Abstract
Dispositional anxiety is a well-established risk factor for the development of anxiety and other emotional disorders. These disorders are common, debilitating, and challenging to treat, pointing to the need to understand the more elementary neurocognitive mechanisms that confer elevated risk. Importantly, many of the maladaptive behaviors characteristic of anxiety, such as worry, occur when threat is absent. This raises the possibility that worry reflects difficulties gating threat-related information from working memory, a limited capacity workspace that supports the maintenance, recall, and manipulation of information, and facilitates goal-directed thoughts and actions. Here, we tested for the first time whether trait-like individual differences in worry, a key facet of the anxious phenotype, reflect difficulties gating threat and neutral-related distracters from working memory. Results indicated that both dispositional worry and anxiety individually predicted the combined filtering cost of threat and neutral distracters. Importantly, worry was associated with inefficient filtering of threat-related but not neutral distracters from working memory. In contrast, dispositional anxiety was related to a similar level of threat and neutral filtering cost. Furthermore, dispositional anxiety’s relationship to filtering of threat was predominantly driven by differences in worry. These results suggest that the propensity to worry is characterized by a failure to gate task-irrelevant threat from working memory. These results provide a framework for understanding the mechanisms underlying chronic worry and more broadly, the cognitive architecture of dispositional anxiety.
Keywords: worry, anxiety, working memory, attention, cognition-emotion interactions
Anxiety disorders are highly prevalent, debilitating, and associated with substantial morbidity and mortality, making them a growing concern for clinicians, health economists, and public policy makers (Collins et al., 2011; Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, 2012). High levels of dispositional anxiety is a well-established risk factor for the development of anxiety disorders as well as co-morbid depression and substance abuse (Barlow, Sauer-Zavala, Carl, Bullis, & Ellard, 2014; Kotov, Gamez, Schmidt, & Watson, 2010), underscoring the need to dissect and understand the more elementary neurocognitive mechanisms underlying the anxious phenotype (Eysenck & Derakshan, 2011).
The hallmark of extreme anxiety is exaggerated distress and arousal in the absence of genuine danger (Grupe & Nitschke, 2013). Chronically elevated anxiety partially reflects anxious individuals’ overreliance on maladaptive cognitive coping strategies, such as worry (Barlow, 2004). Like other strategies aimed at avoiding or escaping distress, worry paradoxically serves to elevate negative affect, arousal, and neuroendocrine activity (Newman, Llera, Erickson, Przeworski, & Castonguay, 2013). Importantly, worry appears to contribute to functional impairment across a range of psychiatric disorders; it prolongs distress, disrupts concentration, evokes interpersonal conflict, and disturbs sleep (Kertz, Bigda-Peyton, Rosmarin, & Björgvinsson, 2012; Newman et al., 2013). Despite the clinical importance of worry, the basic cognitive mechanisms underlying this transdiagnostic marker are unclear. Existing therapeutic strategies for anxiety and other emotional disorders are inconsistently effective or associated with significant adverse effects (Bystritsky, 2012), highlighting the importance of understanding worry’s neurocognitive underpinnings.
Like anxiety more generally, worry occurs in the absence of clear and imminent threat; it represents “the myriad anxious ‘What if …’ mental representations of [past and] possible future events that are common in daily life” (Borkovec, 1985). From a cognitive neuroscience perspective, these features suggest that worry may reflect difficulties gating threat-related information from working memory. Working memory is the “blackboard of the mind” (Goldman-Rakic, 1996, p. 13473), a limited capacity workspace that supports the maintenance, recall, and manipulation of information (Baddeley, 2012). These internal representations of task sets and other kinds of goals play a central role in maintaining goal-directed cognition when sources of potential distraction are encountered (Miller & Cohen, 2001). Once threat-related information enters working memory, it can continue to bias attention, information processing (e.g., memory retrieval), and action after it is no longer present in the external environment, promoting worry and its adverse downstream consequences.
