Abstract
Problem
Group B Streptococcus (GBS) is a leading cause of neonatal morbidity and mortality. We tested the hypothesis that the choriodecidua plays a role in GBS-stimulated human beta defensin (HBD)-2 increases in amnion cells through a secreted factor of choriodecidual origin.
Method of Study
Human amnion epithelial cells were treated with choriodecidual GBS conditioned medium, live GBS, lipoteichoic acid (LTA), or lipopolysaccharide (LPS), with and without IL-1 inhibitors.
Results
Choriodecidual tissue punches released IL-1α and IL-1β in response to GBS and this medium significantly stimulated release of HBD-2 by amnion cell cultures. Inhibitors of IL-1 significantly impaired the release of HBD-2 from amnion cells treated with GBS choriodecidual conditioned medium. Direct stimulation of amnion cells with GBS, LTA, or LPS did not increase HBD-2 release.
Conclusions
Paracrine signaling involving IL-1 of choriodecidual origin is likely a critical driver for amnion HBD-2 increases in response to GBS infection of extraplacental membranes.
Introduction
Streptococcus agalactiae or Group B Streptococcus (GBS) is the leading cause of infectious neonatal morbidity and mortality in the United States [1]. GBS in the gravid female reproductive tract are associated with adverse birth outcomes such as sepsis and meningitis. The ascending pathway of infection begins with colonization of the vagina. GBS then passes through the cervix and enters the uterine cavity where it can cross the extraplacental membranes and infect the neonate. Despite the importance of the extraplacental membranes, the mechanisms by which GBS colonizes the membranes and causes infection remain poorly understood.
Human beta defensins (HBDs) are an important part of the innate immune system and play critical roles responding to infectious microorganisms [2–4]. HBDs are expressed throughout the reproductive tract, including the extraplacental membranes [5]. HBDs are considered a first defense during pregnancy because they can kill bacteria directly through membrane disruption, pore formation in the membrane wall, and polarization [2, 3, 6, 7]. Furthermore, HBDs can promote chemotaxis of immune cells. HBD-2 has been shown to be higher in amniotic fluid from women with intrauterine microbial infection compared to women without intrauterine infection [8]. In addition, HBD-2 concentrations in second trimester amniotic fluid have been positively correlated with preterm premature rupture of the extraplacental membranes [9]. However, infants born preterm had lower HBD-2 levels measured in cord blood compared to term neonates [10]. Infants that suffered from late onset sepsis tended to have lower levels of HBD-2 in cord blood suggesting HBD-2 is critical for effectively fighting infections. Despite the importance of HBD-2 for pregnancy- related infections, few studies have looked at potential stimuli and mechanisms governing HBD-2 expression in the extraplacental membranes and amnion epithelial cells. Pathogens increase HBD-2 in ex vivo extraplacental membranes models, yet little is known about how the pathogens are interacting with the tissue or which cells are primarily responsible for the HBD-2 production [11–13]. In addition, recombinant IL-1β has been shown to stimulate HBD-2 secretion in amnion epithelial cell cultures [14].
Recently, we demonstrated in an in vitro two-compartment model of full thickness human extraplacental membranes that HBD-2 is stimulated in the amnion epithelial cells following GBS inoculation on the decidual side of the membranes [15]. No bacteria were observed invading or crossing the tissue, suggesting a trans-tissue signaling mechanism. Here, we utilized separated extraplacental membranes co-cultured with GBS to test our hypothesis that the choriodecidua plays a necessary role in GBS-stimulated HBD-2 increases in amnion epithelial cells through a secreted factor of choriodecidual origin. Moreover, we provide evidence that IL-1α and IL-1β are the choriodecidual signaling molecules critical for the HBD-2 response in amnion epithelial cells.
Materials and Methods
Reagents and Materials
The GBS strain used in this study (A909, construct RS020, a gift from Amanda Jones, University of Washington), was initially isolated from a septic newborn [16]. GBS was grown at 37 °C in culture using Todd Hewitt Broth (THB, Becton-Dickinson, Franklin Lakes, NJ) or on sheep’s blood agar plates (Blood Agar Base #2, Remel, Lenexa, KS and BBL defibrinated sheep blood, Franklin Lakes, NJ) with 5 μg/mL erythromycin (Hemostat Labs, Dixon, CA). Media (DMEM catalog # 21063 and DMEM:F12 catalog #11039), buffers, fetal bovine serum (FBS; catalog #10438), trypsin-EDTA (catalog #25200), and penicillin/streptomycin (pen/strep; catalog #15140) were from GIBCO (Grand Island, NY). Epidermal growth factor (EGF), and recombinant cytokines (IL-1β, IL-6, IL-8, IL-17, TNF-α) were from Peprotech (Rocky Hill, NJ). Lipoteichoic acid (LTA) from Staphylococcus aureus, IL-1β neutralizing antibody, and IgA isotype control were from Invivogen (San Diego, CA). Lipopolysaccaride (LPS) from Salmonella typhimurium was from List Biological Laboratories (Campbell, CA). IL-1Ra was from Sigma-Aldrich (Saint Louis, MO).
Culture of Extraplacental Choriodecidual Membranes
According to previously published methods, human extraplacental membranes were collected from healthy, non-smoking, singleton pregnancies undergoing scheduled cesarean delivery prior to onset of labor at the University of Michigan Von Voigtlander Women’s Hospital Birth Center [15]. The University of Michigan Institutional Review Board approved this research (IRBMED#HUM0037054).
Membranes were transported to the lab in Dulbecco’s phosphate-buffered saline (DPBS) following delivery and rinsed with medium to remove blood clots. Membranes were then blunt dissected to separate the choriodecidua from the amnion. After dissection, the choriodecidua was punched using a 12-mm biopsy punch. Tissue punches were placed in 12-well plates with 1 mL medium containing Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 1% FBS and pen/strep. Cultures were incubated at 37 °C and 5% CO2. After 4 h, the medium was exchanged for DMEM/1% FBS without antibiotics. Treatments were balanced across subjects with tissue punches from each woman assigned to each treatment, including control cultures that were not exposed to GBS or other treatments.
