Abstract
The human T-cell leukemia retrovirus type-1 (HTLV-1) p30II protein is a multifunctional latency-maintenance factor that negatively regulates viral gene expression and deregulates host signaling pathways involved in aberrant T-cell growth and proliferation. We have previously demonstrated that p30II interacts with the c-MYC oncoprotein and enhances c-MYC-dependent transcriptional and oncogenic functions. However, the molecular and biochemical events that mediate the cooperation between p30II and c-MYC remain to be completely understood. Herein we demonstrate that p30II induces lysine-acetylation of the c-MYC oncoprotein. Acetylation-defective c-MYC Lys→Arg substitution mutants are impaired for oncogenic transformation with p30II in c-myc−/− HO15.19 fibroblasts. Using dual-chromatin-immunoprecipitations (dual-ChIPs), we further demonstrate that p30II is present in c-MYC-containing nucleoprotein complexes in HTLV-1-transformed HuT-102 T-lymphocytes. Moreover, p30II inhibits apoptosis in proliferating cells expressing c-MYC under conditions of genotoxic stress. These findings suggest that c-MYC-acetylation is required for the cooperation between p30II/c-MYC which could promote proviral replication and contribute to HTLV-1-induced carcinogenesis.
Keywords: HTLV-1, p30, c-MYC, acetylation, transformation, apoptosis
Introduction
The HTLV-1 is a complex oncoretrovirus that infects CD4+ T-lymphocytes and causes adult T-cell leukemia/lymphoma (ATLL) –an aggressive and often fatal hematological malignancy that is resistant to most anticancer treatments (Johnson et al., 2001; Kannian and Green., 2010). Similar to other transforming viruses, such as Epstein-Barr virus (EBV), hepatitis B and C viruses (HBV and HCV), human papillomavirus (HPV), and Kaposi sarcoma herpesvirus/human herpesvirus-8 (KSHV/HHV-8), the HTLV-1 induces viral latency associated with the establishment of persistent long-term infections which can lead to the development of malignant disease (Lairmore et al., 2012; Jeang, K.T., 2011; Lairmore et al., 2011; Cesarman and Mesri, 2007; Chen et al., 2013). Importantly, the mechanisms by which HTLV-1 deregulates host signaling pathways and gene expression to promote aberrant T-cell lymphoproliferation are not yet completely defined. The HTLV-1 provirus encodes at least seven regulatory/nonstructural and accessory proteins (Tax, Rex, p8I, p12I, p13II, p30II, HBZ) within a highly conserved 3′ nucleotide sequence, known as pX (Koralnik et al., 1993; Nicot et al., 2005; Cereseto et al., 1997; Edwards et al., 2011; Silic-Benussi et al., 2010; Bai and Nicot, 2012; Van Prooyen et al., 2010; Ma et al., 2013; Arnold et al., 2006). The Tax transactivator protein interacts with cellular transcription factors (CREB/ATF-1, NF-kappaB, and SRFp67, p300/CREB-binding protein and the p300/CBP-associated factor) and regulates the expression of proviral and cellular genes (Harrod et al., 1998; Tang et al., 1998; Harrod et al., 2000; Nicot and Harrod, 2000; Currer et al., 2012; Ho et al., 2012; Zhao and Giam, 1992; Georges et al., 2003; Wu and Sun, 2007). While Tax is generally considered to be the major oncoprotein of HTLV-1 involved in early T-cell immortalization and leukemic transformation, the HTLV-1 basic domain/leucine zipper (HBZ) protein could also contribute to retroviral carcinogenesis (Ma et al., 2013; Arnold et al., 2006; Zhi et al., 2011; Zhao et al., 2013; Zhao et al., 2011; Arnold et al., 2008; Kuhlmann et al., 2007; Matsuoka and Jeang, 2007; Satou et al., 2006). The HBZ product is translated from an antisense pX transcript made from the 3′ long-terminal repeat (LTR; Matsuoka and Jeang, 2007; Satou et al., 2006), and has been shown to induce expression of the human telomerase reverse transcriptase (hTERT) gene through the activation of JunD, associated with aberrant T-cell lymphoproliferation and immortalization (Kuhlmann et al., 2007; Matsuoka and Jeang, 2007; Satou et al., 2006). Zhi et al. (2011) have demonstrated that HBZ counters Tax-induced cellular senescence by inhibiting NF-kappaB activation. Moreover, HBZ-expressing transgenic mice exhibit systemic inflammation, particularly, of the skin and lungs, and develop T-cell lymphomas (Satou et al., 2011). In addition to these findings, we have previously demonstrated that the p30II accessory protein cooperates with the c-MYC oncoprotein and activates c-MYC dependent transcription –associated with increased S-phase cell-cycle entry, multinucleation, and oncogenic transformation/foci-formation by p30II/c-MYC (Awasthi et al., 2005).
The HTLV-1 encodes three latency-maintenance factors: p30II, HBZ, and p13II (Johnson et al., 2001; Kannian and Green, 2010; Lairmore et al., 2012; Nicot et al., 2005; Edwards et al., 2011; Silic-Benussi et al., 2010; Bai and Nicot, 2012; Arnold et al., 2006; Awasthi et al., 2005; Li et al., 2009; Bai et al., 2010; Anupam et al., 2013; Michael et al., 2006; Zhang et al., 2001; Zhang et al., 2000; Nicot et al., 2004; Younis et al., 2004; Younis et al., 2006; Bartoe et al., 2000; Silverman et al., 2004; Clerc et al., 2008; Lemasson et al., 2007; Andresen et al., 2011), which negatively regulate viral gene expression and may help HTLV-1-infected cells evade host immune-surveillance pathways as a prerequisite for viral persistence and the development of malignant disease after many years (Bartoe et al., 2000; Silverman et al., 2004). The antisense protein, HBZ, interacts with the kinase-inducible exchange (KIX) and histone acetyltransferase (HAT) domains of the transcriptional coactivators, p300/CBP, and inhibits Tax-dependent transactivation from the HTLV-1 promoter by interfering with Tax-p300/CBP binding (Wurm et al., 2012; Clerc et al., 2008). Lemasson et al. (2007) have also shown that HBZ directly interacts with CREB/ATF-1 transcription factors and prevents their recruitment into Tax-CREB nucleoprotein complexes on the viral promoter. The p30II protein (also known as Tax-Open Reading Frame II, or Tof-II) is comprised of 241 amino acid residues and contains arginine- and serine/threonine-rich regions which share sequence similarities with POU-family homeodomain transcription factors, Oct-1/2 and Pit-1, and is translated from an alternative doubly-spliced pX mRNA (Johnson et al., 2001; Koralnik et al., 1993; Nicot et al., 2005; Bai and Nicot, 2012; Bai et al., 2010; Anupam et al., 2013). p30II contains three nuclear localization signals: NLS1 (residues 66–73), NLS2 (residues 91–98), and NLS3 (residues 200–220) and two putative nucleolar localization/retention signals: NoLS1 (residues 73–78) and NoLS2 (residues 91–98) (Koralnik et al., 1993; Nicot et al., 2005; Bai et al., 2010; Anupam et al., 2013; Ghorbel et al., 2006), as well as a functional transcriptional activation domain, spanning amino acid residues 62–220 which was characterized using Gal4 (DBD)-p30II fusion/UAS transactivation experiments (Zhang et al., 2001; Zhang et al., 2000). The p30II protein suppresses the expression of HTLV-1 antigens through distinct transcriptional and posttranscriptional mechanisms. Zhang et al. (2001) have demonstrated p30II binds to the KIX domain of p300/CBP and inhibits Tax-dependent transactivation from the viral promoter through competitive interactions with p300/CBP. The ability of p30II to interfere with Tax-dependent transactivation was dependent upon a single lysine residue at position 106 (K106) within the p30II protein and also required p300 HAT activity (Michael et al., 2006). By contrast, Nicot et al. (2004) and Younis et al. (2004) have shown that p30II inhibits HTLV-1 gene expression by sequestering tax/rex mRNAs within nucleoli, thereby preventing their nuclear export. The related p28II protein of HTLV-2 similarly inhibits the nuclear export of tax/rex transcripts (Younis et al., 2004; Younis et al., 2006). Although the mechanism(s) by which p30II posttranscriptionally regulates viral and cellular gene expression remains to be fully elucidated (Nicot et al., 2004; Younis et al., 2004; Younis et al., 2006; Taylor et al., 2009), Younis et al. (2006) have demonstrated that p30II is recruited to Tax-containing transcription complexes on the HTLV-1 promoter and travels together with the RNA Pol II elongation complex until it reaches a downstream target mRNA structural element. The p13II protein, which corresponds to amino acids 155–241 of the carboxyl-terminus of p30II, in its ubiquitinated form, has also been shown to negatively regulate HTLV-1 gene expression by inhibiting the ability of Tax to bind to the transcriptional coactivators p300/CBP (Andresen et al., 2011).
In addition to its role in maintaining viral latency, we have demonstrated that p30II cooperates with c-MYC and augments c-MYC-dependent transcriptional and oncogenic functions through interactions with the MYST-family acetyltransferase, TIP60, on c-MYC-responsive gene promoters (Awasthi et al., 2005). Acute and lymphoma-stage ATLL patient isolates frequently over-express c-MYC, as a result of 8q24 chromosomal translocations and/or c-myc locus gene amplification, associated with an aggressive disease phenotype and poor clinical outcomes (Awasthi et al., 2005; Mengle-Gaw and Rabbitts, 1987; Okumura et al., 2012; Koizumi et al., 1989; Saggioro et al., 1999; Hall et al., 1998; Miyazaki et al., 1996; Duyao et al., 1992). The viral Tax protein transactivates NF-kappaB elements within the c-myc promoter (Duyao et al., 1992). However, Gabet et al. (2003) have shown that Tax represses c-MYC-dependent transactivation from the hTERT gene promoter, and Semmes et al. (1996) have also demonstrated Tax inhibits c-MYC-dependent transcription and anchorage-independent cell growth. Interestingly, tumors in tax transgenic mice exhibit high levels of apoptosis associated with increased oncogene expression, including c-MYC (Saggioro et al., 1999; Hall et al., 1998). Thus, the cooperation between p30II and c-MYC may provide a mechanism whereby the viral pX proteins (p30II, Tax, and/or HBZ) coordinately regulate oncogene-activation, while inhibiting c-MYC-induced apoptosis, to promote proviral replication and the proliferation of HTLV-1-infected T-cells.
The c-MYC oncoprotein is acetylated on multiple lysine residues by the transcriptional cofactors/acetyltransferases, p300/CBP, PCAF/GCN5, and TIP60 (Faiola et al., 2005; Zhang et al., 2005; Vervoorts et al., 2003; Patel et al., 2004; Vervoorts et al., 2006; Mao et al., 2011). Faiola et al. (2005) have demonstrated that p300-mediated lysine-acetylation of c-MYC is associated with increased turnover and degradation of the c-MYC protein. This is in contrast to the role of acetylation by PCAF/GCN5 and TIP60, which has been shown to stabilize and significantly increase the half-life of c-MYC (Patel et al., 2004). Conversely, the Max protein, another basic domain/helix-loop-helix/leucine zipper (bHLHZip)-family transcription factor that regulates c-MYC functions, is acetylated on lysine residues K57, K144, and K145 which is important for its nuclear localization in mammalian cells (Faiola et al., 2007). It is important to note that, as many of the acetylation sites within c-MYC have been identified through in vitro biochemical studies, the role of site-specific acetylation in c-MYC biological functions remains to be fully determined.