Here, we tested for the first time whether trait-like individual differences in worry reflect difficulties gating threat-related distracters from working memory. Building on prior work focused on trait anxiety (Stout, Shackman, & Larson, 2013), we used an emotional variant of the well-established change detection working memory task (Vogel, McCollough, & Machizawa, 2005). Subjects were instructed to selectively retain one or more emotional faces while ignoring others. Faces were either threat-related (i.e., fearful; Whalen, 1998) or emotionally-neutral. This procedure allowed us to quantify the cost of distracter filtering; defined here as the impact of distracter processing on the storage of task-relevant targets. Critically, it also enabled us to test whether individuals characterized by higher levels of worry, measured using the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990), are less efficient at gating threat-related distracters from working memory. To assess whether these relations are specific to worry, we performed additional analyses using the State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), a broad-band measure of dispositional anxiety. In particular, we used factor analytic techniques to decompose the STAI into worry and non-worry components and then examined whether gating deficits predominantly reflect the worry component of the anxious phenotype.
Method
Participants
Fifty-eight participants (44 women) from the University of Wisconsin-Milwaukee completed this study (mean age = 20.2 years, SD = 2.45). Participants provided informed consent and were compensated with course extra-credit or $10 per hour. Data from seven participants were removed from further analysis due to missing data (n = 4) or chance performance (n = 3).
Anxiety and Mood Measures
In addition to the PSWQ and STAI, individual differences in depression (Beck Depression Inventory; BDI; Beck, Steer, Ball, & Ranieri, 1996) were assessed to enable us to test the specificity of worry-related effects. Mean (SD) scores were: PSWQ = 43.3 (16.0); STAI = 37.6 (9.7); BDI = 8.1 (7.6). As expected, these measures were strongly correlated, rs > .63, p < .001.
Task and Procedures
A lateralized change detection working memory task, adapted from prior event-related potential (ERP) research by our group (Stout et al., 2013) and others (Sessa, Luria, Gotler, Jolicœur, & Dell'Acqua, 2011), enabled us to estimate the number of threat-related and emotionally-neutral faces stored in working memory (See Figure 1). This design enabled direct comparison with prior ERP research (e.g., Stout et al., 2013).
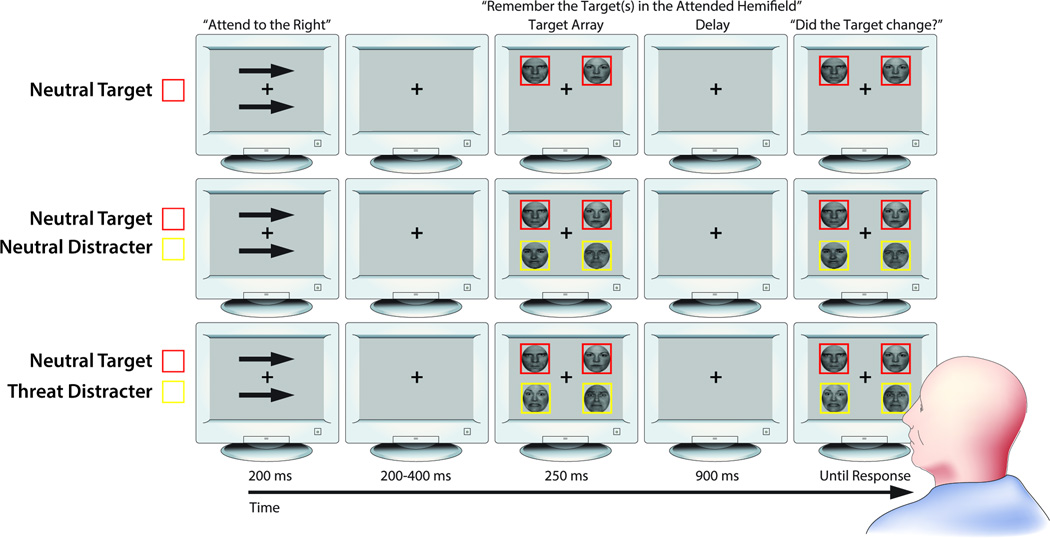
Figure 1.