GBS Coculture with Extraplacental Choriodecidual Membranes
GBS in early exponential growth phase was diluted with DMEM/1% FBS to an estimated 1×106 colony forming units/mL (CFU/mL). Inoculant concentrations were validated by overnight growth on 5% sheep blood agar with erythromycin. GBS was heat killed by incubating bacterial culture at 70 °C for 15 min. Lack of viability was confirmed by plating GBS on 5% sheep blood agar. LTA and LPS treatments were made in DMEM/1% FBS. Following a 24-h acclimation, the medium of the choriodecidual punches was replaced with 1 mL GBS inoculant (~1×106 CFU/mL), LTA (1 μg/mL), LPS (100 ng/mL), or fresh DMEM/1%FBS (control). Following 24 h of incubation with GBS, medium from the choriodecidual punches was filtered through a 0.2 μm pore filter to remove the bacteria, and then stored at −80 °C: this medium is referred to as GBS conditioned choriodecidual medium in this study. Medium from choriodecidual punches treated with LTA or LPS for 24 h was also 0.2 μm filtered and then stored at −80 °C: this medium is referred to as LTA or LPS conditioned choriodecidual medium. Coculture experiments were conducted in triplicate using extraplacental membranes from a minimum of seven women.
Amnion Epithelial Cell Isolation
Amnion epithelial cells were isolated from the same membranes used for choriodecidual punch cultures, using methods adapted from three protocols [17–19]. Primary isolated amnion epithelial cells instead of amnion tissue explants were used to ensure cell type and cell number across experiments. Briefly, blunt-dissected amnion was digested with 0.25% trypsin-EDTA at 37 °C for 30 minutes. Amnion tissue pieces were transferred to fresh trypsin-EDTA and the digestion was repeated. Following each digestion, the trypsin-EDTA was neutralized in the digest with medium (DMEM:F12) supplemented with 10% FBS and pen/strep. Cells were pelleted by centrifugation at 128 g for 5 minutes, suspended in medium, repelleted, and resuspended in medium containing EGF (DMEM:F12 supplemented with 10% FBS, pen/strep, and 10 ng/mL EGF). Amnion epithelial cells were plated at 500,000 cells/well (12-well plates) in 1 mL medium, and grown to 70–80% confluence. Medium was changed two days after seeding, and cells were treated on day 3.
Amnion epithelial cells were treated with either GBS choriodecidual conditioned medium, LTA (10 μg/mL), LPS (100 ng/mL), live GBS (~1×106 CFU/mL), or one of the following recombinant cytokines: IL-1α (1 ng/mL), IL-1β (1 ng/mL), IL-6 (100 ng/mL), IL-8 (100 ng/mL), IL-17 (100 ng/mL), or TNF-α (100 ng/mL). In addition, cells were untreated (controls), treated with increasing concentrations of IL-1α (12.5–1000 pg/mL), IL-1β (12.5–1000 pg/mL), or co-treated with IL-1α + IL-1β. Cells were incubated with treatments for 24 h. The medium used for these amnion epithelial cell treatments was the same as that used with choriodecidual punches (DMEM supplemented with 1% FBS and pen/strep), except that no pen/strep antibiotic was included for cultures with live GBS. No changes in cellular morphology, cytokine secretion, or HBD-2 secretion were noted with the medium change. Experiments were conducted in triplicate using extraplacental membranes from a minimum of five women.
IL-1 Inhibitors
To inhibit IL-1 activity, amnion epithelial cells were treated with GBS choriodecidual conditioned medium for 24 h with and without IL-1β neutralizing antibody (1000 ng/mL), IgA isotype control (1000 ng/mL), or IL-1 receptor antagonist (IL-1Ra; 100 ng/mL; 5.8 nM). IL-1β neutralizing antibody was incubated with the GBS choriodecidual conditioned medium for 30 minutes prior to incubation with the amnion epithelial cells. The concentration used for IL-1β neutralizing antibody and IL-1Ra was determined by concentration-response curves generated by treating amnion epithelial cells with 1 ng/mL IL-1α or IL-1β and increasing concentrations of IL-1β neutralizing antibody or IL-1Ra (Supplemental Figures 1 and 2). Reported Kd values for IL-1Ra range from 0.2 – 14 nM [20, 21]. Experiments were conducted in triplicate using amnion epithelial cells from five women.
Cytokine and HBD ELISAs
HBD-2 concentrations in GBS choriodecidual conditioned medium were measured using a commercially available enzyme linked immunosorbant assay (ELISA) kit according to manufacturer’s instructions (Peprotech, Rocky Hill, NJ). The HBD-2 ELISA detection range was 16–2000 pg/mL. Cytokine concentrations in GBS choriodecidual conditioned medium and amnion epithelial cell medium were measured by the University of Michigan Immunology Core using commercially available ELISA kits (R&D Systems). Cytokine detection ranges were as follows: 7.81–500 pg/mL for IL-1α; 2.91–2500 pg/mL for IL-1β; 9.38–125,000 pg/mL for IL-6; 31.2–2000 pg/mL for IL-8; and 15.6–5000 pg/mL for TNF-α. Cytokine and HBD-2 concentrations are reported as pg or ng protein/mL medium.
Statistical Analysis
Data are expressed as mean ± SEM and were analyzed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA) or SigmaStat 3.5 software (SigmaStat Software, San Jose, CA). ANOVAs with Tukey’s post hoc test were performed. Data were considered significant if the P value was < 0.05. When values were below the ELISA kit limit of detection (LOD), values were transformed to LOD/√2 prior to statistical analysis [22]. For HBD-2 assays, no-treatment controls, LTA and LPS treatment generally fell below the limit of detection, while GBS choriodecidual conditioned medium treatment was above the limit of detection. Cytokine values for all treatment conditions generally fell above the limit of detection.