The present study provides new insight into the mechanism(s) of cooperation between p30II and c-MYC, and demonstrates that p30II induces acetylation of the c-MYC oncoprotein which is required for oncogenic foci-formation by p30II/c-MYC (Awasthi et al., 2005). These findings allude to a possible role for c-myc oncogene-activation and its cooperation with retroviral accessory proteins during proviral replication and HTLV-1-associated T-cell leukemogenesis.
Results
Transactivation of the human cyclin D2 promoter and induction of endogenous Cyclin D2 by HTLV-1 p30II-GFP
To determine whether an HTLV-1 p30II-GFP fusion induces expression of the endogenous Cyclin D2 protein, we cotransfected 293A HEK cells with increasing amounts of the CMV-HTLV-1 p30II-GFP (Nicot et al., 2004) or pcDNA3.1-GFP expression constructs. The relative expression of p30II-GFP and GFP was determined by immunoblotting (Fig. 1A). As shown in Figure 1A, higher levels of Cyclin D2 were observed to coincide with increasing amounts of HTLV-1 p30II-GFP, relative to the GFP control. The subcellular distribution of p30II-GFP was predominantly nuclear/nucleoplasmic, whereas GFP exhibited a whole-cell fluorescence pattern (Fig. 1A, right panels). We have previously demonstrated that the HTLV-1 p30II (HA-tagged) protein interacts with the c-MYC oncoprotein and transactivates c-MYC-responsive E-box enhancer elements within the human cyclin D2 promoter, dependent upon recruitment of the MYST-family acetyltransferase TIP60 (Awasthi et al., 2005). Therefore, we next tested whether the p30II-GFP fusion similarly modulates c-MYC-dependent transactivation. Results in Figure 1B demonstrate that increasing amounts of the CMV-HTLV-1 p30II-GFP expression construct transcriptionally activate a human cyclin D2 promoter-luciferase reporter gene, as compared to the pcDNA3.1-GFP control. The ability of p30II-GFP to transactivate the cyclin D2 promoter was dependent upon the conserved c-MYC-responsive E-box enhancer elements, as a mutant cyclin D2MUT promoter-luciferase reporter lacking the E-boxes (Vervoorts et al., 2003) was not transactivated by increasing p30II-GFP (Fig. 1C). Consistent with our earlier findings that p30II stabilizes the recruitment of the transcriptional cofactor/acetyltransferase TIP60 to p30II/c-MYC nuclear complexes (Awasthi et al., 2005), over-expression of wildtype TIP60 resulted in significantly higher transactivation of the cyclin D2 promoter by p30II-GFP (Fig. 1D). A dominant-negative TIP60Q377E/G380E mutant, defective for acetyltransferase activity (Ikura et al., 2000), inhibited p30II-GFP-mediated transactivation (Fig. 1D). We next tested the effects of inhibiting TIP60 expression using a small-interfering RNA targeted against tip60 transcripts (siRNA-tip60) upon p30II-GFP-dependent transactivation from the cyclin D2 promoter. A non-specific RNA (nsRNA) was included as a negative control. Results in Figure 1E demonstrate that increasing amounts of transfected siRNA-tip60 inhibited p30II-GFP-dependent transcriptional activation, compared to the nsRNA. The inhibition of endogenous TIP60 protein expression by siRNA-tip60 was verified by immunoblotting (Fig. 1E, bottom right). The p30II-GFP and GFP proteins were visualized by direct-fluorescence microscopy (Fig. 1E, upper right panels). These results collectively agree with our previous observations in Awasthi et al. (2005) and further demonstrate that the p30II-GFP fusion behaves in a similar transcriptional manner as the p30II (HA-tagged) protein. To rule out the possibility that p30II-GFP might influence expression of the FLAG-tagged TIP60wildtype or TIP60Q377E/G380E mutant proteins in co-transfected cells, we performed immunofluorescence-microscopy using an anti-FLAG antibody, and then quantified the relative fluorescence intensities of the TIP60-FLAG proteins within individual cells with Zeiss Axiovision 4.8 software. As shown in Figures 1F and 1G, the TIP60-FLAG proteins were comparably expressed in co-transfected 293A cells containing either CMV-HTLV-1 p30II-GFP or the CβS empty vector.
Fig. 1.

HTLV-1 p30II-GFP transactivates c-MYC-responsive E-box enhancer elements within the human cyclin D2 promoter and induces Cyclin D2 expression. (A) 293A HEK cells were transfected with increasing amounts (1.0, 2.0 μg) of CMV-HTLV-1 p30II-GFP or a pcDNA3.1-GFP control. Expression of p30II-GFP and GFP was detected by direct fluorescence-microscopy (top panels) and immunoblotting. The subcellular distributions of HTLV-1 p30II-GFP and GFP were determined by direct fluorescence-microscopy. Scale bars in the micrographs represent 100 μm. (B) Cells were co-transfected with a human cyclin D2 promoter-luciferase reporter plasmid (0.5 μg) and increasing amounts (1.0, 2.0 μg) of CMV-HTLV-1 p30II-GFP or pcDNA3.1-GFP. (C) The cells were co-transfected as in B with a mutant cyclin D2MUT promoter-luciferase construct which lacks conserved E-box elements in the presence of increasing CMV-HTLV-1 p30II-GFP or pcDNA3.1-GFP. (D) Cells were co-transfected with a human cyclin D2 promoter-luciferase reporter plasmid and CMV-HTLV-1 p30II-GFP in the presence of increasing amounts of CMV-wildtype TIP60 or CMV-TIP60Q377E/G380E (1.0, 3.0 μg) (Ikura et al., 2000). (E) 293A HEK cells were co-transfected with a cyclin D2 promoter-luciferase reporter plasmid and CMV-HTLV-1 p30II-GFP. The cells were also repeatedly transfected with an siRNA targeted against tip60 (siRNA-tip60) or a non-specific RNA (nsRNA) control. The HTLV-1 p30II-GFP and GFP proteins were visualized by direct-fluorescence microscopy (upper right panels). The inhibition of endogenous TIP60 protein expression by the transfected siRNA-tip60 was demonstrated by immunoblotting (lower panels). Actin is shown as a control for equivalent protein loading. A tk-renilla luciferase construct was co-transfected as a control for transfection efficiencies (not shown) and dual-luciferase measurements were performed. The reported activities were normalized for equivalent renilla-luciferase activities. All luciferase assays were performed in duplicate (n=2) or triplicate (n=3) and results from representative experiments are shown. The Students t-distributions (alpha = 0.05) for luciferase assay results were B: 49,001.95; C: 10,293.99; D: 15,534.79; and E: 3,892.06. (F) The expression of the FLAG-tagged TIP60wildtype and TIP60Q377E/G380E mutant proteins in HTLV-1 p30II-GFP-expressing cells was detected by immunofluorescence-microscopy using an anti-FLAG monoclonal antibody and quantified using Zeiss Axiovision 4.8 software to measure relative fluorescence intensities within individual selected cells. (G) Graphical representation of experimental results in F. Five individual cells were analyzed for each data point. The Student’s t-distribution (alpha = 0.05) is 324.03. (H) The cellular acetyltransferases TIP60 and p300 are present in HTLV-1 p30II/c-MYC transcription complexes recruited to the endogenous cyclin D2 promoter in HTLV-1-transformed T-lymphocytes. Chromatin-immunoprecipitations were carried out on HTLV-1-transformed HuT-102 lymphocytes using antibodies against HTLV-1 p30II (peptide B; Koralnik et al., 1993), p300, c-MYC, and TIP60 as in Awasthi et al. (2005). Non-specific rabbit IgG was included as a control. The precipitated oligonucleosomal DNA fragments were detected by PCR amplification using the prm primer pair; the utr primers were used as a negative control (top panels). Dual-ChIPs (lower panels) were performed by re-immunoprecipitating nucleoprotein complexes using individual antibodies against HTLV-1 p30II, p300, c-MYC, TIP60, or non-specific IgG as described in Vakoc et al. (2005). The re-precipitated DNA fragments were detected by PCR using the prm primers.
Recruitment of the acetyltransferases TIP60 and p300 to p30II/c-MYC transcription complexes in HTLV-1-transformed T-cells
Using chromatin-immunoprecipitations (ChIPs), we have shown that the retroviral p30II accessory protein is recruited, together with c-MYC and its associated transcriptional cofactors TIP60, p300, and TRRAP/p434, to E-box enhancer elements within the cyclin D2 promoter in HTLV-1-transformed T-lymphocytes (Awasthi et al., 2005). Now, extending these findings, we have used dual-ChIPs (Vakoc et al., 2005) to determine if p30II, c-MYC, TIP60, and p300 are present within the same nucleoprotein complex assembled on E-box elements in the cyclin D2 promoter in HTLV-1-transformed HuT-102 T-cells. In the dual-ChIP assay, cross-linked bound oligonucleosomal protein complexes are initially immunoprecipitated using individual antibodies and Protein A-agarose in a standard ChIP. However, the ChIP-ed complexes are then re-immunoprecipitated from each sample using a second series of antibodies to detect factors that are present together in a single nucleoprotein complex (Vakoc et al., 2005). As shown in Figure 1H, we now provide the first evidence that p30II (immunoprecipitated using the rabbit polyclonal anti-HTLV-1 p30II, Peptide B, antibody – kindly provided by Dr. G. Franchini, NCI/NIH; Koralnik et al., 1993) is recruited with c-MYC, TIP60, and p300 to E-boxes in the cyclin D2 promoter in HTLV-1-transformed T-lymphocytes. Non-specific rabbit IgG was included as a negative ChIP control (Fig. 1H). Intriguingly, in complementary dual-ChIPs, TIP60 and p300 seemed to only weakly associate, which suggests these individual factors may be separated in the p30II/c-MYC complex (Fig. 1H).
Acetylation-defective Lys→Arg c-MYC substitution mutants are impaired for oncogenic cellular transformation by p30II/c-MYC
To determine if lysine-acetylation of the c-MYC oncoprotein is required for its cooperation with p30II (Awasthi et al., 2005), we performed in vitro cell transformation/foci-formation assays using c-myc−/− HO15.19 rodent fibroblasts which are knocked out for the c-myc gene (Albihn et al., 2006; Mateyak et al., 1997). The cultures were cotransfected with CMV-HTLV-1 p30II-GFP or a pcDNA3.1-GFP control, and either CβF-wildtype c-MYC or expression constructs for the acetylation-impaired Lys→Arg mutants, c-MYC K323R/K417R (Patel et al., 2004) or c-MYC R5 (Faiola et al., 2005). Colony formation –indicative of a loss of cellular contact-inhibition associated with oncogenic transformation (Awasthi et al., 2005), was observed following 2 weeks and the transformed foci were stained with methylene blue for enhanced visualization (Figs. 2A and 2B). The c-myc−/− HO15.19 fibroblasts grow as a monolayer and typically exhibit a senescence-like cellular morphology and reduced proliferative rate, as compared to the parental TGR-1 rat fibroblasts which are more spindle-shaped (Fig. 2B, right panels). The co-expression of wildtype c-MYC together with the GFP control did not result in significant foci-formation (approximately 6 colonies for c-MYC/GFP, versus 7 colonies for untransfected cells; Figs. 2A and 2C). In agreement with our previous data in Awasthi et al. (2005), the c-MYC oncoprotein cooperated with HTLV-1 p30II to induce oncogenic cellular transformation and resulted in approximately 42 colonies (Figs. 2A–2C). The expression of p30II-GFP within transformed colonies was visualized by direct fluorescence-microscopy (Fig. 2B). Importantly, the acetylation-impaired Lys→Arg c-MYC mutants, K323/K417R and R5, were defective for oncogenic transformation and cooperation with p30II (Fig. 2C), suggesting that acetylation of the c-MYC oncoprotein might be required for its molecular interactions with p30II (Awasthi et al., 2005; Faiola et al., 2005; Vervoorts et al., 2003; Patel et al., 2004). Consistent with these findings, the co-expression of increasing amounts of various dominant-negative acetyltransferase mutants: TIP60Q377E/G380E, PCAFΔHAT, and p300ΔE1A (Moser et al., 2000; Ikura et al., 2000; Chakravarti et al., 1999; Yang et al., 1996), inhibited HTLV-1 p30II/c-MYC-induced oncogenic cellular transformation in vitro in the wrn−/− WS AG11395 fibroblast cell-line, which was the line used to originally demonstrate the oncogenic cooperation between p30II and c-MYC (Fig. 2D; Awasthi et al., 2005). We recognize, however, that these dominant-negative coactivator mutants are likely to have a pleiotropic negative impact upon cellular transcription as well as cell proliferation in general.