Working memory task. Rows depict conditions from the change detection task (top row: NT1, middle row: NT1ND1, bottom row: NT1FD1). The trial sequence began with a fixation cross (500 ms). Next, attention was directed to the to-be-remembered hemifield with arrow cues (200ms). After a brief inter-stimulus interval (200–400), a bilateral display of two or four faces was briefly presented (250 ms). Participants were instructed to attend to one or two target faces in the cued hemifield, which were outlined with red (or yellow) borders and ignore distracter faces outlined in yellow (or red). Colors selected for targets and distracters were counterbalanced. After a delay interval (900 ms) representing the maintenance period of working memory, a whole-display probe was presented (until response) where participants were asked to identify whether the target face changed identity (equiprobable). On change trials, the identity of the target face changed while the expression (i.e., fearful or neutral) did not. Portions of this figure were reprinted by permission from Macmillan Publishers, Ltd: Nature Reviews Neuroscience (Houdé & Tzourio-Mazoyer, 2003; Peelen & Downing, 2007) and adapted from a figure originally published in Stout et al. (2013).
To assess the influence of distracter face expression and individual differences in worry on the efficiency of gating task-irrelevant faces from working memory, the task included conditions in which threat-related distracters (1 Neutral Target and 1 Fear Distracter [NT1FD1]), neutral distracters (1 Neutral Target and 1 Neutral Distracter [NT1ND1]), or a single neutral target (1 Neutral Target [NT1]) were present. These conditions allowed us to calculate “filtering cost” scores for each expression. To confirm that our behavioral measures were sensitive to the number of faces held in working memory, the task also included conditions in which set size was varied and only task-relevant targets were presented (see Supplemental Material for these results). Subjects completed 20 practice trials and 40 experimental trials/condition (5 blocks).
Hypothesis Testing (Filtering Cost)
Prior to hypothesis testing, a focused ANOVA incorporating the NT1FD1, NT1ND1, and NT1 conditions was used to assess whether threat-related and emotionally-neutral distracters similarly degrade working memory capacity. Working memory capacity (K) was estimated as S× [(H −FA) / (1 −FA)]; where S is set-size (i.e., the number of target faces), H is hit rate, and FA is the false alarm rate (Pashler, 1988).
To test whether individuals with higher levels of worry fail to govern threat's access to working memory, an index of “filtering cost” was computed separately for the threat and neutral distracter conditions. We also computed a general filtering cost score by averaging the threat and neutral filtering cost scores. Prior work indicates that such behavioral indices of filtering cost are related to ERP measures (i.e., CDA) that are sensitive to the unnecessary storage of distracters in working memory (Fukuda & Vogel, 2009). Filtering cost for threat-related distracters was calculated as the difference in working memory capacity (K) between trials in which a neutral target was paired with a fear distracter (NT1FD1) and trials in which a single neutral target was presented (NT1). A larger filtering cost indicates greater degradation of working memory capacity (i.e., for the task-relevant neutral face) in the face of competition from a task-irrelevant threat-related distracter (for similar applications, see Lee, Cowan, Vogel, Rolan, Valle-Inclán, & Hackley, 2010). Filtering cost for emotionally-neutral distracters was computed using trials in which a neutral target was paired with a neutral distracter (NT1ND1). Data were rank-transformed to correct non-Gaussian distribution of the residuals (cf. Weng et al., 2013). Examinations of the Z-scores revealed that all of the rank-transformed cases were < 2 SDs from the mean. Scatterplots were visually inspected for outliers using Scatterize (http://webtasks.keck.waisman.wisc.edu/scatterize).
A secondary aim of this study was to assess whether filtering of threat-related distracters predominantly reflects the worry component of the more complex, multidimensional anxious phenotype. To examine this, we identified sub-factors from a factor analysis on the STAI (see Supplemental Material). This allowed us to separate and test the unique influence of the worry vs. non-worry sub-components of dispositional anxiety by running separate regressions predicting threat filtering cost for worry and non-worry anxiety.