Results
GBS infection of the choriodecidua induces HBD-2 expression in amnion epithelial cells via paracrine signaling
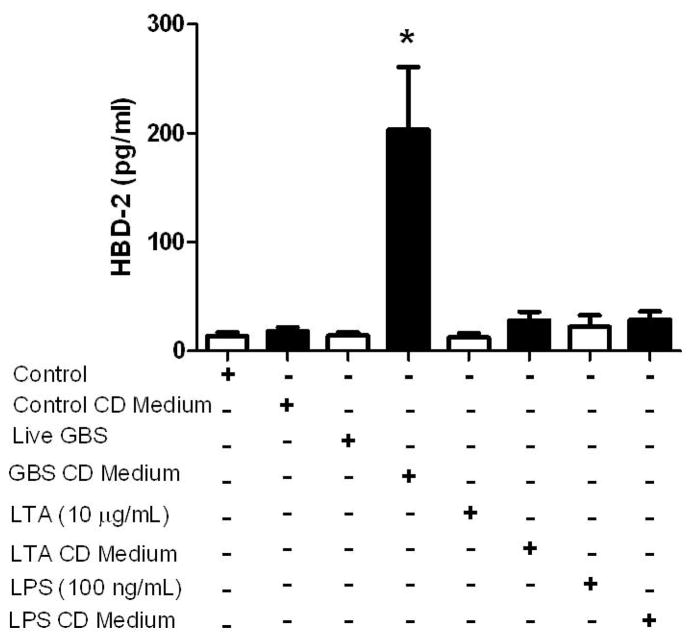
To determine if choriodecidua secretes factors that stimulate HBD-2 production in amnion epithelial cells, GBS was applied to choriodecidual punch cultures that had been stripped from the amnion, and the GBS choriodecidual conditioned medium was added to the amnion epithelial cell cultures. Amnion epithelial cells exposed to the GBS choriodecidual conditioned medium for 24 h showed a robust (11.2 fold) increase in HBD-2 release (Figure 1; P < 0.05) compared to amnion epithelial cells exposed to control choriodecidual conditioned medium. LTA and LPS were also used to determine if a similar host response could be provoked by bacterial cell wall/membrane components and to determine if host response was pathogen specific. In contrast to live GBS, neither LTA choriodecidual conditioned medium nor LPS choriodecidual conditioned medium stimulated release of HBD-2 from amnion epithelial cells suggesting the importance of the whole bacteria to stimulate the host response. Furthermore, amnion epithelial cells directly stimulated with live GBS, LTA, or LPS did not exhibit an increased HBD-2 response. HBD-2 was not detected in choriodecidual medium alone (data not shown), indicating that the HBD-2 increases observed with GBS choriodecidual conditioned medium were the result of HBD-2 secretion from amnion epithelial cells in culture.
Figure 1.
HBD-2 release in human amnion epithelial cell cultures treated directly with pathogenic stimuli (live GBS, LTA, or LPS) or with choriodecidual (CD) conditioned medium generated by culturing CD tissue punches with live GBS, LTA, or LPS for 24 h. HBD-2 protein in the medium was measured by ELISA. Columns are mean ± SEM (N=24 women for GBS CD conditioned medium, N=5 women for live GBS, and N=7 women for remaining treatment groups). The asterisk (*) represents a significant difference between treatment and control when compared by Tukey’s post hoc test following ANOVA (P < 0.05).
GBS is a potent stimulant for choriodecidual cytokines
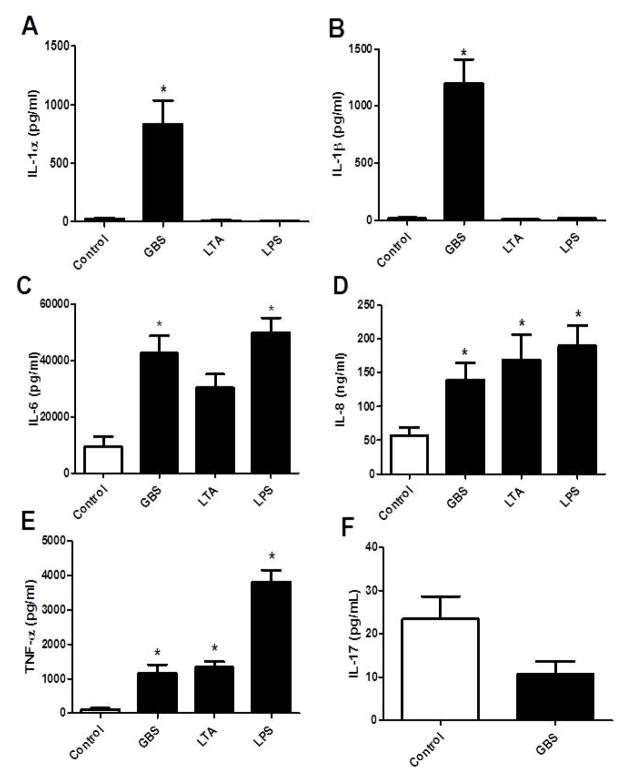
Preliminary experiments demonstrated that amnion epithelial cells did not release cytokines when stimulated with GBS directly or filtered GBS supernatant (data not shown). Cytokines were then measured in the medium of cultured choriodecidual punches exposed to pathogenic stimuli, in order to identify cytokines that may contribute to the HBD-2 response in amnion epithelial cells. First, heat-killed GBS and live GBS were compared for stimulation of IL-1β and TNF-α release. Heat-killed GBS failed to stimulate secretion of IL-1β or TNF-α from choriodecidual punches, in contrast to live GBS which elicited a strong IL-1β (287.2 ± 44.7 pg/mL) and TNF-α (1969 ± 456.7 pg/mL) response (Figure 2; P < 0.05). Additional cytokines were then probed to compare stimulated release by live GBS, LTA, and LPS. Live GBS significantly increased IL-1α (38.5 fold), IL-1β (71.1 fold), TNF-α (10.2 fold), IL-6 (4.5 fold), and IL-8 (2.4 fold) compared to controls (Figure 3A–E; P < 0.05). Live GBS did not change IL-17 secretion from choriodecidual punches compared to control (Figure 3F). LTA significantly increased only IL-8 (2.9 fold) and TNF-α (11.7 fold), and LPS significantly increased IL-6 (5.3 fold), IL-8 (3.3 fold), and TNF-α (33.4 fold). Neither LTA nor LPS stimulated increased release of IL-1α or IL-1β.