Fig. 2.
The acetylation-defective Lys→Arg c-MYC mutants, K323R/K417R and R5, are impaired for oncogenic cellular transformation by HTLV-1 p30II-GFP/c-MYC. (A) c-myc−/− HO15.19 Fibroblasts were co-transfected with CβF-wildtype c-MYC and pcDNA3.1-GFP or CMV-HTLV-1 p30II-GFP. The transfected cultures were monitored microscopically over a two week period and foci-formation/cellular transformation was quantified by direct counting. Transformed colonies were stained with methylene blue for visualization. (B) An enlarged field with multiple foci is shown in the top left panel. Expression of HTLV-1 p30II-GFP in transformed colonies was visualized by direct fluorescence-microscopy. The c-myc−/− HO15.19 cells exhibit a senescent morphology compared to their parental rodent TGR-1 fibroblasts. (C) c-myc−/− HO15.19 Fibroblasts were co-transfected with CMV-HTLV-1 p30II-GFP or pcDNA3.1-GFP and CβF-wildtype c-MYC or the Lys→Arg c-MYC mutants, K323R/K417R or R5 (Faiola et al., 2005; Patel et al., 2004). Foci-formation was quantified by direct counting as described in A. Error bars represent standard deviations. The Student’s t-distribution (alpha = 0.05) is 1.014. (D) Effects of various dominant-negative acetyltransferase mutants upon foci-formation/cellular transformation by HTLV-1 p30II (HA)/c-MYC were determined by co-transfecting immortalized human wrn−/− WS AG11395 fibroblasts with CMV-HTLV-1 p30II (HA)/CβF-c-MYC and increasing amounts (1.0, 2.0 μg) of CMV-TIP60Q377E/G380E, CMV-PCAFΔHAT, or CMV-p300ΔE1A. The Student’s t-distribution (alpha = 0.05) is 1.903.
The HTLV-1 p30II protein interacts with the Lys→Arg c-MYC mutants and induces acetylation of the c-MYC oncoprotein
We next sought to determine if the impairment in oncogenic transformation by p30II and the acetylation-defective Lys→Arg c-MYC mutants, K323R/K417R and R5, is due to reduced interactions between p30II and c-MYC (Awasthi et al., 2005). Both of the Lys→Arg c-MYC mutant proteins colocalized with HTLV-1 p30II-GFP, similar to the wildtype c-MYC, in nuclei of cotransfected c-myc−/− HO15.19 fibroblasts or HeLa cells (Figs. 3A and 3B). The expression of HTLV-1 p30II-GFP, GFP, and c-MYC proteins was detected by immunofluorescence-microscopy. Untransfected c-myc−/− HO15.19 or HeLa cells (UT) and pcDNA3.1-GFP were included as negative controls (Figs. 3A and 3B). The p30II-GFP fusion localized to the nucleus/nucleolus with a subfraction distributed throughout the cytoplasm, whereas GFP exhibited a whole-cell fluorescence pattern (Figs. 3A and 3B). Further, we demonstrated that the p30II-GFP co-immunoprecipitates with FLAG epitope-tagged wildtype c-MYC, as well as the acetylation-defective Lys→Arg c-MYC mutants, K323R/K417R and R5, in whole-cell extracts prepared from cotransfected 293T HEK cells (Fig. 4A). These results suggest the impairment in the cooperation between HTLV-1 p30II and the Lys→Arg c-MYC mutants (Fig. 2C) is not derived from impaired molecular interactions with p30II, but, rather, could reflect the defective acetylation status of the c-MYC mutant proteins (Faiola et al., 2005; Patel et al., 2004).
Fig. 3.

The acetylation-impaired Lys→Arg c-MYC mutant proteins, K323R/K417R and R5, colocalize with nuclear HTLV-1 p30II-GFP. (A) The subcellular distribution of HTLV-1 p30II-GFP and wildtype c-MYC, or the acetylation-defective c-MYC K323R/417R and R5 mutant proteins, was observed in cotransfected HeLa cells and (B) c-myc−/− HO15.19 fibroblasts by immunofluorescence-microscopy. A pcDNA3.1-GFP expression construct was included as a negative control. The graphs at right were generated using Zeiss AxioVision 4.8 software and represent a heat-map of colocalization for the indicated areas (dotted lines). DIC phase-contrast images with DAPI nuclear-staining are shown for reference. The scale bars represent 10 μM. UT, untransfected cells.
Fig. 4.
HTLV-1 p30II induces Lys-acetylation of the c-MYC oncoprotein. (A) The HTLV-1 p30II-GFP was immunoprecipitated with FLAG-tagged wildtype c-MYC, c-MYC K323R/K417R and R5 mutant proteins using a monoclonal anti-FLAG M2 antibody (Sigma) and Protein-G agarose (Invitrogen). 293T HEK cells were co-transfected with CβF-wildtype c-MYC or the Lys→Arg mutants K323R/K417R or R5, and CMV-HTLV-1 p30II-GFP or a pcDNA3.1-GFP control. Expression of the over-expressed wildtype and mutant c-MYC proteins and Actin was detected by immunoblotting. The bound immunoprecipitated HTLV-1 p30II-GFP was detected using a rabbit polyclonal anti-GFP antibody (Sigma). Non-specific bands are indicated, ns. (B) Diagram of the c-MYC oncoprotein (MBI/MBII, MYC boxes I and II; A, acidic region; B, basic region; HLH, helix-loop-helix motif; LZ, leucine zipper motif; TAD, transcriptional activation domain; NLS, nuclear localization sequence). Specific Lys residues acetylated by p300/CBP (solid triangles), PCAF/hGCN5 and TIP60 (open circles) acetyltransferases are indicated (Faiola et al., 2005; Patel et al., 2004; Veroorts et al., 2003; Zhang et al., 2005). (C) The HTLV-1 p30II-GFP induces Lys-acetylation of c-MYC. 293 HEK cells were cotransfected with CβF-c-MYC and increasing amounts of CMV-HTLV-1 p30II-GFP or an empty vector. The expression of HTLV-1 p30II-GFP and c-MYC was detected by immunoblotting. The over-expressed FLAG-tagged c-MYC protein was immunoprecipitated using an anti-FLAG M2 antibody. Actin is shown as a control for equivalent protein loading. The Lys-acetylated c-MYC protein was immunoprecipitated using an anti-Acetyl-Lysine antibody (Millipore) and detected by immunoblotting as described in Patel et al. (2004). (D) The c-MYC oncoprotein is acetylated in HTLV-1-transformed HuT-102 and MJG11 T-lymphocytes. Uninfected Jurkat E6.1 T-lymphocytes are shown as a negative control. Input levels of endogenous p30II, c-MYC, and Actin were determined by immunoblotting. Immunoprecipitations were performed using an anti-Acetyl-Lysine antibody (left lanes) or monoclonal anti-GFP antibody control (right lanes). Lysine-acetylated c-MYC was detected by immunoblotting. A non-specific band, ns, is indicated. (E) Effects of the HDAC-inhibitor, trichostatin A (TSA), upon HTLV-1 p30II transactivation were determined by co-transfecting 293A cells with a cyclin D2 promoter-luciferase reporter plasmid (0.5 μg) and CMV-HTLV-1 p30II-GFP or pcDNA3.1-GFP in the presence of increasing TSA (200, 400 ng/ml). The Student’s t-distribution (alpha = 0.05) is 8153.91. The expression of HTLV-1 p30II-GFP and GFP was detected by direct fluorescence-microscopy (not shown).
A diagram of the c-MYC oncoprotein depicting its acetylation sites is shown in Figure 4B (Faiola et al., 2005; Vervoorts et al., 2003; Patel et al., 2004). The p30II-GFP fusion induces lysine-acetylation of FLAG epitope-tagged c-MYC in cotransfected 293 HEK cells, as compared to an empty vector control (Fig. 4C). The acetylated c-MYC protein was immunoprecipitated using an anti-Acetyl-Lysine antibody (Millipore) and detected by immunoblotting as described in Patel et al. (2004; Fig. 4C). We also observed that c-MYC is acetylated in HTLV-1-transformed T-cell-lines, HuT-102 and MJG11 (Fig. 4D). Uninfected Jurkat lymphoblastic leukemia cells were included for comparison (Fig. 4D). The endogenous p30II protein was detected in HTLV-1-infected cells using the rabbit polyclonal Peptide B antibody (Koralnik et al., 1993). The acetylated c-MYC oncoprotein was immunoprecipitated using an anti-Acetyl-Lysine antibody as described; and an anti-GFP antibody was included as a control for IP specificity (Fig. 4D, lower panel). These findings together suggest that acetylation of the c-MYC protein is required for its oncogenic cooperation with p30II. Consistent with this notion, a chemical histone deacetylase (HDAC)-inhibitor, Trichostatin A (TSA), significantly increased the transcriptional activation from a human cyclin D2 promoter-luciferase reporter plasmid by p30II-GFP in cotransfected cells (Fig. 4E; Awasthi et al., 2005; Bouchard et al., 2001).