Results
Distracters degrade working memory capacity
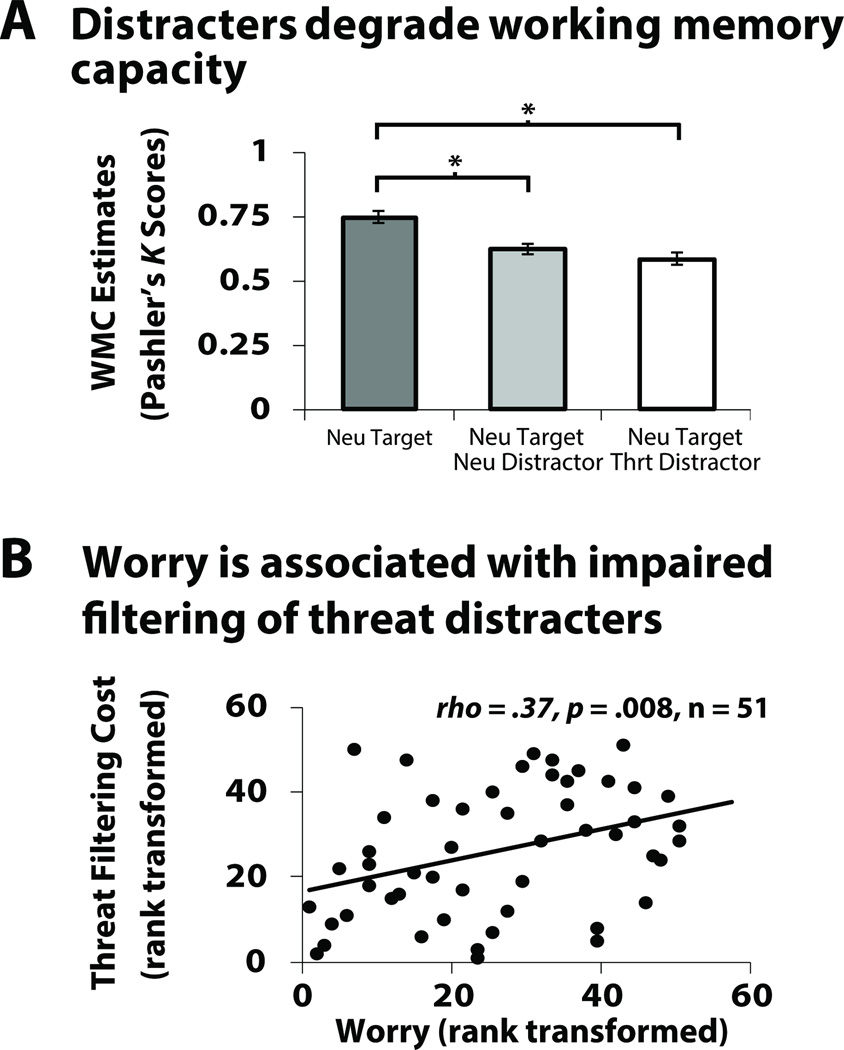
As shown in Figure 2a, an omnibus analysis indicated that working memory capacity was altered in the face of distraction, F(2, 50) = 28.1, p < .001, . Planned contrasts confirmed that both threat-related (t(50) = 6.65, p < .001) and emotionally-neutral distracters (t(50) = 5.95, p < .001) degraded working memory capacity for the task-relevant target, without significantly differing from one another, t(50) = 1.76, p = .09.
Figure 2.
(A) Mean filtering cost scores (Pashler’s K estimates) for the one neutral target (NT1), neutral target plus neutral distracter (NT1ND1), and neutral target and threat distracter (NT1FD1) conditions. Asterisks represent significant pairwise mean differences (p < .05). Error bars reflect the probability that the null hypothesis was rejected by chance: p < .05 (non-overlapping error bars; see Shackman, McMenamin, Maxwell, Greischar, and Davidson (2010) for discussion of this method for calculating error bars). (B) Spearman’s (rho) correlation (two-tailed) between rank transformed threat filtering cost (NT1 – NT1FD1) and dispositional worry using the PSWQ (rank transformed).