Figure 2.
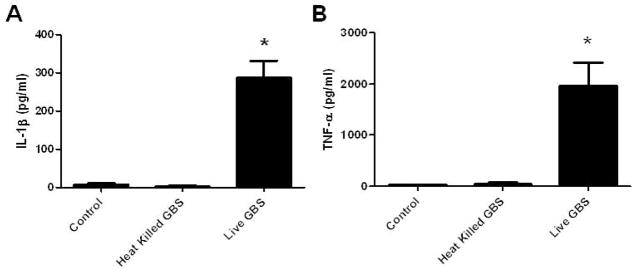
IL-1β and TNF-α release by choriodecidual punch cultures treated with medium alone (control), heat-killed GBS, or live GBS. IL-1β (A) and TNF-α (B) in the medium were measured by ELISA. Columns are mean ± SEM (N=5 women). Asterisks (*) represent significant differences between treatment and control when compared by Tukey’s post hoc test following ANOVA (P < 0.05).
Figure 3.
Cytokine release into medium by choriodecidual punch cultures treated with medium alone (control), live GBS, LTA, or LPS. IL-1α (A), IL-1β (B), IL-6 (C), IL-8 (D), and TNF-α (E) in the medium were measured by ELISA. IL-17 (F) release was measured after treatment with medium alone (control) or live GBS. Columns are mean ± SEM (N=21 women for Control and live GBS treatment groups; N=6 women for LTA and LPS treatment groups). Asterisks (*) represent significant differences between treatment and control when compared by Tukey’s post hoc test following ANOVA (P < 0.05).
IL-1 neutralizing antibody and IL-1 receptor antagonist inhibit GBS choriodecidual conditioned medium stimulated HBD-2 release from amnion epithelial cells
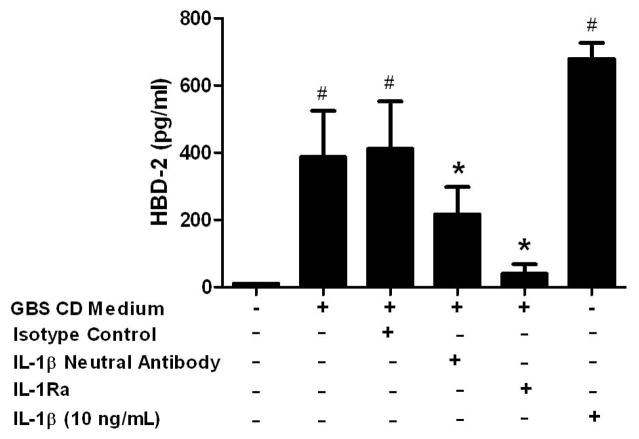
To test the hypothesis that IL-1α and IL-1β secreted from choriodecidua mediates amnion-secreted HBD-2, amnion epithelial cell cultures were treated with GBS choriodecidual conditioned medium containing IL-1β neutralizing antibody or IL-1Ra as inhibitors of IL-1 signaling. To determine the appropriate concentration of inhibitors, we established concentration-response curves for each inhibitor in amnion epithelial cells treated with 1 ng/mL IL-1α or 1 ng/mL IL-1β (Supplemental Figures 1 and 2). The IL-1β neutralizing antibody partially suppressed HBD-2 secretion stimulated by GBS choriodecidual conditioned medium (1.8 fold reduction), whereas IL-1Ra almost abolished the response (9.7 fold reduction) (Figure 4; P < 0.05). IL-1β (10 ng/mL) was used as positive control for HBD-2 secretion from amnion epithelial cells.
Figure 4.
The effect of IL-1 inhibitors on HBD-2 release by amnion epithelial cells. Amnion epithelial cells were treated with choriodecidual (CD) conditioned medium with and without IL-1 inhibitors for 24 h. IL-1β (10 ng/mL) treatment for 24 h was used as a positive control for HBD-2 secretion from amnion epithelial cells. HBD-2 protein in the amnion cell culture medium was measured by ELISA. Columns are mean ± SEM (N=5 women). Pound symbol (#) represents significant differences between treatment and medium only control and asterisks (*) represent significant differences between treatment and GBS CD Medium when compared by Tukey’s post hoc test following ANOVA (P < 0.05).