HTLV-1 p30II inhibits c-MYC-dependent cellular apoptosis in the presence of genotoxic stress
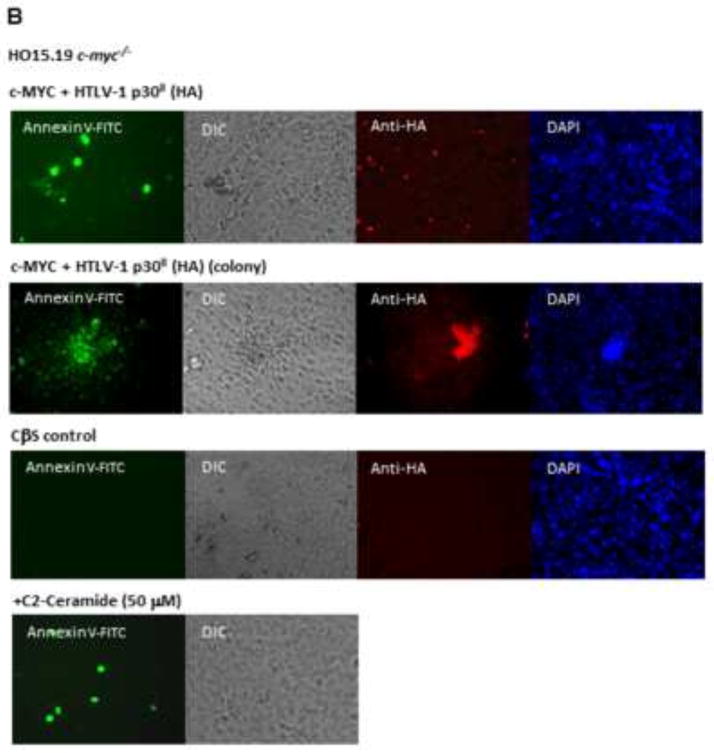
To determine if p30II and acetylation of the c-MYC protein influence c-MYC-dependent programmed cell death, we cotransfected c-myc−/− HO15.19 fibroblasts with expression constructs for wildtype c-MYC or the Lys→Arg acetylation-defective mutants, K323R/K417R and R5, together with HTLV-1 p30II (HA) or an empty CβS vector control. Cells were also treated with the sphingolipid C2-ceramide as a positive control for apoptosis. Programmed cell death was detected and quantified by staining the cotransfected cultures with Annexin-V-FITC and propidium iodide (BD-Pharmingen) and subsequent analysis by flow cytometry. We did not observe significant differences in cellular apoptosis for the wildtype c-MYC or acetylation-defective mutant proteins, relative to the control, when expressed alone in the c-myc−/− HO15.19 background (Fig. 5A). Apoptosis was also visualized by Annexin-V-FITC-staining and immunofluorescence microscopy in cotransfected c-myc−/− HO15.19 cells as well as transformed foci expressing p30II (HA)/c-MYC (Fig. 5B). Surprisingly, with prolonged 12-hr labeling of the cotransfected cells with bromodeoxyuridine (BrdU) for flow cytometric cell-cycle analyses, we found that c-MYC-expressing cells exhibited significantly more apoptosis than p30II/c-MYC-expressing cells, which also contained polyploidy as noted in Awasthi et al. (2005; Fig. 5C). We therefore cotransfected HO15.19 fibroblasts to express various combinations of wildtype c-MYC or the Lys→Arg acetylation-defective mutants, K323R/K417R and R5, together with HTLV-1 p30II(HA) or empty vector control, and subsequently treated the cultures for 12-hr with BrdU. Then the cells were stained with Annexin-V-FITC and apoptosis was measured by visually counting, in triplicate, the numbers of Annexin-V-FITC-positive cells per field (Fig. 5D). Results shown in Figure 5D and graphed in Figure 5E demonstrate that the c-MYC/empty CβS vector combination induced high levels of apoptosis, as compared to cells expressing p30II(HA)/c-MYC (Figs. 5D and 5E). The K323R/K417R and R5 acetylation-defective mutants did not induce significant apoptosis in the presence of prolonged BrdU treatment (Fig. 5E; data not shown), which may reflect a general functional impairment of these mutants in their ability to promote cellular proliferation. A representative experiment is shown in Figure 5F which demonstrates that p30II significantly protects against c-MYC-dependent apoptosis in the presence of prolonged genotoxic stress induced by BrdU-treatment. The micrographs in the right panels depict the Annexin-V-FITC-staining results in Figure 1F.
Fig. 5.
The HTLV-1 p30II protein inhibits c-MYC-dependent apoptosis induced by genotoxic stress. (A) Cellular apoptosis was quantified in co-transfected c-myc−/− HO15.19 fibroblasts expressing c-MYC or the acetylation-defective Lys→Arg c-MYC mutants, and HTLV-1 p30II. The cells were transfected with a CβS vector control, CMV-HTLV-1 p30II (HA), or CβF-c-MYC alone (1.5 μg each), or in combination with wildtype c-MYC or the K323R/K417R and R5 mutants. Certain cultures were treated with C2-ceramide (50 μM) as a positive control for apoptosis. Programmed cell-death was measured by staining the co-transfected cells with Annexin-V-FITC/PI (BD-Pharmingen) and performing flow-cytometry. (B) To visualize apoptosis in adherent cultures by microscopy, c-myc−/− HO15.19 fibroblasts were co-transfected with CβF-c-MYC and CMV-HTLV-1 p30II (HA) or a CβS vector. The c-MYC/HTLV-1 p30II (HA)-induced colonies were also analyzed to determine the level of apoptosis in transformed foci. C2-ceramide-treated cells were included as a positive control for apoptosis (bottom). The transfected cells were stained with Annexin-V-FITC (BD-Pharmingen) and apoptosis was detected by direct fluorescence-microscopy. Expression of the HTLV-1 p30II (HA) protein was detected using a monoclonal anti-HA antibody (CA5, Roche Molecular Biochemicals). (C) c-myc−/− HO15.19 Fibroblasts were co-transfected with CβF-wildtype c-MYC (or the acetylation-defective c-MYC mutant, K323R/K417R) and CMV-HTLV-1 p30II (HA) or a CβS control. Apoptosis and cell-cycle progression were measured by labeling cultures with BrdU for 12 hr and subsequently staining permeablized/fixed cells with an anti-BrdU-FITC-conjugated antibody and 7-AAD (BD-Pharmingen) and performing flow-cytometric analyses. Cell populations with diploid (2N, 4N) or polyploid (4N, 8N, 16N) DNA content are indicated. (D) To visualize the effects of HTLV-1 p30II (HA) upon apoptosis induced by c-MYC or Lys→Arg c-MYC mutants in the presence of genotoxic stress (i.e., prolonged exposure to BrdU), c-myc−/− HO15.19 fibroblasts were co-transfected with various combinations of CβS vector, CMV-HTLV-1 p30II (HA), CβF-wildtype c-MYC or acetylation-defective Lys→Arg c-MYC mutants and exposed to BrdU for 12 hr. Staurosporine (1 μM) and C2-ceramide (60 μM)-treated cultures were included as positive controls for apoptosis. The transfected cells were stained using Annexin-V-FITC and analyzed by direct fluorescence-microscopy. (E) The Apoptotic Index (number of Annexin V-FITC-positive cells/Field) was obtained by counting and averaging the number of Annexin V-FITC-positive cells in three separate microscopic fields. The graph shows average data from duplicate experiments and error bars representing standard deviations are provided. A representative experiment is shown in (F). The average percentages of Annexin-V-FITC-positive cells per field were determined by counting three microscopic fields for each sample. Standard deviations are represented by the error bars. The Student’s t-distribution (alpha = 0.05) is 1.700. Representative micrographs depicting the Annexin V-FITC-staining (green) and DIC phase contrast in merged images are shown at right.
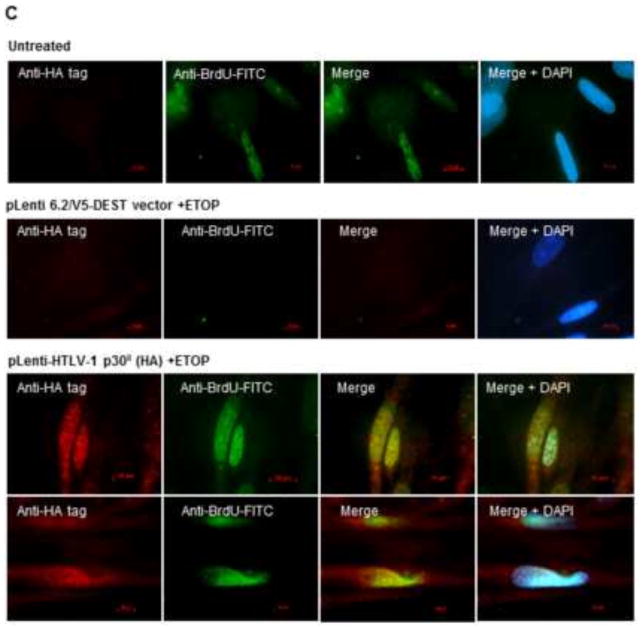
Datta et al. (2007) have previously reported that p30II protects primary T-cell-lines expressing the HTLV-1 proviral clone ACH.1 against Camptothecin-induced apoptosis, compared to cells containing the ACH.30.1 mutant defective for p30II production. Similar to Camptothecin, genomic BrdU-incorporation produces DNA-damage (single strand breaks) and genotoxic stress and cytotoxicity (Ackland et al., 1988) that is countered by the HTLV-1 p30II protein. To further test the effects of p30II in the presence of genotoxic stress, we transfected HFL1 human fetal lung fibroblasts with CMV-HTLV-1 p30II-GFP or a pcDNA3.1-GFP control and treated certain cultures with subinhibitory concentrations of the chemical Topoisomerase II-inhibitor, etoposide, and subsequently monitored them for the induction of multinucleate cells. A low, but detectable level of multinucleation was observed in the GFP-expressing control cells treated with the chemical inhibitor (approximately 2%, Figs. 6A and 6B). Consistent with our previous observations (Awasthi et al., 2005), p30II-GFP expression resulted in significantly more multinucleation in the presence of etoposide (> 6%, Figs. 6A and 6B), which could possibly be attributed to the induction of c-MYC and aberrant DNA-endoreduplication following p53-dependent G2/M-arrest (Awasthi et al., 2005; Datta et al., 2007). We and others have shown that p30II induces the accumulation of cells in the G2-phase of the cell cycle (Awasthi et al., 2005; Datta et al., 2007). As p53 regulates the G2/M checkpoint through the induction of regulatory factors, including 14-3-3σ and p21Waf/Cip1 (Chan et al., 2000), we therefore examined p53 expression in p30II-GFP-expressing cells in the presence or absence of etoposide. These studies revealed that p53 protein levels were increased in both p30II-GFP-expressing and etoposide-treated HFL1 fibroblasts (Fig. 6B). To determine whether p30II might promote c-MYC-dependent DNA endoreduplication in etoposide-treated cells, we next transduced HFL1 fibroblasts with a lentiviral vector expressing HTLV-1 p30II (HA-tagged), or an empty lentiviral vector, and then treated the cultures with etoposide as described. The cells were subsequently labeled with BrdU for 6 hours and stained with an anti-BrdU-FITC antibody (BD Pharmingen) to identify cells in the S-phase of the cell cycle. The HTLV-1 p30II (HA) protein was detected by immunofluorescence-microscopy (Fig. 6C, lower panels). These results demonstrate that p30II-expressing cells incorporated the BrdU deoxynucleotide analog into their genome under conditions of etoposide-treatment (Fig. 6C). We were not able to detect c-MYC-acetylation in the etoposide-treated cells, which express low levels of full-length c-MYC (data not shown). Conacci-Sorrell et al. (2014) recently reported the majority of c-MYC is cleaved by the Calpain protease into a truncated form (designated ‘MYC-nick’), containing amino acid residues 1–298, in the presence of etoposide. This proteolytic processing would remove lysine residues K323 and K417 which were identified as putative targets for acetylation by TIP60 (Patel et al., 2004). Therefore, the levels of acetylated full-length c-MYC may be too low to detect using our current IP methods in etoposide-treated cells.
Fig. 6.
HTLV-1 p30II induces multinucleation in the presence of subinhibitory concentrations of etoposide. (A) HFL1 Human fetal lung fibroblasts were transfected with CMV-HTLV-1 p30II-GFP or a pcDNA3.1-GFP control and certain cultures were treated with subinhibitory concentrations (≤ 1 μM) of the genotoxin, etoposide. The cultures were incubated for 96 hr at 37°C and 5% CO2 and multinucleation was quantified in the GFP-positive cells. Error bars represent standard deviations in triplicate assays. The Student’s t-distribution (alpha = 0.05) is 0.128. (B) HFL1 Fibroblasts were transfected as in A and cultured in the absence or presence of subinhibitory levels of etoposide. Multinucleation was visualized in HTLV-1 p30II-GFP and GFP-expressing cells by fluorescence-microscopy. The cells were immunostained to detect induction of the p53 tumor suppressor; and nuclei were visualized by DAPI-staining. Actin was stained using Texas-Red (TR)-Phalloidin. The open arrows indicate multinucleation in the GFP-positive cells. (C) Cells were transduced with a lentiviral vector expressing the HTLV-1 p30II (HA-tagged) protein, or an empty lentiviral vector, and then treated with etoposide as described. The cultures were subsequently labeled with BrdU for 6 hrs and stained with an anti-BrdU-FITC antibody (BD Pharmingen) to detect cells in S-phase. The p30II (HA) protein was visualized in transduced cells by immunofluorescence-microscopy using an anti-HA (12CA5) monoclonal antibody (lower panels). DAPI-nuclear-staining is shown in the merged images for comparison.