Worry predicts increased filtering cost in the face of threat-related distracters
Individuals characterized by heightened worry showed a larger general filtering cost in the presence of distracters, rs(51) = .34, p = .02. After dissecting general filtering cost into separate expression-specific filtering cost scores, we found that relations between worry and the filtering cost were specific to threat, rs(51) = .37, p = .008. This association remained significant after controlling for filtering cost in the presence of emotionally-neutral distracters (prs(49) = .31, p = .03); nuisance variation in overall working memory capacity, mean-centered age, and sex (ps < .01); as well as self-reported depression (prs(49) = .29, p = .04); and was not significant for emotionally-neutral distracters (rs(51) = .21, p = .14). We next examined the contribution of depression on the relationship between worry and general filtering cost due to the overlapping nature of these constructs (Nitschke, Heller, Imig, McDonald, & Miller, 2001). Worry remained significant, prs = .33, t = 2.08, p = .04; but the BDI did not significantly contribute to the model, prs = .10, t = 0.68, p = .50.1
Relations between trait anxiety and threat filtering predominantly reflect worry
Anxious individuals tend to allocate excessive working memory storage to task-irrelevant information (Qi et al., 2014; Stout et al., 2013). Consistent with this, higher levels of trait anxiety were associated with increased general filtering costs, rs (51) = .34, p = .02. Filtering cost was quantitatively stronger in the face of threat-related distracters, rs (51) = .32, p = .02 (neutral distracters: rs (51) = .26, p = .07). However, after controlling for neutral-filtering cost, the relationship between trait anxiety and threat filtering cost was no longer significant, prs = .24, p = .09.
Next, we assessed whether the association between trait anxiety and threat filtering predominantly reflects the influence of worry. This would be consistent with evidence that the STAI is a complex, multidimensional measure that reflects dissociable individual differences in negative emotionality, depression/fatigue, and worry (Bieling, Antony, & Swinson, 1998; Kelly, 2004; Nitschke et al., 2001). As a first test, we computed the partial correlation between the STAI and filtering cost while controlling for variation in worry, indexed by the PSWQ. In this case, trait anxiety no longer predicted the cost of general filtering (prs(48) = .17, p = .24), or threat-filtering (prs (48) = .13, p = .39).
As a second test, we decomposed the STAI into worry-related and non-worry-related components and separately assessed their relations with distracter filtering costs. To define the two components, a factor analysis was performed using data from an independent sample of individuals who had completed the STAI (see the Supplement). Consistent with prior work in a smaller sample (Kelly, 2004), this identified a subset of items that clearly mapped onto the worry component of trait anxiety (e.g., ‘I worry too much over something that really doesn’t matter’), allowing us to define separate STAI-Worry and STAI-Nonworry scales in the primary sample. As expected, the STAI-Worry scale was correlated with the PSWQ, rs = .67, p < .001. Like the PSWQ, STAI-Worry predicted general filtering cost (rs (51) = .32, p = .02) and the cost of filtering threat-related distracters (rs (51) = .36, p = .01), but not the cost of filtering emotionally-neutral distracters, rs (51) = .20, p =.17.
The STAI-Nonworry scale was significantly related to general filtering cost, rs (51) = .32, p = .02. In contrast to STAI-Worry, STAI-Nonworry did not significantly predict the cost of filtering threat (rs (51) = .25, p = .08), but did predict the cost of filtering neutral distracters (rs (51) = .28, p = .05).
Discussion
These results provide compelling evidence that the propensity to worry is associated with difficulty gating threat distracters from working memory. This was not evident for neutral distracters and could not be explained by individual differences in age, sex, depressive symptoms, or maximum working memory capacity. Furthermore, while high levels of trait anxiety also predicted threat-gating deficits, this appears to predominantly reflect the worry component of trait anxiety. In contrast, the non-worry component was associated with deficits filtering neutral distracters. These findings provide convergent evidence that the worry component of the anxious phenotype reflects aberrant control over the contents of working memory for threat-related information; whereas the non-worry facets appear to be more closely related to previously documented anxiety-related inhibitory deficits for neutral stimuli (Berggren & Derakshan, 2013; Bishop, 2007).