Recombinant IL-1α and IL-1β Stimulate HBD-2 Release from Amnion Epithelial Cells
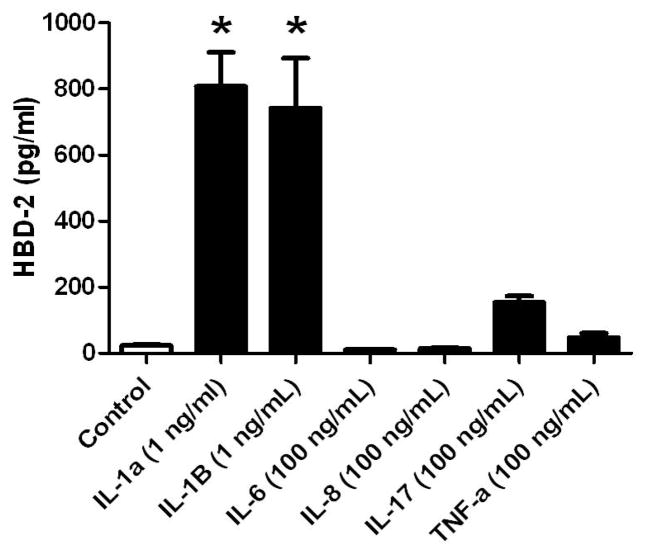
Because experiments with IL-1 inhibitors suggested a role for IL-1α and IL-1β in the HBD-2 response in amnion epithelial cells, we treated amnion epithelial cells with recombinant cytokines (Figure 5). HBD-2 release was significantly increased in a concentration-dependent manner by either IL-1α or IL-1β (Figure 5 and 6; P < 0.05), demonstrating that IL-1α and IL-1β directly stimulated HBD-2 release. However, co-treatment with equal concentrations of each IL-1α and IL-1β did not produce an increased HBD-2 response compared with exposure to either recombinant cytokine alone (Figure 6). Treatment with recombinant cytokines, IL-6, IL-8, IL-17, or TNF-α had no significant effect on HBD-2 from amnion epithelial cell cultures (Figure 5).
Figure 5.
HBD-2 release into medium by primary amnion epithelial cells treated with recombinant cytokines after 24 h. HBD-2 protein in the medium was measured by ELISA. Columns are mean ± SEM (N=3–6 women). Asterisks (*) represent significant differences between treatment and control when compared by Tukey’s post hoc test following ANOVA (P < 0.05).
Figure 6.
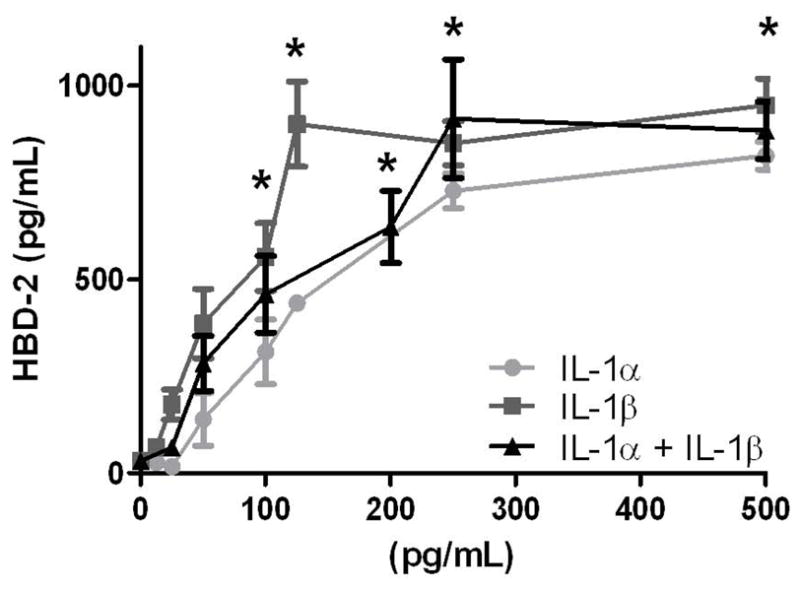
HBD-2 release by amnion epithelial cells treated with recombinant IL-1α and IL-1β after 24 h. Equal concentrations of each cytokine were used for the IL-1α + IL-1β treatment group. HBD-2 protein in the medium was measured by ELISA. Columns are mean ± SEM (N=7 women). Asterisks (*) represent significant differences compared to medium only control when compared by Tukey’s post hoc test following ANOVA (P < 0.05). No statistical differences were observed between cytokines treatments at any given concentration.
Discussion
As one of the leading causes of neonatal morbidity and mortality, GBS remains a serious public health problem [1]. The mechanisms by which GBS interacts with the host immune response have yet to be fully elucidated in gestational tissues. We previously reported that expression of the antimicrobial peptide HBD-2 increases in amnion epithelial cells of full thickness extraplacental membranes following GBS inoculation on the decidual side of the membranes in vitro, in the apparent absence of direct contact with the amnion epithelial cells[15]. Zaga, et al. reported similar HBD-2 results in full thickness human extraplacental membranes treated with GBS [23]. Here, we show for the first time that soluble factors from GBS-stimulated choriodecidual cultures were essential for secretion of HBD-2 from the amnion, and present evidence that IL-1β may play a critical role as a soluble factor in the tissue signaling necessary for the GBS-stimulated HBD-2 response in amniotic cells.
The present study utilized a combination of approaches to investigate GBS-stimulated intercellular signal transduction for increased amniotic expression of HBD-2. To study contributions specifically from the choriodecidua, we dissected choriodecidua from amnion, cultured choriodecidual punches with various treatments, and then used the sterile-filtered choriodecidual conditioned media to expose human amnion epithelial cell cultures. The large increase in IL-1α and IL-1β in the medium from GBS-treated choriodecidual tissue cultures suggests that IL-1 may have a role in GBS-stimulated responses in extraplacental membranes. Inhibition of stimulated HBD-2 secretion from amnion epithelial cells using an IL-1β neutralizing antibody and IL-1Ra, a nonselective IL-1 receptor antagonist, confirmed a role for IL-1 in the HBD-2 pathway. As we observed near complete inhibition of HBD-2 secretion when using IL-1Ra and only partial inhibition when using IL-1β neutralizing antibody, IL-1α and IL-1β may have redundant functions in HBD-2 secretion from amnion epithelial cells. Because live GBS failed to stimulate HBD-2 release from cultured amnion epithelial cells, GBS is not likely the source of the soluble factors responsible for stimulating HBD-2 release from amnion epithelial cells. Moreover, sterile-filtered supernatant from GBS cultures did not simulate HBD-2 in amnion epithelial cells in preliminary studies (data not shown), consistent with the conclusion that the choriodecidua, and not GBS, is the source of the soluble factor(s) necessary for stimulating the HBD-2 response in amnion cells. A similar mechanism, demonstrating the necessity of IL-1β in stimulating HBD-2 secretion, has been proposed for both uterine and pulmonary epithelial cells [24, 25]. Previous studies suggest that amnion epithelial cells are incapable of producing IL-1, lending further support for choriodecidua as the IL-1 source for HBD-2 production in amnion epithelial cells [26, 27].