Our results allude to a mechanistic model whereby p30II induces acetylation of the c-MYC oncoprotein to augment its transcriptional activity, and inhibits c-MYC-dependent cellular apoptosis in the presence of genotoxic stress –which could lead to the increased accumulation of somatic mutations and genomic instability associated with oncogenic transformation and viral carcinogenesis (Fig. 7; Awasthi et al., 2005). As DNA-damage-inducing agents, such as etoposide, hydroxydaunorubicin, and cyclophosphamide are used in the CHOP (cyclophosphamide, hydroxydaunorubicin, oncovin, prednisolone) anticancer chemotherapy regimen for the clinical management of ATLL, our present findings suggest that retroviral proteins, such as p30II, may interfere with the cytopathic killing effects of these drugs and could promote the accumulation of somatic mutations which could contribute to disease progression.
Fig. 7.
Model depicting c-MYC-acetylation on multiple lysines by cellular acetyltransferases and its role in retroviral carcinogenesis. The HTLV-1 p30II accessory protein interacts with TIP60 and p300/CBP, induces acetylation of the c-MYC oncoprotein, and augments c-MYC transcriptional activity and oncogenic potential. p30II also inhibits c-MYC-dependent apoptosis induced by genotoxic stress, which could promote continuous DNA-replication and the acquisition of genetic lesions that support oncogenic transformation.
Discussion
We are studying how the persistently expressed latency-maintenance factors (i.e., p30II, HBZ, p13II) of HTLV-1 influence host cellular pathways to promote proviral replication and contribute to disease progression during viral carcinogenesis (Nicot et al., 2005; Bai and Nicot, 2012; Arnold et al., 2006; Awasthi et al., 2005; Li et al., 2009; Bai et al., 2010; Anupam et al., 2013; Michael et al., 2006; Zhang et al., 2001; Nicot et al., 2004; Younis et al., 2004; Younis et al., 2006; Bartoe et al., 2000; Silverman et al., 2004; Clerc et al., 2008; Lemasson et al., 2007; Andresen et al., 2011). Whereas the transactivator protein, Tax, is recognized to play a pivotal role during the earliest stages of T-cell immortalization and oncogenesis (Johnson et al., 2001; Kannian and Green, 2010; Currer et al., 2012; Hasegawa et al., 2006; Swaims et al., 2010; Rauch et al., 2009; Mitra-Kaushik et al., 2004; Grossman and Ratner, 1997), other viral pX factors (p30II, p13II, and HBZ) have been shown to negatively regulate proviral gene expression and inhibit Tax-dependent transactivation of the HTLV-1 5′ LTR (Nicot et al., 2005; Bai and Nicot, 2012; Arnold et al., 2006; Bai et al., 2010; Anupam et al., 2013; Michael et al., 2006; Zhang et al., 2001; Nicot et al., 2004; Younis et al., 2004; Younis et al., 2006; Silverman et al., 2004; Clerc et al., 2008; Lemasson et al., 2007; Andresen et al., 2011). These proteins help to maintain latency by suppressing the expression of viral antigens which is required for HTLV-1-infected T- lymphocytes to evade host immune-surveillance pathways and establish long-term proviral persistence (Johnson et al., 2001; Nicot et al., 2005; Edwards et al., 2011; Bai and Nicot, 2012; Arnold et al., 2006; Li et al., 2009; Bai et al., 2010; Anupam et al., 2013; Bartoe et al., 2000; Silverman et al., 2004). The p30II accessory protein contains a transcriptional activation domain between amino acid residues 62–220 and interacts with the transcriptional cofactors, p300/CBP and TIP60 (Awasthi et al., 2005; Michael et al., 2006; Zhang et al., 2001; Zhang et al., 2000). We and others have published microarray gene expression analyses of cellular target genes whose expression is activated or repressed by p30II (Awasthi et al., 2005; Taylor et al., 2009; Michael et al., 2004). In the Awasthi et al. (2005) study, by including a dominant-negative TIP60Q377E/G380E HAT mutant (Ikura et al., 2000), we identified several downstream target genes transcriptionally activated by p30II whose expression is dependent upon the TIP60 acetyltransferase. p30II differentially regulates CREB-dependent transcription, and inhibits Tax-quaternary complex formation with CREB and p300 on 21-bp-repeat Tax-responsive elements (TREs) through binding to the KIX domain of p300 (Michael et al., 2006; Zhang et al., 2001; Zhang et al., 2000). Datta et al. (2006) have also shown that p30II inhibits DNA-binding by PU.1 and represses (Toll-like receptor-4) TLR4 inflammatory signaling in macrophages. These studies were recently extended by Fenizia et al. (2014), who demonstrated that p30II inhibits the expression of interferon-responsive genes following lipopolysaccharide-stimulation of TLR3 and TLR4 in monocytes and dendritic cells, which may contribute to a permissive cellular phenotype during early-stage infection as well as throughout the course of persistent disease.
We have previously demonstrated that p30II interacts with TIP60 and enhances c-MYC-dependent transcriptional activation and oncogenic potential (Awasthi et al., 2005). However, the molecular mechanism(s) by which p30II cooperates with c-MYC remains to be fully elucidated. Herein, we have shown that p30II is recruited to c-MYC/TIP60/p300 transcriptional complexes on E-box enhancer elements within the endogenous cyclin D2 promoter in HTLV-1-transformed HuT-102 T-lymphocytes using dual-ChIPs (Fig. 1H). p30II induces acetylation of the c-MYC oncoprotein in transfected 293 cells (Fig. 4C); and c-MYC is also strongly acetylated in the HTLV-1-infected T-cell-lines HuT-102 and MJG11 (Fig. 4D). In the Awasthi et al. (2005) study, we demonstrated that amino acid residues 99–154 of p30II interact with the TIP60 acetyltransferase, which functions as a transcriptional cofactor and has been shown to acetylate the c-MYC protein (Patel et al., 2004; Frank et al., 2003). The specific sites of TIP60-mediated acetylation within c-MYC are yet to be identified (Patel et al., 2004). Furthermore, we found that the acetylation-defective Lys→Arg substitution mutants of c-MYC (R5 and K323R/K417R) are impaired for oncogenic cellular transformation/foci formation with p30II in cotransfected c-myc−/− HO15.19 fibroblasts (Figs. 2A–2C).
We did not observe discernable differences in cellular apoptosis induced by wildtype c-MYC or the acetylation-defective c-MYC mutants K323/R/K417R or R5 in the presence of p30II in cotransfected c-myc−/− HO15.19 fibroblasts (Figs. 5A and 5B). As Datta et al. (2007) have reported that p30II expression inhibits apoptosis induced by genotoxic stress in Camptothecin-treated primary T-lymphocytes transduced with an HTLV-1 ACH proviral clone, we next tested whether c-MYC-acetylation plays a role in the ability of p30II to protect against cell-death induced by DNA-damage-inducing agents. Interestingly, p30II significantly inhibited c-MYC-dependent apoptosis caused by prolonged exposure to BrdU, which induces single-strand DNA breaks (Figs. 5C–F; Ackland et al., 1988). Neither of the acetylation-defective c-MYC mutants resulted in increased apoptosis in BrdU-treated cells (Figs. 5C–F). This could be attributed to an inability of these mutants to restore normal cell-cycle functions and genomic replication in transfected c-myc−/− HO15.19 fibroblasts, as compared to wildtype c-MYC. We also observed that p30II results in an increased number of multinucleate cells in the presence of the genotoxic chemical, etoposide (Figs. 6A and 6B). These findings collectively agree with our previous results (Awasthi et al., 2005) and those in Datta et al. (2007), as well as the report by Baydoun et al. (2011) that p30II promotes error-prone nonhomologous-end-joining DNA-repair.
Doueiri et al. (2012) have reported a list of proteins detected in the interactomes of S-tagged HTLV-1 p30II and related HTLV-2 p28II proteins, based upon S-tag affinity purification and mass spectrometric/proteomic analyses. Noticeably, however, these studies failed to detect interactions between p30II and TIP60, c-MYC, or other known p30II-binding partners including p300/CBP, CREB, PU.1, and large ribosomal subunit protein L18a (Awasthi et al., 2005; Michael et al., 2006; Zhang et al., 2001; Zhang et al., 2000; Ghorbel et al., 2006; Datta et al., 2006; Doueiri et al., 2012). Using the I-TASSER computational protein-folding and structure prediction algorithm (University of Michigan, http://zhanglab.ccmb.med.umich.edu/I-TASSER/), we determined that the TIP60-binding domain (aa residues 99–154; Awasthi et al., 2005) of the S-tagged p30II protein is predicted to be misfolded and likely inaccessible, compared to the wildtype p30II protein structure (Supplement Fig. S1). Thus, albeit intriguing, the significance of the interacting factors identified through this proteomic screen remains to be determined until the biological functionality (e.g., inhibition of Tax-dependent transactivation, cooperation with oncoproteins, or nuclear sequestration of tax/rex mRNA) of the S-tagged p30II protein has been demonstrated (Doueiri et al., 2012).
The c-MYC oncoprotein is frequently overexpressed in acute and lymphoma-stage ATLL clinical isolates, as a result of 8q24 chromosomal translocations and/or c-myc locus gene amplification (Awasthi et al., 2005; Mengle-Gaw et al., 1987; Okumura et al., 2012; Koizumi et al., 1989; Saggioro et al., 1999; Hall et al., 1998; Miyazaki et al., 1996; Duyao et al., 1992). c-MYC regulates cellular proliferation and differentiation (Link et al., 2012; Takahashi et al., 2007; Soufi et al., 2012), and has been shown to function as a non-linear amplifier of transcriptionally active genes in lymphocytes and embryonic stem cells (Nie et al., 2012). While it is assumed the primary purpose of p30II’s cooperation with c-MYC is to promote proviral replication in HTLV-1-infected cells (Awasthi et al., 2005), these molecular interactions could contribute to disease progression associated with c-MYC deregulation in acute T-cell leukemia or lymphoma-stage ATLL (Mengle-Gaw et al., 1987; Okumura et al., 2012; Koizumi et al., 1989; Saggioro et al., 1999; Hall et al., 1998; Miyazaki et al., 1996; Duyao et al., 1992). Our findings further suggest that p30II (and other HTLV-1 proteins) may interfere with the cytopathic effects of CHOP-regimen DNA-damage-inducing chemotherapy drugs, such as etoposide, hydroxydaunorubicin, or cyclophosphamide, used in the clinical management of ATLL and could promote the accumulation of somatic mutations which may lead to the development of advanced acute- or lymphoma-stage disease. Therefore, it is intriguing to speculate that it may become possible to sensitize ATLL tumor lymphocytes to oncogene-induced apoptosis in the presence of DNA-damage-inducing chemotherapy drugs by targeting the latency-maintenance factor p30II and/or its interactions with host cellular components.
Materials and methods
Cell culture and transfections
293 HEK cells (ATCC, CRL-1573), 293A (Quantum Biotechnology) and 293T/17 (ATCC, CRL-11268) cells were seeded at 2 × 105 cells/well in six-well plates or 60 mm2 tissue-culture dishes (Nalge) and cultured in ATCC 46-X medium or Eagle’s minimum essential medium (EMEM; ATCC) supplemented with sodium bicarbonate (Invitrogen), 10% fetal bovine serum (FBS; Atlanta Biologicals, Biowest), 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate (Invitrogen) at 37°C under 5% CO2. For certain experiments, 293A HEK cells were cultured in the presence of 2% FBS. Immortalized human wrn−/− WS fibroblasts (AG11395) (Moser et al., 2000), parental TGR1 and c-myc−/− HO15.19 rat fibroblasts (Albihn et al., 2006; Mateyak et al., 1997), and HeLa cells (ATCC, CCL-2) were grown in Dulbecco’s modified Eagle’s medium (DMEM; ATCC) supplemented as described. The HFL1 human fetal lung fibroblast cell-line (ATCC, CCL-153) was cultured in F-12K medium. HTLV-1-transformed T-cell lines, HuT-102 (ATCC, TIB-162) and MJG11 (ATCC, CRL-8294), and Jurkat E6.1 T-lymphocytes (ATCC, TIB-152) were cultured in RPMI 1640 medium (ATCC) supplemented with 20% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate (Invitrogen), and 20 μg/ml gentamicin sulfate (SIGMA-Aldrich).