Worry is a central feature in neurocognitive theories of anxiety (Eysenck & Derakshan, 2011). On the basis of the current results and prior work by our group (Stout et al., 2013), we propose that the propensity to worry is associated with difficulties governing threat’s access to working memory. If irrelevant threat-related information unnecessarily enters working memory, the distressing thoughts and memories characteristic of worry and pathological anxiety are likely to be mentally rehearsed and compete with goal-related thoughts and actions. Coupled with research suggesting that dispositional and clinically anxious individuals identify worry as a coping strategy (Barlow, 2004; Newman et al., 2013), threat-related information is likely to be given priority in working memory—enabling a state of worry that is sustained long after the threat is no longer present in the immediate environment. According to the attentional control theory of anxiety (ACT; Eysenck & Derakshan, 2011; Eysenck, Derakshan, Santos, & Calvo, 2007), this state of worry, in turn, causes the working memory and attentional deficits characteristic of the anxious phenotype.
Dispositional worry and anxiety are important risk factors for the development of anxiety disorders as well as co-morbid depression and substance abuse (Barlow et al., 2014; Kotov et al., 2010). The present study provides compelling evidence that trait-like individual differences in worry are associated with difficulty gating threat-related distracters from working memory, the capacity-limited workspace underlying goal-directed thoughts and behavior. Once in working memory, threat-related information is well positioned to bias the stream of information processing when it is no longer present in the external environment, promoting worry and other maladaptive cognitions that contribute to functional impairment (Thiruchselvam, Hajcak, & Gross, 2012). From a translational perspective, these findings provide a framework for understanding the cognitive mechanisms underlying elevated worry and set the stage for research aimed at delineating the underlying neural circuitry. A key challenge will be to more fully dissect the influence of worry from other features of the anxious phenotype, such as negative emotionality and behavioral inhibition, that can influence working memory (Robinson, Vytal, Cornwell, & Grillon, 2013; Shackman, Sarinopoulos, Maxwell, Pizzagalli, Lavric, & Davidson, 2006). From the perspective of basic psychological science, our results also provide new insights into the cognitive architecture of stable individual differences in anxiety, a core facet of personality and temperament (Caspi, Roberts, & Shiner, 2005).
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health (NIMH) grant MH086809 (to C. L. L.) and the University of Maryland. Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the MacArthur Foundation.
Footnotes
The authors declare no conflicts of interest.
Recent research suggest that elevated depressive symptoms are associated with poor working memory filtering (Owens, Koster, & Derakshan, 2012, 2013; Stout & Rokke, 2010). In our sample, the BDI did not contribute to the relationship between dispositional anxiety (Trait Anxiety, STAI-Worry, & STAI-Nonworry) and either general or expression-specific filtering cost scores (prs < .23, ps > .10).
References
- Baddeley A. Working memory: Theories, models, and controversies. Annual Review of Psychology. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- Barlow DH. The nature of anxious apprehension. In: Barlow DH, editor. Anxiety and its disorders: The nature and treatment of anxiety and panic. New York, NY: The Guilford Press; 2004. pp. 64–104. [Google Scholar]
- Barlow DH, Sauer-Zavala S, Carl JR, Bullis JR, Ellard KK. The nature, diagnosis, and treatment of neuroticism: Back to the future. Clinical Psychological Science. 2014;2:344–365. [Google Scholar]
- Beck A, Steer R, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berggren N, Derakshan N. Attentional control deficits in anxiety: Why you see them and why you don’t. Biological Psychology. 2013;92:440–446. doi: 10.1016/j.biopsycho.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait Version: Structure and content re-examined. Behaviour Research and Therapy. 1998;36:777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Bishop S. Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences. 2007;11:307–216. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Borkovec TD. Worry: A potentially valuable concept. Behaviour Research and Therapy. 1985;23:481–482. doi: 10.1016/0005-7967(85)90178-0. [DOI] [PubMed] [Google Scholar]