Previous studies have shown that both IL-1α and IL-1β are present in placenta and extraplacental membranes of healthy women [28–30]. We demonstrated that recombinant IL-1β stimulated HBD-2 in amnion epithelial cell cultures, consistent with previous studies [14]. In addition, we showed for the first time that recombinant IL-1α also stimulated HBD-2 secretion in human amnion cells, and did so at concentrations similar to IL-1α concentrations detected in medium of GBS-stimulated choriodecidual tissue punch cultures. Our results are consistent with increases in IL-1α reported in bladder tissue and urine of mice treated with pathogenic GBS [31]. Furthermore, IL-1α and IL-1β are implicated in stimulating HBD-2 secretion from other cell and tissue types including human keratinocytes, uterine macrophages, corneal epithelial cells and middle ear epithelial cells [32–35].
Exposure of human amnion epithelial cell cultures to heat-killed GBS failed to stimulate release of either IL-1β or TNF-α cytokines, in contrast to the strong stimulatory response observed with live GBS. This finding suggests that the GBS response may be dependent on more than an immunogenic response to the bacterial cell wall. Consistent with the latter explanation, neither the gram negative bacterial cell wall component LPS nor the gram positive bacterial cell wall component LTA stimulated HBD-2 release from amnion cell cultures. Likewise, neither LPS nor LTA stimulated IL-1α or IL-1β release from choriodecidual punch cultures. In contrast, both LTA and LPS stimulated release of IL-6, IL-8 and TNF-α. Because IL-1α and IL-1β were the only cytokines assessed in the present study that required live GBS for stimulation, and because live GBS was required for stimulating the HBD-2 response in amnion cells, these cytokine results lend further, albeit indirect, support for IL-1α and IL-1β as possible mediators of GBS signaling for the amniotic HBD-2 response. Although LTA is most relevant to gram positive GBS, LPS was included as an additional exposure because of its widespread use as an immunogenic stimulus in experiments with gestational tissues [37–39].
In our study, bacteria cell wall or membrane components LTA and LPS did not stimulate IL-1α or IL-1β secretion from the choriodecidual tissue. Other studies have observed an increase in IL-1β secretion from full thickness extraplacental membranes treated with LPS [36–39]. In contrast to our study which used only choriodecidual tissue, these studies used full thickness membranes and the magnitude of the changes was modest and varied among studies. In addition, the source and concentration of the LPS we used was different and could have influenced the results. In agreement with our study, King, et al. found that LPS and LTA had no effect on antimicrobial peptide mRNA expression in human endometrial epithelial cells [40]. Likewise, we observed that heat-killed GBS failed to elicit IL-1β or TNF-α secretion from the choriodecidual tissue, suggesting that cytokine stimulation relies on either a heat-liable protein or internalization of the live bacterium. The latter results are similar to those showing that live S. aureus engages inflammatory pathways and cytokine secretion differently than heat-killed S. aureus in mononuclear cells [41]. Although, not tested here, cellular internalization of live GBS may be critical for cytokine secretion in our model, as has been demonstrated previously in mouse dendritic cells [42].
Cytokines mediate parturition-activating pathways by increasing prostaglandins (PGs), matrix metalloproteinases, and recruitment of neutrophils and macrophages in the gestational tissues. In particular, IL-1β has been implicated in adverse birth outcomes. Recently, Mitchell, et al. linked maternal recto-vaginal GBS colonization during pregnancy with increased IL-1β in maternal and fetal serum and early term births [43]. Non-human primates inoculated with GBS had increased amniotic fluid concentrations of IL-1β, as well as IL-6, and TNF-α, PGE2 and PGF2α, prior to the onset of contractions and parturition [44]. Furthermore, direct infusion with IL-1β stimulated the onset of contractions and preterm labor in primates [45]. Fewer studies have examined the role of IL-1α in adverse birth outcomes. One study found that IL-1α treated pregnant mice had increased PGE2 and fetal deaths [46].
Although recombinant IL-1α and IL-1β each stimulated HBD-2 release in amnion epithelial cells, other cytokines (IL-6, IL-8, IL-17, and TNF-α) did not significantly increase HBD-2 secretion. The concentrations of IL-6, IL-17, and TNF-α we used to treat amnion epithelial cells were higher than the concentrations we observed in the medium of the GBS-stimulated choriodecidual punch cultures. IL-17 is secreted from Th17 cells and implicated in HBD-2 secretion in pulmonary epithelium and keratinocytes [47–49]. Although IL-17 appears to produce a slight increase in HBD-2 (not statistically significant) in our amnion epithelial cells, no IL-17 was detected when choriodecidual punches were treated with GBS. Since IL-17 was not elevated in the choriodecidual punches treated with GBS we did not test the ability of LTA or LPS to stimulate IL-17. Regardless, IL-17 does not appear to be an important immune mediator of HBD-2 release in human extraplacental membranes ex vivo and is likely because of minimal immune cells present in the healthy tissue we collect.
While contributing new information to the understanding of microbial infection in gestational tissue, our study has limitations, nonetheless. Because we did not directly assess microbial contamination of the donor tissue, it is possible that the tissues used in these experiments had GBS or other microbial contamination. However, no microbial growth was observed and cytokine levels in the medium remained low in no-treatment controls, suggesting that infection was not present prior to treatment [50]. In addition, the women donors received antibiotics prior to cesarean section surgery, as is practice at the University of Michigan hospitals, and antibiotics were present in medium for an initial 4-h culture period. Using the same tissue collection and culture protocols as in the present study, we did not observe microbial growth from extraplacental tissue extracts in agar [15]. Furthermore, our data clearly show that GBS, LTA, and LPS elicit specific robust cellular responses.