The N3-CMV-HTLV-1 p30II-GFP and pMH-CMV-HTLV-1 p30II (HA-tagged) expression constructs (Nicot et al., 2004), human cyclin D2 promoter-luciferase and cyclin D2MUT-luciferase reporter plasmids (Vervoorts et al., 2003; Bouchard et al., 2001), pOZ-CMV-TIP60 and pOZ-CMV-TIP60Q377E/G380E (Ikura et al., 2000), pSI-CMV-p300ΔE1A and pCX-CMV-PCAFΔHAT (Chakravarti et al., 1999; Yang et al., 1996), CβF-CMV-wildtype c-MYC (FLAG-tagged) and CβF-CMV-Lys→Arg c-MYC mutants: K323R/K417R and R5 (Faiola et al., 2005; Patel et al., 2004; McMahon et al., 1998) have been previously described. The c-MYC R5 combinatorial mutant contains the following Lys→Arg substitution mutations: K144R/K149R/K158R/K317R/K323R (Faiola et al., 2005). All transfections were carried out using SuperFect (Qiagen) or Lipofectamine-Plus (Invitrogen) reagents as recommended in the manufacturers’ protocols. Luciferase assays were performed using the Dual-Glo Luciferase Assay System (Promega, Inc.).
To inhibit endogenous TIP60 protein expression using small interfering-RNAs (siRNAs), 293A HEK cells were repeatedly transfected with increasing amounts (0.25 and 0.5 μg) of a bridged nucleic acid (BNA) RNA oligonucleotide targeted against tip60 transcripts (siRNA-tip60): 5′-AACCCCUCCACCUUCCG+T-phosphorothioate-3′, or a non-specific RNA (nsRNA) BNA oligonucleotide: 5′-2-O-methyl-UUACCGAGACCGUACGUA-2-O-methyl-U-3′ (Biosynthesis, Inc.) as a negative control. All RNA transfections were performed using the HiPerfect transfection reagent (Qiagen, Inc.) as recommended in the manufacturer’s protocol.
Chromatin-immunoprecipitations and dual-ChIPs
Chromatin-immunoprecipitations were performed by cross-linking c-MYC-containing nucleoprotein complexes in vivo by treating cultures with 270 μl of 37% formaldehyde for 10 minutes at 37°C under 5% CO2. The cells were pelleted by centrifugation and resuspended in 200 μl SDS-Lysis Buffer (Upstate Biotechnology). Chromatin DNA was fragmented by sonication, and oligonucleosomal/nucleoprotein complexes were precipitated using primary antibodies and 60 μl salmon sperm DNA/Protein A-agarose (Upstate Biotechnology). The precipitated complexes were washed, cross-links were reversed, and DNA fragments were amplified by PCR using oligonucleotide DNA primers that anneal to nucleotide sequences that flank conserved E-box enhancer elements within the human cyclin D2 promoter (prm primers: 5′-CCCCTTCCTCCTGGAGTGAAATAC-3′ and 5′-CGTGCTCTAACGCATCCTTGAGTC-3′); the control utr primers (5′-ATCAGACCCTATTCTCGGCTCAGG-3′ and 5′-CAGTCAGTAAGGCACTTTATTTCCCC-3′) anneal to an untranslated (utr) region of the cyclin D2 gene (Awasthi et al., 2005; Vervoorts et al., 2003). PCR products were electrophoresed through a 2% Tris-acetate-EDTA agarose gel and visualized using ethidum bromide. Dual-ChIPs were performed using HTLV-1-transformed HuT-102 lymphocytes as described in Vakoc et al. (2005). Briefly, following the initial immunoprecipitation, nucleoprotein complexes were eluted with 10 mM DTT at 37°C for 30 minutes. Samples were then diluted in ChIP-Dilution Buffer (Upstate Biotechnology) and re-immunoprecipitated using primary antibodies and 60 μl salmon sperm DNA/Protein A-agarose. The re-immunoprecipitated complexes were washed, crosslinks were reversed, and bound DNA fragments were amplified by PCR using the prm oligonucleotide primers as described (Awasthi et al., 2005).
Foci-formation/cellular transformation
To assess cellular transformation by HTLV-1 p30II-GFP (or p30II-HA) and wildtype c-MYC or the acetylation-defective Lys→Arg c-MYC K323R/K417R and R5 mutants, 2 × 105 c-myc−/− HO15.19 rat fibroblasts (Albihn et al., 2006; Mateyak et al., 1997) were seeded in 60 mm2 tissue-culture dishes and co-transfected with CβF-wildtype c-MYC or Lys→Arg c-MYC mutants and CMV-HTLV-1 p30II-GFP or a pcDNA3.1-GFP control. Transfected cultures were monitored microscopically over a two-week period until transformed colonies were visible –evidenced by the loss of contact-inhibition and pronounced cell clustering (Awasthi et al., 2005). The plates were fixed in 70% ethanol-PBS and stained with methylene blue for improved visualization of colonies. DAPI-nuclear staining was also included in certain experiments to verify cellular foci/nuclear aggregation. The expression of HTLV-1 p30II-GFP in transformed foci was confirmed by direct fluorescence-microscopy. Foci-formation experiments using immortalized human wrn−/− WS AG11395 fibroblasts (Moser et al., 2000) to determine the effects of dominant-negative acetyltransferase mutants, TIP60Q377E/G380E, p300ΔE1A, and PCAFΔHAT (Ikura et al., 2000; Chakravarti et al., 1999; Yang et al., 1996), upon HTLV-1 p30II(HA)/c-MYC-induced cellular transformation were performed as in Awasthi et al. (2005).
Annexin-V-FITC apoptosis assays and BrdU-induced cytotoxicity
Apoptosis was measured in co-transfected c-myc−/− HO15.19 fibroblasts, expressing the HTLV-1 p30II (HA) and wildtype or acetylation-defective Lys→Arg c-MYC mutant proteins, by staining the cultures with Annexin-V-FITC/PI (BD-Pharmingen) and performing flow cytometric analyses on a BD FACSCaliber instrument. Alternatively, the adherent c-myc−/− HO15.19 fibroblasts and HTLV-1 p30II(HA)/c-MYC-transformed colonies were stained with Annexin-V-FITC and apoptotic cells were visualized and quantified by direct fluorescence-microscopy. C2-Ceramide and staurosporine (Sigma-Aldrich) were included as positive apoptosis controls.
To evaluate the effects of HTLV-1 p30II and wildtype c-MYC, or acetylation-defective c-MYC K323R/K417R and R5 mutants, upon programmed cell-death induced by prolonged exposure to genotoxic stress, the co-transfected c-myc−/− HO15.19 cultures were treated with bromodeoxyuridine (BrdU) for 12 hr. Subsequently, the cells were washed, fixed/permeabilized and immunostained using an anti-BrdU-FITC-conjugated antibody. The DNA was stained with 7-AAD (BD-Pharmingen) and the cells were analyzed by flow cytometry (Awasthi et al., 2005). Cytotoxicity induced by long-term BrdU-exposure was observed by staining the co-transfected cultures with Annexin-V-FITC and apoptosis was quantified by direct fluorescence-microscopy.
Detecting c-MYC-acetylation
To observe Lys-acetylation of the c-MYC oncoprotein, 293 HEK cells were co-transfected with CβF-CMV-c-MYC (FLAG-tagged) in the presence of increasing amounts of CMV-HTLV-1 p30II-GFP or an empty vector control. The cells were pelleted by centrifugation at 4° C, resuspended in RIPA buffer (phosphate-buffered saline, PBS, 1% v/v IGEPAL-CA630, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate, SDS) containing protease inhibitors (antipain dihydrochloride, bestatin, leupeptin, aprotinin, chymostatin, and pepstatin (Roche Applied Sciences), and lysed by sonication at 70% duty cycle using a microprobe. Acetylated cellular proteins were immunoprecipitated using an anti-Acetyl-Lysine antibody (Millipore)/Protein G-agarose (Invitrogen), centrifuged and washed, resolved by SDS-PAGE, and the bound acetylated-c-MYC protein was detected by immunostaining as in Patel et al., 2004. The status of c-MYC-acetylation in HTLV-1-transformed HuT-102 and MJG11 T-lymphocytes was determined by immunoprecipitating cellular acetylated proteins using an anti-Acetyl-Lysine antibody/Protein G-agrose. As a negative IP control, immunoprecipitations were carried out in parallel using an anti-GFP antibody (Sigma-Aldrich). The acetylated-c-MYC was detected by immunoblotting as described (Patel et al., 2004). Uninfected Jurkat E6.1 T-lymphocytes were included as a control.
BrdU-incorporation and lentiviral transductions
To determine if HTLV-1 p30II-expressing cells enter the S-phase and replicate their genome in the presence of etoposide, HFL1 human fibroblasts (ATCC, CCL-153) were transduced with lentiviral particles expressing the HTLV-1 p30II (HA-tagged) protein: pLenti-6.2/V5-DEST-HTLV-1 p30II HA, or empty pLenti-6.2/V5-DEST vector particles (Invitrogen, Inc.) as a negative control. The transduced cultures were treated with etoposide and then labeled with BrdU for 6 hrs. The cells were subsequently fixed, permeabilized, and stained using an anti-BrdU-FITC antibody (BD Pharmingen), and immunofluorescence-microscopy was performed to detect anti-BrdU-FITC-staining in the p30II (HA)-expressing or vector control transduced cells.
Statistical analysis
The statistical significance and confidence intervals of experimental data sets were analyzed by determining their Student’s t-distribution values (alpha = 0.05).
