, Sakai K, Hirai T, Kato T, Tomizawa S, Enomoto M. Risk factors for early reconstruction failure of multilevel cervical corpectomy with dynamic plate fixation. Spine. 2011;36:582–87. doi: 10.1097/BRS.0b013e3181e0f06a. [DOI] [PubMed] [Google Scholar]
- 30.Hee HT, Majd ME, Holt RT, Whitecloud TS, III, Pienkowski D. Complications of multilevel cervical corpectomies and reconstruction with titanium cages and anterior plating. J Spinal Disord Tech. 2003;16:1–8. doi: 10.1097/00024720-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Wang JC, Hart RA, Emery SE, Bohlman HH. Graft migration or displacement after multilevel cervical corpectomy and strut grafting. Spine. 2003;28:1016–21. doi: 10.1097/01.BRS.0000061998.62204.D7. [DOI] [PubMed] [Google Scholar]
- 32.Byung-Wan Choi, Kyung-Jin Song, Han Chang. Ossification of the posterior longitudinal ligament: A review of literature. Asian Spine Journal. 2011;5:267–76. doi: 10.4184/asj.2011.5.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasso RC, Ruggiero RA, Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28:140–42. doi: 10.1097/00007632-200301150-00009. [DOI] [PubMed] [Google Scholar]
- 34.Nancy E. Epstein. Circumferential cervical surgery for ossification of theposterior longitudinal ligament, a multianalytic outcome study. Spine. 2004;29:1340–45. doi: 10.1097/01.brs.0000127195.35180.08. [DOI] [PubMed] [Google Scholar]
- 35.Epstein N. Posterior approaches in the management of cervical spondylosis and ossification of the posterior longitudinal ligament. Surg Neurol. 2002;58:194–208. doi: 10.1016/s0090-3019(02)00819-4. [DOI] [PubMed] [Google Scholar]
- 36.Shin JH, Steinmetz MP, Benzel EC, Krishnaney AA. Dorsal versus ventral surgery for cervical ossification of the posterior longitudinal ligament: considerations for approach selection and review of surgical outcomes. Neurosurg Focus. 2011;30:E8:1–8. doi: 10.3171/2010.12.FOCUS10270. [DOI] [PubMed] [Google Scholar]
- 37.Komotar RJ, Mocco J, Kaiser MG. Surgical management of cervical myelopathy: Indications and techniques for laminectomy and fusion. The Spine Journal. 2006;6:252–67. doi: 10.1016/j.spinee.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Ryken TC, Heary RF, Matz PG, Anderson PA, Groff MW, Holly NT, Kaiser MG, Mummaneni PV, Choudhri TF, Vresilovic EAJ, Resnick DK. Cervical laminectomy for the treatment of cervical degenerative myelopathy. J Neurosurg: Spine. 2009;11:142–49. doi: 10.3171/2009.1.SPINE08725. [DOI] [PubMed] [Google Scholar]
- 39.Kaminsky SB, Clark CR, Traynelis VC. Operative treatment of cervical spondylotic myelopathy and radiculopathy: A comparison of laminectomy and laminoplasty at five year average follow-up. Iowa Orthop J. 2004;24:95–105. [PMC free article] [PubMed] [Google Scholar]
- 40.Michael P. Steinmetz, Daniel K. Resnick. Cervical laminoplasty. The Spine Journal. 2006;6:274–81. doi: 10.1016/j.spinee.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Guo Y, Chen D, Lu X, Wang X, Tian H, et al. Diagnosis and surgery of ossification of posterior longitudinal ligament associated with dural ossification in the cervical spine. Eur Spine J. 2009;18:15–47. doi: 10.1007/s00586-009-1029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizuno J, Nakagawa H. Outcome analysis of anterior decompressive surgery and fusion for cervical ossification of the posterior longitudinal ligament: Report of 107 cases and review of the literature. Neurosurg Focus. 2001;10:1–8. doi: 10.3171/foc.2001.10.4.7. [DOI] [PubMed] [Google Scholar]
- 43.Iwasaki M, Okuda S, Miyauchi A, Sakaura H, Mukai Y, Yonenobu K, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: Part 2: Advantages of anterior decompression and fusion over laminoplasty. Spine (Phila Pa 1976) 2007;32:654–60. doi: 10.1097/01.brs.0000257566.91177.cb. [DOI] [PubMed] [Google Scholar]
- 44.Kim K, Isu T, Sugawara A, Morimoto D, Matsumoto R, Isobe M, et al. Treatment of cervical OPLL by cervical anterior fusion using autologous vertebral bone grafts. Acta Neurochir (Wien) 2009;151:1549–5. doi: 10.1007/s00701-009-0478-z. [DOI] [PubMed] [Google Scholar]
- 45.Masaki Y, Yamazaki M, Okawa A, Aramomi M, Hashimoto M, Koda M, et al. An analysis of factors causing poor surgical outcome in patients with cervical myelopathy due to ossification of the posterior longitudinal ligament: Anterior decompression with spinal fusion versus laminoplasty. J Spinal Disord Tech. 2007;20:7–13. doi: 10.1097/01.bsd.0000211260.28497.35. [DOI] [PubMed] [Google Scholar]
- 46.Mizuno J, Nakagawa H. Outcome analysis of anterior decompressive surgery and fusion for cervical ossification of the posterior longitudinal ligament: Report of 107 cases and review of the literature. Neurosurg Focus. 2001;10:1–7. doi: 10.3171/foc.2001.10.4.7. [DOI] [PubMed] [Google Scholar]
- 47.Cho WS, Chung CK, Jahng TA, Kim HJ. Post-laminectomy kyphosis in patients with cervical ossification of the posterior longitudinal ligament: Does it cause neurological deterioration? J Korean Neurosurg Soc. 2008;43:259–64. doi: 10.3340/jkns.2008.43.6.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato Y, Iwasaki M, Fuji T, Yonenobu K, Ochi T. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89:217–23. doi: 10.3171/jns.1998.89.2.0217. [DOI] [PubMed] [Google Scholar]