In conclusion, this study utilized human extraplacental membranes to show that GBS treated choriodecidual tissue secrete IL-1α and IL-1β. Furthermore, we show that IL-1α and IL-1β from the choriodecidual tissues are important for GBS stimulated HBD-2 secretion from the amnion epithelial cells.
Supplementary Material
Acknowledgments
We thank the Loch-Caruso lab members for help with tissue culture experiments and helpful discussions. This research was supported by a research grant from the National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (NIH), (R56 AI100903) and a grant from the Global Alliance to Prevent Prematurity and Stillbirth, an initiative of Seattle Children’s and the Bill & Melinda Gates Foundation, to D.M.A., R.L.-C., and C.X., as well as a research grant from the National Institute of Environmental Health Sciences (NIEHS), NIH, (R01 ES014860) and a project supported by the NIEHS Superfund Research Program PROTECT Center to R.L.-C. and R.W.L., M.C.C. (P42 ES017198). D.M.A. was supported by an Investigators in the Pathogenesis of Infectious Disease grant from the Burroughs Wellcome Fund. K.A.H. was supported, in part, by postdoctoral training fellowships through the National Center for Advancing Translational Sciences, NIH, (UL1 TR000433) and an Institutional Training Grant from NIEHS (T32 ES007062). This research benefitted from additional support from the NIEHS, NIH, for the University of Michigan Lifestage Exposures and Adult Disease Center (P30 ES017885).
Footnotes
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Erica Boldenow, Email: boldenow@umich.edu.
Kelly A. Hogan, Email: kahogan@umich.edu.
Mark C. Chames, Email: mchames@med.umich.edu.
David M. Aronoff, Email: d.aronoff@vanderbilt.edu.
Chuanwu Xi, Email: cxi@umich.edu.
Rita Loch-Caruso, Email: rlc@umich.edu.
References
- 1.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2010;59(RR-10):1–36. [PubMed] [Google Scholar]
- 2.King AE, et al. Innate immune defences in the human uterus during pregnancy. Placenta. 2007;28(11–12):1099–106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 3.King AE, et al. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta. 2007;28(2–3):161–9. doi: 10.1016/j.placenta.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, et al. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci. 2005;40(2):123–32. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Horne AW, Stock SJ, King AE. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008;135(6):739–49. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 6.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends in immunology. 2009;30(3):131–41. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frew L, Stock SJ. Antimicrobial peptides and pregnancy. Reproduction. 2011;141(6):725–35. doi: 10.1530/REP-10-0537. [DOI] [PubMed] [Google Scholar]
- 8.Soto E, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2007;20(1):15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iavazzo C, et al. The role of human beta defensins 2 and 3 in the second trimester amniotic fluid in predicting preterm labor and premature rupture of membranes. Archives of gynecology and obstetrics. 2010;281(5):793–9. doi: 10.1007/s00404-009-1155-4. [DOI] [PubMed] [Google Scholar]
- 10.Olbrich P, et al. Association of human beta-defensin-2 serum levels and sepsis in preterm neonates*. Pediatr Crit Care Med. 2013;14(8):796–800. doi: 10.1097/PCC.0b013e3182975e0f. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Lopez G, Flores-Espinosa P, Zaga-Clavellina V. Tissue-specific human beta-defensins (HBD)1, HBD2, and HBD3 secretion from human extra-placental membranes stimulated with Escherichia coli. Reprod Biol Endocrinol. 2010;8:146. doi: 10.1186/1477-7827-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaga-Clavellina V, Martha RV, Flores-Espinosa P. In vitro secretion profile of pro-inflammatory cytokines IL-1beta, TNF-alpha, IL-6, and of human beta-defensins (HBD)-1, HBD-2, and HBD-3 from human chorioamniotic membranes after selective stimulation with Gardnerella vaginalis. Am J Reprod Immunol. 2012;67(1):34–43. doi: 10.1111/j.1600-0897.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- 13.Zaga-Clavellina V, et al. Tissue-specific human beta-defensins (HBD)-1, HBD-2 and HBD-3 secretion profile from human amniochorionic membranes stimulated with Candida albicans in a two-compartment tissue culture system. Reprod Biol Endocrinol. 2012;10:70. doi: 10.1186/1477-7827-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stock SJ, et al. Natural antimicrobial production by the amnion. American journal of obstetrics and gynecology. 2007;196(3):255 e1–6. doi: 10.1016/j.ajog.2006.10.908. [DOI] [PubMed] [Google Scholar]
- 15.Boldenow E, et al. Antimicrobial peptide response to group B Streptococcus in human extraplacental membranes in culture. Placenta. 2013;34(6):480–5. doi: 10.1016/j.placenta.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lancefield RC, McCarty M, Everly WN. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975;142(1):165–79. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilancheran S, et al. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577–88. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 18.Pratama G, et al. Changes in culture expanded human amniotic epithelial cells: implications for potential therapeutic applications. PLoS One. 2011;6(11):e26136. doi: 10.1371/journal.pone.0026136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, et al. Human amniotic epithelial cell feeder layers maintain mouse embryonic stem cell pluripotency via epigenetic regulation of the c-Myc promoter. Acta Biochim Biophys Sin (Shanghai) 2010;42(2):109–15. doi: 10.1093/abbs/gmp115. [DOI] [PubMed] [Google Scholar]
- 20.Symons JA, Young PR, Duff GW. Soluble type II interleukin 1 (IL-1) receptor binds and blocks processing of IL-1 beta precursor and loses affinity for IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 1995;92(5):1714–8. doi: 10.1073/pnas.92.5.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arend WP. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991;88(5):1445–51. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croghan C, Egeghy P. Methods of dealing with values below the limit of detection using SAS. Southern SAS User Group; St. Petersburg, FL: 2003. [Google Scholar]