- Bystritsky A. Treatment-resistant anxiety disorders. Molecular Psychiatry. 2006;11:805–814. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: Stability and change. Annual Review of Psychology. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Otero-Ojeda A. Grand challenges in global mental health. Nature. 2011;475(7354):27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N. New perspectives in attentional control theory. Personality and Individual Differences. 2011;50:955–960. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. Human variation in overriding attentional capture. Journal of Neuroscience. 2009;29:8726–8733. doi: 10.1523/JNEUROSCI.2145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences, U.S.A. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdé O, Tzourio-Mazoyer N. Neural foundations of logical and mathematical cognition. Nature Reviews Neuroscience. 2003;4:504–514. doi: 10.1038/nrn1117. [DOI] [PubMed] [Google Scholar]
- Kelly WE. Examining the relationship between worry and trait anxiety. College Student Journal. 2004;38:370. [Google Scholar]
- Kertz SJ, Bigda-Peyton JS, Rosmarin DH, Björgvinsson T. The importance of worry across diagnostic presentations: Prevalence, severity and associated symptoms in a partial hospital setting. Journal of Anxiety Disorders. 2012;26:126–133. doi: 10.1016/j.janxdis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depression, and substance use disorders: A meta-analysis. Psychological Bulletin. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Lee E, Cowan N, Vogel EK, Rolan T, Valle-Inclán F, Hackley SA. Visual working memory deficits in patients with Parkinson’s disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain. 2010;133:2677–2689. doi: 10.1093/brain/awq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Newman MG, Llera SJ, Erickson TM, Przeworski A. Worry and generalized anxiety disorder: A review and theoretical synthesis of evidence on nature, etiology, mechanisms, and treatment. Annual Review of Clinical Psychology. 2013;9:275–297. doi: 10.1146/annurev-clinpsy-050212-185544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research. 2001;25:1–22. [Google Scholar]
- Owens M, Koster EHW, Derakshan N. Impaired filtering efficiency in dysphoria: An ERP study. Social, Cognitive, and Affective Neuroscience. 2012;7:752–763. doi: 10.1093/scan/nsr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M, Koster EWH, Derakshan N. Improving attention control in dysphoria through cognitive training: Effects on working memory capacity and filtering efficiency. Psychophysiology. 2013;50:297–307. doi: 10.1111/psyp.12010. [DOI] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Perception & Psychophysics. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Downing PE. The neural basis of visual body perception. Nature Reviews Neuroscience. 2007;8:636–648. doi: 10.1038/nrn2195. [DOI] [PubMed] [Google Scholar]
- Qi S, Ding C, Li H. Neural correlates of inefficient filtering of emotionally neutral distracters from working memory in trait anxiety. Cognitive, Affective, and Behavioral Neuroscience. 2014;14:253–265. doi: 10.3758/s13415-013-0203-5. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Vytal K, Cornwell BR, Grillon C. The impact of anxiety upon cognition: Perspectives from human threat of shock studies. Frontiers in Human Neuroscience. 2013;7:203. doi: 10.3389/fnhum.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa P, Luria R, Gotler A, Jolicœur P, Dell’ Acqua R. Interhemispheric ERP asymmetries over inferior parietal cortex reveal differential visual working memory maintenance for fearful versus neutral face identities. Psychophysiology. 2011;48:187–197. doi: 10.1111/j.1469-8986.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010;51:1319–1333. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Spielberger CC, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stout DM, Rokke PD. Components of working memory predict levels of distress. Cognition and Emotion. 2010;24:1293–1303. [Google Scholar]
- Stout DM, Shackman AJ, Larson CL. Failure to filter: anxious individuals show inefficient gating of threat from working memory. Frontiers in Human Neuroscience. 2013;7:58. doi: 10.3389/fnhum.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchselvam R, Hajcak G, Gross JJ. Looking inward: Shifting attention within working memory representations alters emotional responses. Psychological Science. 2012;23:1461–1466. doi: 10.1177/0956797612449838. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Weng HY, Fox AS, Shackman AJ, Stodola DE, Caldwell JZK, Olson MC, Rogers GM, Davidson RJ. Compassion training alters altruism and the neural responses to suffering. Psychological Science. 2013;24:1171–80. doi: 10.1177/0956797612469537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.