Supplementary Material
Acetylation of c-MYC is required for oncogenic transformation by HTLV-1 p30II/c-MYC
Acetylation-defective c-MYC mutants are impaired for foci-formation by p30II/c-MYC
The HTLV-1 p30II protein induces lysine-acetylation of c-MYC
p30II is present in c-MYC nucleoprotein complexes in HTLV-1-transformed T-cells
HTLV-1 p30II inhibits apoptosis in c-MYC-expressing proliferating cells
Acknowledgments
This work was supported by National Cancer Institute/National Institutes of Health grants 1R15CA158945-01A1 and 1R15CA139425-01 to RH. We thank SB McMahon (The Wistar Institute) for the CβF-CMV-c-MYCK417R/K323R expression construct, and Yoshihiro Nakatani (Dana-Farber Cancer Institute) for the pOZ-CMV-TIP60, pOZ-CMV-TIP60Q377E/G380E, pSI-CMV-p300ΔE1A, and pCX-CMV-PCAFΔHAT plasmids, and RJ Monnat (University of Washington) for immortalized wrn−/− WS (AG11395) fibroblasts. We also thank Rick Jones (SMU) for helpful comments and suggestions. Other members of the Harrod lab, A Malu, T Hutchison, O Nguyen, R Gardner, J Chu, A White, C Hazen, M Hancock, N Rao, H Yang, S Awasthi, M Loh, K Nelson, N Tayeh, and B Fawcett, are acknowledged for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackland SP, Schilsky RL, Beckett MA, Weichselbaum RR. Synergistic cytotoxicity and DNA strand break formation by bromodeoxyuridine and bleomycin in human tumor cells. Cancer Res. 1988;48:4244–4249. [PubMed] [Google Scholar]
- Albihn A, Loven J, Ohlsson J, Osorio LM, Henriksson M. c-Myc-dependent etoposide-induced apoptosis involves activation of Bax and caspases, and PKCdelta signaling. J Cell Biochem. 2006;98:1597–1614. doi: 10.1002/jcb.20816. [DOI] [PubMed] [Google Scholar]
- Andresen V, Pise-Masison CA, Sinha-Datta U, Bellon M, Valeri V, Washington Parks R, Cecchinato V, Fukumoto R, Nicot C, Franchini G. Suppression of HTLV-1 replication by Tax-mediated rerouting of the p13 viral protein to nuclear speckles. Blood. 2011;118:1549–1559. doi: 10.1182/blood-2010-06-293340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anupam R, Doueiri R, Green PL. The need to accessorize: molecular roles of HTLV-1 p30 and HTLV-2 p28 accessory proteins in the viral life cycle. Front Microbiol. 2013;4(275):1–9. doi: 10.3389/fmicb.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J, Yamamoto B, Li M, Phipps AJ, Younis I, Lairmore MD, Green PL. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood. 2006;107:3976–3982. doi: 10.1182/blood-2005-11-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood. 2008;112:3788–3797. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Sharma A, Wong K, Zhang J, Matlock EF, Rogers L, Motloch P, Takemoto S, Taguchi H, Cole MD, Lüscher B, Dittrich O, Tagami H, Nakatani Y, McGee M, Girard AM, Gaughan L, Robson CN, Monnat RJ, Jr, Harrod R. A human T-cell lymphotropic virus type 1 enhancer of Myc transforming potential stabilizes Myc-TIP60 transcriptional interactions. Mol Cell Biol. 2005;25:6178–6198. doi: 10.1128/MCB.25.14.6178-6198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai XT, Baydoun HH, Nicot C. HTLV-I p30: a versatile protein modulating virus replication and pathogenesis. Mol Aspects Med. 2010;31:344–349. doi: 10.1016/j.mam.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai XT, Nicot C. Overview on HTLV-1 p12, p8, p30, p13: accomplices in persistent infection and viral pathogenesis. Front Microbiol. 2012;3(400):1–9. doi: 10.3389/fmicb.2012.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoe JT, Albrecht B, Collins ND, Robek MD, Ratner L, Green PL, Lairmore MD. Functional role of pX open reading frame II of human T-lymphotropic virus type 1 in maintenance of viral loads in vivo. J Virol. 2000;74:1094–1100. doi: 10.1128/jvi.74.3.1094-1100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun HH, Pancewicz J, Nicot C. Human T-lymphotropic virus type 1 virus p30 inhibits homologous recombination and favors unfaithful DNA repair. Blood. 2011;117:5897–5906. doi: 10.1182/blood-2010-08-304600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, Luscher B. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev. 2001;15:2042–2047. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereseto A, Berneman Z, Koralnik I, Vaughn J, Franchini G, Klotman ME. Differential expression of alternatively spliced pX mRNAs in HTLV-I-infected cell lines. Leukemia. 1997;11:866–870. doi: 10.1038/sj.leu.2400665. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Mesri EA. Kaposi sarcoma-associated herpesvirus and other viruses in human lymphomagenesis. Curr Top Microbiol Immunol. 2007;312:263–287. doi: 10.1007/978-3-540-34344-8_10. [DOI] [PubMed] [Google Scholar]
- Chakravarti D, Ogryzko V, Kao HY, Nash A, Chen H, Nakatani Y, Evans RM. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell. 1999;96:393–403. doi: 10.1016/s0092-8674(00)80552-8. [DOI] [PubMed] [Google Scholar]
- Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 2000;14:1584–1588. [PMC free article] [PubMed] [Google Scholar]
- Chen HS, Lu F, Lieberman PM. Epigenetic regulation of EBV and KSHV latency. Curr Opin Virol. 2013;3:251–259. doi: 10.1016/j.coviro.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc I, Polakowski N, Andre-Arpin C, Cook P, Barbeau B, Mesnard JM, Lemasson I. An interaction between the human T cell leukemia virus type 1 basic leucine zipper factor (HBZ) and the KIX domain of p300/CBP contributes to the down-regulation of tax-dependent viral transcription by HBZ. J Biol Chem. 2008;283:23903–23913. doi: 10.1074/jbc.M803116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Ngouenet C, Anderson S, Brabletz T, Eisenman RN. Stress-induced cleavage of Myc promotes cancer cell survival. Genes Dev. 2014;28:689–707. doi: 10.1101/gad.231894.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currer R, Van Duyne R, Jaworski E, Guendel I, Sampey G, Das R, Narayanan A, Kashanchi F. HTLV tax: a fascinating multifunctional co-regulator of viral and cellular pathways. Front Microbiol. 2012;3(406):1–24. doi: 10.3389/fmicb.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Silverman L, Phipps AJ, Hiraragi H, Ratner L, Lairmore MD. Human T-lymphotropic virus type-1 p30 alters cell cycle G2 regulation of T lymphocytes to enhance cell survival. Retrovirology. 2007;4:49. doi: 10.1186/1742-4690-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Sinha-Datta U, Dhillon NK, Buch S, Nicot C. The HTLV-I p30 interferes with TLR4 signaling and modulates the release of pro- and anti-inflammatory cytokines from human macrophages. J Biol Chem. 2006;281:23414–23424. doi: 10.1074/jbc.M600684200. [DOI] [PubMed] [Google Scholar]
- Doueiri R, Anupam R, Kvaratskhelia M, Green KB, Lairmore MD, Green PL. Comparative host protein interactions with HTLV-1 p30 and HTLV-2 p28: insights into difference in pathobiology of human retroviruses. Retrovirology. 2012;9:64. doi: 10.1186/1742-4690-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyao MP, Kessler DJ, Spicer DB, Sonenshein GE. Transactivation of the c-myc gene by HTLV-1 tax is mediated by NFκB. Curr Top Microbiol Immunol. 1992;182:421–424. doi: 10.1007/978-3-642-77633-5_53. [DOI] [PubMed] [Google Scholar]
- Edwards D, Fenezia C, Gold H, de Castro-Amarante MF, Buchmann C, Pise-Masison CA, Franchini G. Orf-I and orf-II-encoded proteins in HTLV-1 infection and persistence. Viruses. 2011;3:861–885. doi: 10.3390/v3060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiola F, Liu X, Lo S, Pan S, Zhang K, Lymar E, Farina A, Martinez E. Dual regulation of c-Myc by p300 via acetylation-dependent control of Myc protein turnover and coactivation of Myc-induced transcription. Mol Cell Biol. 2005;25:10220–10234. doi: 10.1128/MCB.25.23.10220-10234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiola F, Wu YT, Pan S, Zhang K, Farina A, Martinez E. Max is acetylated by p300 at several nuclear localization residues. Biochem J. 2007;403:397–407. doi: 10.1042/BJ20061593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenizia C, Fiocchi M, Jones K, Parks RW, Ceribelli M, Chevalier SA, Edwards D, Ruscetti F, Pise-Masison CA, Franchini G. Human T-cell leukemia/lymphoma virus type 1 p30, but not p12/p8, counteracts Toll-like receptor 3 (TLR3) and TLR4 signaling in human monocytes and dendritic cells. J Virol. 2014;88:393–402. doi: 10.1128/JVI.01788-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, Livingston DM, Amati B. MYC recruits the TIP60 acetyltransferase complex to chromatin. EMBO Rep. 2003;4:575–580. doi: 10.1038/sj.embor.embor861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabet AS, Mortreux F, Charneau P, Riou P, Duc-Dodon M, Wu Y, Jeang KT, Wattel E. Inactivation of hTERT transcription by Tax. Oncogene. 2003;22:3734–3741. doi: 10.1038/sj.onc.1206468. [DOI] [PubMed] [Google Scholar]
- Georges SA, Giebler HA, Cole PA, Luger K, Laybourn PJ, Nyborg JK. Tax recruitment of CBP/p300, via the KIX domain, reveals a potent requirement for acetyltransferase activity that is chromatin dependent and histone tail independent. Mol Cell Biol. 2003;23:3392–3404. doi: 10.1128/MCB.23.10.3392-3404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbel S, Sinha-Datta U, Dundr M, Brown M, Franchini G, Nicot C. Human T-cell leukemia virus type I p30 nuclear/nucleolar retention is mediated through interactions with RNA and a constituent of the 60 S ribosomal subunit. J Biol Chem. 2006;281:37150–37158. doi: 10.1074/jbc.M603981200. [DOI] [PubMed] [Google Scholar]
- Giam CZ, Jeang KT. HTLV-1 Tax and adult T-cell leukemia. Front Biosci. 2007;12:1496–1507. doi: 10.2741/2163. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Ratner L. Cytokine expression and tumorigenicity of large granular lymphocytic leukemia cells from mice transgenic for the tax gene of human T-cell leukemia virus type I. Blood. 1997;90:783–794. [PubMed] [Google Scholar]
- Hall AP, Irvine J, Blyth K, Cameron ER, Onions DE, Campbell ME. Tumours derived from HTLV-I tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J Pathol. 1998;186:209–214. doi: 10.1002/(SICI)1096-9896(1998100)186:2<209::AID-PATH162>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Harrod R, Kuo YL, Tang Y, Yao Y, Vassilev A, Nakatani Y, Giam CZ. P300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax in a multi-histone acetyltransferase/activator-enhancer complex. J Biol Chem. 2000;275:11852–11857. doi: 10.1074/jbc.275.16.11852. [DOI] [PubMed] [Google Scholar]
- Harrod R, Tang Y, Nicot C, Lu HS, Vassilev A, Nakatani Y, Giam CZ. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Sawa H, Lewis MJ, Orba Y, Sheehy N, Yamamoto Y, Ichinohe T, Tsunetsugu-Yokota Y, Katano H, Takahashi H, Matsuda J, Sata T, Kurata T, Nagashima K, Hall WW. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat Med. 2006;12:466–472. doi: 10.1038/nm1389. [DOI] [PubMed] [Google Scholar]