- 23.Zaga-Clavellina V, Garcia-Lopez G, Flores-Espinosa P. Evidence of in vitro differential secretion of human beta-defensins-1, -2, and -3 after selective exposure to Streptococcus agalactiae in human fetal membranes. J Matern Fetal Neonatal Med. 2012;25(4):358–63. doi: 10.3109/14767058.2011.578695. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi-Ishii Y, Nagaoka I. Modulation of human beta-defensin-2 transcription in pulmonary epithelial cells by lipopolysaccharide-stimulated mononuclear phagocytes via proinflammatory cytokine production. J Immunol. 2003;170(8):4226–36. doi: 10.4049/jimmunol.170.8.4226. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer TM, et al. IL-1beta-mediated proinflammatory responses are inhibited by estradiol via down-regulation of IL-1 receptor type I in uterine epithelial cells. J Immunol. 2005;175(10):6509–16. doi: 10.4049/jimmunol.175.10.6509. [DOI] [PubMed] [Google Scholar]
- 26.Reisenberger K, et al. Cytokine and prostaglandin production by amnion cells in response to the addition of different bacteria. Am J Obstet Gynecol. 1998;178(1 Pt 1):50–3. doi: 10.1016/s0002-9378(98)70625-8. [DOI] [PubMed] [Google Scholar]
- 27.Menon R, et al. Expression of inflammatory cytokines (interleukin-1 beta and interleukin-6) in amniochorionic membranes. Am J Obstet Gynecol. 1995;172(2 Pt 1):493–500. doi: 10.1016/0002-9378(95)90562-6. [DOI] [PubMed] [Google Scholar]
- 28.Hu XL, Yang Y, Hunt JS. Differential distribution of interleukin-1 alpha and interleukin-1 beta proteins in human placentas. J Reprod Immunol. 1992;22(3):257–68. doi: 10.1016/0165-0378(92)90047-8. [DOI] [PubMed] [Google Scholar]
- 29.Young A, et al. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2):445–9. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 30.Keelan JA, et al. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999;181(6):1530–6. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 31.Ulett GC, et al. Group B Streptococcus (GBS) urinary tract infection involves binding of GBS to bladder uroepithelium and potent but GBS-specific induction of interleukin 1alpha. J Infect Dis. 2010;201(6):866–70. doi: 10.1086/650696. [DOI] [PubMed] [Google Scholar]
- 32.Liu AY, et al. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118(2):275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 33.Pioli PA, et al. Lipopolysaccharide-induced IL-1 beta production by human uterine macrophages up-regulates uterine epithelial cell expression of human beta-defensin 2. Journal of immunology. 2006;176(11):6647–55. doi: 10.4049/jimmunol.176.11.6647. [DOI] [PubMed] [Google Scholar]
- 34.McDermott AM, et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Investigative ophthalmology & visual science. 2003;44(5):1859–65. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon SK, et al. Activation of a Src-dependent Raf-MEK1/2-ERK signaling pathway is required for IL-1alpha-induced upregulation of beta-defensin 2 in human middle ear epithelial cells. Biochim Biophys Acta. 2002;1590(1–3):41–51. doi: 10.1016/s0167-4889(02)00196-9. [DOI] [PubMed] [Google Scholar]
- 36.Hoang M, et al. Human Fetal Membranes Generate Distinct Cytokine Profiles in Response to Bacterial Toll-like Receptor and Nod-like Receptor Agonists. Biol Reprod. 2014 doi: 10.1095/biolreprod.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortunato SJ, et al. Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. American journal of obstetrics and gynecology. 1996;174(6):1855–61. doi: 10.1016/s0002-9378(96)70221-1. discussion 1861–2. [DOI] [PubMed] [Google Scholar]
- 38.Miller MF, Loch-Caruso R. Comparison of LPS-stimulated release of cytokines in punch versus transwell tissue culture systems of human gestational membranes. Reprod Biol Endocrinol. 2010;8:121. doi: 10.1186/1477-7827-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaga V, et al. Secretions of interleukin-1beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod. 2004;71(4):1296–302. doi: 10.1095/biolreprod.104.028621. [DOI] [PubMed] [Google Scholar]
- 40.King AE, et al. Regulation of natural antibiotic expression by inflammatory mediators and mimics of infection in human endometrial epithelial cells. Molecular human reproduction. 2002;8(4):341–9. doi: 10.1093/molehr/8.4.341. [DOI] [PubMed] [Google Scholar]
- 41.Strunk T, et al. Method of bacterial killing differentially affects the human innate immune response to Staphylococcus epidermidis. Innate Immun. 2011;17(6):508–16. doi: 10.1177/1753425910379840. [DOI] [PubMed] [Google Scholar]
- 42.Costa A, et al. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol. 2012;188(4):1953–60. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell K, et al. Group B Streptococcus colonization and higher maternal IL-1beta concentrations are associated with early term births. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2013;26(1):56–61. doi: 10.3109/14767058.2012.725789. [DOI] [PubMed] [Google Scholar]
- 44.Gravett MG, et al. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171(6):1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 45.Sadowsky DW, et al. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. American journal of obstetrics and gynecology. 2006;195(6):1578–89. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 46.Silver RM, et al. Bacterial lipopolysaccharide-mediated murine fetal death: the role of interleukin-1. Am J Obstet Gynecol. 1997;176(3):544–9. doi: 10.1016/s0002-9378(97)70545-3. [DOI] [PubMed] [Google Scholar]
- 47.Pennino D, et al. IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes. J Immunol. 2010;184(9):4880–8. doi: 10.4049/jimmunol.0901767. [DOI] [PubMed] [Google Scholar]
- 48.Kao CY, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173(5):3482–91. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 49.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castro-Leyva V, et al. Preserved ex vivo inflammatory status in decidual cells from women with preterm labor and subclinical intrauterine infection. PloS one. 2012;7(8):e43605. doi: 10.1371/journal.pone.0043605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.