- Ho YK, Zhi H, DeBiaso D, Philip S, Shih HM, Giam CZ. HTLV-1 tax-induced rapid senescence is driven by the transcriptional activity of NF-κB and depends on chronically activated IKKα and p65/RelA. J Virol. 2012;86:9474–9483. doi: 10.1128/JVI.00158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, Scully R, Qin J, Nakatani Y. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–473. doi: 10.1016/s0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- Jeang KT. Human T-cell leukemia virus type 1 (HTLV-1) latency: death signaling awakens the sleeping retrovirus. Cell Cycle. 2011;10:3824–3825. doi: 10.4161/cc.10.22.18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Harrod R, Franchini G. Molecular biology and pathogenesis of the human T-cell leukaemia/lymphotropic virus type 1 (HTLV-1) Int J Exp Pathol. 2001;82:135–147. doi: 10.1046/j.1365-2613.2001.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannian P, Green PL. Human T lymphotropic virus type 1 (HTLV-1): molecular biology and oncogenesis. Viruses. 2010;2:2037–2077. doi: 10.3390/v2092037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi T, Nakao Y, Kawanishi M, Maeda S, Sugiyama T, Fujita T. Suppression of c-myc mRNA expression by steroid hormones in HTLV-I-infected T-cell line, KH-2. Int J Cancer. 1989;44:701–706. doi: 10.1002/ijc.2910440425. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann AS, Villaudy J, Gazzolo L, Castellazzi M, Mesnard JM, Duc Dodon M. HTLV-1 HBZ cooperates with JunD to enhance transcription of the human telomerase reverse transcriptase gene (hTERT) Retrovirology. 2007;4:92. doi: 10.1186/1742-4690-4-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore MD, Anupam R, Bowden N, Haines R, Haynes RA, 2nd, Ratner L, Green PL. Molecular determinants of human T-lymphotropic virus type 1 transmission and spread. Viruses. 2011;3:1131–1165. doi: 10.3390/v3071131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lairmore MD, Haines R, Anupam R. Mechanisms of human T-lymphotropic virus type 1 transmission and disease. Curr Opin Virol. 2012;2:474–481. doi: 10.1016/j.coviro.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Lemasson I, Lewis MR, Polakowski N, Hivin P, Cavanaugh MH, Thebault S, Barbeau B, Nyborg JK, Mesnard JM. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J Virol. 2007;81:1543–1553. doi: 10.1128/JVI.00480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Kesic M, Yin H, Yu L, Green PL. Kinetic analysis of human T-cell leukemia virus type 1 gene expression in cell culture and infected animals. J Virol. 2009;83:3788–3797. doi: 10.1128/JVI.02315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link JM, Ota S, Zhou ZQ, Daniel CJ, Sears RC, Hurlin PJ. A critical role for Mnt in Myc-driven T-cell proliferation and oncogenesis. Proc Natl Acad Sci USA. 2012;109:19685–19690. doi: 10.1073/pnas.1206406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Yasunaga J, Fan J, Yanagawa S, Matsuoka M. HTLV-1 bZIP factor dysregulates the Wnt pathways to support proliferation and migration of adult T-cell leukemia cells. Oncogene. 2013;32:4222–4230. doi: 10.1038/onc.2012.450. [DOI] [PubMed] [Google Scholar]
- Mao B, Zhao G, Lv X, Chen HZ, Xue Z, Yang B, Liu DP, Liang CC. Sirt1 deacetylates c-Myc and promotes c-Myc/Max association. Int J Biochem Cell Biol. 2011;43:1573–1581. doi: 10.1016/j.biocel.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Mateyak MK, Obaya AJ, Adachi S, Sedivy JM. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 1997;8:1039–1048. [PubMed] [Google Scholar]
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell. 1998;94:363–374. doi: 10.1016/s0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- Mengle-Gaw L, Rabbitts TH. A human chromosome 8 region with abnormalities in B cell, HTLV-I+ T cell and c-myc amplified tumours. EMBO J. 1987;6:1959–1965. doi: 10.1002/j.1460-2075.1987.tb02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael B, Nair AM, Datta A, Hiraragi H, Ratner L, Lairmore MD. Histone acetyltransferase (HAT) activity of p300 modulates human T lymphotropic virus type 1 p30II-mediated repression of LTR transcriptional activity. Virology. 2006;354:225–239. doi: 10.1016/j.virol.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael B, Nair AM, Hiraragi H, Shen L, Feuer G, Boris-Lawrie K, Lairmore MD. Human T lymphotropic virus type-1 p30II alters cellular gene expression to selectively enhance signaling pathways that activate T lymphocytes. Retrovirology. 2004;1:39. doi: 10.1186/1742-4690-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra-Kaushik S, Harding J, Hess J, Schreiber R, Ratner L. Enhanced tumorigenesis in HTLV-1 tax-transgenic mice deficient in interferon-gamma. Blood. 2004;104:3305–3311. doi: 10.1182/blood-2004-01-0266. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Liu ZJ, Taniguchi T. Selective cooperation of HTLV-1-encoded p40tax-1 with cellular oncoproteins in the induction of hematopoietic cell proliferation. Oncogene. 1996;12:2403–2408. [PubMed] [Google Scholar]
- Moser MJ, Kamath-Loeb AS, Jacob JE, Bennett SE, Oshima J, Monnat RJ. WRN helicase expression in Werner syndrome cell lines. Nucleic Acids Res. 2000;28:648–654. doi: 10.1093/nar/28.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot C, Dundr M, Johnson JM, Fullen JR, Alonzo N, Fukumoto R, Princler GL, Derse D, Misteli T, Franchini G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat Med. 2004;10:197–201. doi: 10.1038/nm984. [DOI] [PubMed] [Google Scholar]
- Nicot C, Harrod R. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 Tax protein. Mol Cell Biol. 2000;20:8580–8589. doi: 10.1128/mcb.20.22.8580-8589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot C, Harrod RL, Ciminale V, Franchini G. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene. 2005;24:6026–6034. doi: 10.1038/sj.onc.1208977. [DOI] [PubMed] [Google Scholar]
- Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, Zhao K, Levens D. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K, Ikebe M, Shimokama T, Takeshita M, Kinjo N, Sugimachi K, Higashi H. An unusual enteropathy-associated T-cell lymphoma with MYC translocation arising in a Japanese patient: a case report. World J Gastroenterol. 2012;18:2434–2437. doi: 10.3748/wjg.v18.i19.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JH, Du Y, Ard PG, Phillips C, Carella B, Chen CJ, Rakowski C, Chatterjee C, Lieberman PM, Lane WS, Blobel GA, McMahon SB. The c-Myc oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol. 2004;24:10826–10834. doi: 10.1128/MCB.24.24.10826-10834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch D, Gross S, Harding J, Niewiesk S, Lairmore MD, Piwnica-Worms D, Ratner L. Imaging spontaneous tumorigenesis: inflammation precedes development of peripheral NK tumors. Blood. 2009;113:1493–1500. doi: 10.1182/blood-2008-07-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggioro D, D’agostino DM, Chieco-Bianchi L. Analysis of Tax-expressing cell lines generated from HTLV-1 tax-transgenic mice: correlation between c-myc overexpression and neoplastic potential. Exp Cell Res. 1999;247:525–533. doi: 10.1006/excr.1998.4381. [DOI] [PubMed] [Google Scholar]
- Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA. 2006;103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou Y, Yasunaga J, Zhao T, Yoshida M, Miyazato P, Takai K, Shimizu K, Ohshima K, Green PL, Ohkura N, Yamaguchi T, Ono M, Sakaguchi S, Matsuoka M. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011;7(e1001274):1–14. doi: 10.1371/journal.ppat.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmes OJ, Barret JF, Dang CV, Jeang KT. Human T-cell leukemia virus type I tax masks c-Myc function through a cAMP-dependent pathway. J Biol Chem. 1996;271:9730–9738. doi: 10.1074/jbc.271.16.9730. [DOI] [PubMed] [Google Scholar]
- Silic-Benussi M, Biasiotto R, Andresen V, Franchini G, D’Agostino DM, Ciminale V. HTLV-1 p13, a small protein with a busy agenda. Mol Aspects Med. 2010;31:350–358. doi: 10.1016/j.mam.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman LR, Phipps AJ, Montgomery A, Ratner L, Lairmore MD. Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: evidence of in vivo reversion. J Virol. 2004;78:3837–3845. doi: 10.1128/JVI.78.8.3837-3845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A, Donohue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaims AY, Khani F, Zhang Y, Roberts AI, Devadas S, Shi Y, Rabson AB. Immune activation induces immortalization of HTLV-1 LTR-Tax transgenic CD4+ T cells. Blood. 2010;116:2994–3003. doi: 10.1182/blood-2009-07-231050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tang Y, Tie F, Boros I, Harrod R, Glover M, Giam CZ. An extended alpha-helix and specific amino acid residues opposite the DNA-binding surface of the cAMP response element binding protein basic domain are important for human T cell lymphotropic retrovirus type I Tax binding. J Biol Chem. 1998;273:27339–27346. doi: 10.1074/jbc.273.42.27339. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Ghorbel S, Nicot C. Genome wide analysis of human genes transcriptionally and post-transcriptionally regulated by the HTLV-I protein p30. BMC Genomics. 2009;10 (311):1–14. doi: 10.1186/1471-2164-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Van Prooyen N, Andresen V, Gold H, Bialuk I, Pise-Masison C, Franchini G. Hijacking the T-cell communication network by the human T-cell leukemia/lymphoma virus type 1 (HTLV-1) p12 and p8 proteins. Mol Aspects Med. 2010;31:333–343. doi: 10.1016/j.mam.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoorts J, Luscher-Firzlaff J, Luscher B. The ins and outs of MYC regulation by posttranslational mechanisms. J Biol Chem. 2006;281:34725–34729. doi: 10.1074/jbc.R600017200. [DOI] [PubMed] [Google Scholar]
- Vervoorts J, Luscher-Firzlaff JM, Rottmann S, Lilischkis R, Walsemann G, Dohmann K, Austen M, Luscher B. Stimulation of c-MYC transcriptional activity and acetylation by recruitment of the cofactor CBP. EMBO Rep. 2003;4:484–490. doi: 10.1038/sj.embor.embor821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Sun SC. Retroviral oncoprotein Tax deregulates NF-kappaB by activating Tak1 and mediating the physical association of Tak1-IKK. EMBO Rep. 2007;8:510–515. doi: 10.1038/sj.embor.7400931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm T, Wright DG, Polakowski N, Mesnard JM, Lemasson I. The HTLV-1-encoded protein HBZ directly inhibits the acetyl transferase activity of p300/CBP. Nucleic Acids Res. 2012;40:5910–5925. doi: 10.1093/nar/gks244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Younis I, Boris-Lawrie K, Green PL. Human T-cell leukemia virus open reading frame II encodes a posttranscriptional repressor that is recruited at the level of transcription. J Virol. 2006;80:181–191. doi: 10.1128/JVI.80.1.181-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis I, Khair L, Dundr M, Lairmore MD, Franchini G, Green PL. Repression of human T-cell leukemia virus type 1 and type 2 replication by a viral mRNA-encoded posttranscriptional regulator. J Virol. 2004;78:11077–11083. doi: 10.1128/JVI.78.20.11077-11083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zane L, Yasunaga J, Mitagami Y, Yedavalli V, Tang SW, Chen CY, Ratner L, Lu X, Jeang KT. Wip1 and p53 contribute to HTLV-1 Tax-induced tumorigenesis. Retrovirology. 2012;9:114. doi: 10.1186/1742-4690-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Faiola F, Martinez E. Six lysine residues on c-Myc are direct substrates for acetylation by p300. Biochem Biophys Res Commun. 2005;336:274–280. doi: 10.1016/j.bbrc.2005.08.075. [DOI] [PubMed] [Google Scholar]
- Zhang W, Nisbet JW, Albrecht B, Ding W, Kashanchi F, Bartoe JT, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) regulates gene transcription by binding CREB binding protein/p300. J Virol. 2001;75:9885–9895. doi: 10.1128/JVI.75.20.9885-9895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Nisbet JW, Bartoe JT, Ding W, Lairmore MD. Human T-lymphotropic virus type 1 p30(II) functions as a transcription factor and differentially modulates CREB-responsive promoters. J Virol. 2000;74:11270–11277. doi: 10.1128/jvi.74.23.11270-11277.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LJ, Giam CZ. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Coutts A, Xu L, Ohshima K, Matsuoka M. HTLV-1 bZIP factor supports proliferation of adult T cell leukemia cells through suppression of C/EBPa signaling. Retrovirology. 2013;10:159. doi: 10.1186/1742-4690-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Satou Y, Sugata K, Miyazato P, Green PL, Imamura T, Matsuoka M. HTLV-1 bZIP factor enhances TGF-B signaling through p300 coactivator. Blood. 2011;118:1865–1876. doi: 10.1182/blood-2010-12-326199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi H, Yang L, Kuo YL, Ho YK, Shih HM, Giam CZ. NF-kB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002025. epub [e1002025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.