Abstract
Myelofibrosis (MF) is characterized by the constitutive mobilization of hematopoietic stem cells (HSC) and progenitor cells (HPC) and the establishment of extramedullary hematopoiesis (EMH). The mechanisms underlying this abnormal HSC/HPC trafficking pattern remain poorly understood. We demonstrated that both splenic and peripheral blood (PB) MF CD34+ cells equally share a defective ability to home to the marrow but not the spleens of NOD/SCID mice. This trafficking pattern could not be attributed to discordant expression of integrins or chemokine receptors other than the down-regulation of CXCR4 by both PB and splenic MF CD34+ cells. The number of both splenic MF CD34+ cells and HPCs that migrated towards splenic MF plasma was, however, significantly greater than the number that migrated towards PB MF plasma. The concentration of the intact HSC/HPC chemo-attractant, CXCL12, was greater in splenic MF plasma than PB MF plasma as quantified using mass spectrometry. Functionally inactive truncated products of CXCL12 which are the product of proteolytic degradation by serine proteases were detected at similar levels in both splenic and PB MF plasma. Treatment with an anti-CXCL12 neutralizing antibody resulted in a reduction in the degree of migration of splenic MF CD34+ cells towards both PB and splenic MF plasma validating the role of CXCL12 as a functional chemo-attractant. Our data indicate that the MF splenic microenvironment is characterized by increased levels of intact, functional CXCL12, which contributes to the localization of MF CD34+ cells to the spleen and the establishment of EMH.
Introduction
In humans, the white pulp of the spleen is primarily a lymphoid compartment that is crucial for immune surveillance and response, since it is the site of formation of antibodies against invading pathogens. The red pulp of the spleen contains macrophages which filter and remove cellular debris and bacteria from the circulation. Recently, limited numbers of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) have been detected in the spleens of normal adults, which have been attributed to the small number of circulating HSCs that migrate through the blood and enter the spleen. 1–3 Stem cell trafficking largely depends on the interaction between integrins and chemokine receptors expressed by HSCs/HPCs and a variety of matrix proteins present within the bone marrow (BM) microenvironment and chemokines elaborated by marrow stromal cells, osteoblasts, or endothelial cells. 4 The interactions between CXCL12, also known as stromal-derived factor-1 (SDF-1), and its receptor CXCR4 play a pivotal role in determining the migration, homing, retention, proliferation, and differentiation of human HSCs/HPCs. 4–6
The clinical spectrum of myelofibrosis (MF) includes primary myelofibrosis (PMF) and MF that develops during the course of essential thrombocythemia (ET) or polycythemia vera (PV). MF is characterized by abnormal trafficking of HSCs and HPCs, resulting in their constitutive mobilization and the establishment of extramedullary sites of hematopoiesis. The constitutive mobilization of MF HSCs/HPCs has been associated with profound alterations in the CXCR4/CXCL12 axis, which occur as a consequence of down-regulation of CXCR4 expression by MF CD34+ cells due to hypermethylation of the CXCR4 promoter and the proteolytic degradation of CXCL12 and vascular adhesion molecule-1 (VCAM-1) due to the elaboration of a number of serine proteases, including matrix metalloproteinase-9 (MMP-9) and neutrophil elastase (NE).7–12 These findings suggest that MF stem cell behavior can be influenced not only by intrinsic properties of the stem cells but also by regulatory signals provided by the MF microenvironment.
Disease progression in MF is frequently accompanied by greater degrees of splenomegaly due to increased extramedullary hematopoiesis (EMH).13–14 For EMH to occur, a permissive microenvironment must be established which supplies the signals that are required for MF HSCs to enter the spleen and then initiate and sustain hematopoiesis. We hypothesized that alterations within the microenvironment of the spleen contribute to the abnormal trafficking of MF-stem cells (MF-SC) leading to the development of EMH. To test this hypothesis, we have quantified the intact and truncated forms (loss of 2 aa, 3 aa, 4 aa, and 5 aa, aa = amino acid) of CXCL12 in paired splenic and PB MF plasmas using mass spectrometry. The homing of MF CD34+ cells to the spleens and marrow of NOD/SCID mice and the migratory ability of splenic MF CD34+ cells towards spleen and PB MF plasma were also evaluated.
Materials and Methods
Patient specimens and cell preparation
Surgically removed spleens were obtained from patients with advanced forms of MF who were undergoing therapeutic splenectomy. Normal spleen tissues were provided by The National Disease Research Interchange (NDRI). Single-cell preparations were prepared from the spleens of six patients who fulfilled the WHO diagnostic criteria for PMF or PV/ET-related MF 14 (Table 1) and from 7 normal individuals according to the method of Barosi and coworkers. 15 PB from these MF patients was collected at the time of splenectomy. Mononuclear cells (MNC) were isolated by density gradient centrifugation of both splenic and PB single-cell suspensions using Ficoll-Paque (GE Healthcare Life Sciences). CD34+ cells were selected from splenic or PB MNCs using a CD34+ cell selection kit (STEMCELL Technologies). The purity of the CD34+ cells was determined using a FACSCanto flow cytometer (BD). CD34+ cells with ≥ 95% purity were used in all experiments. G-CSF–mobilized PB (mPB) CD34+ cells from 6 donors were purchased from STEMCELL Technologies.
Table 1.
Clinical Characteristics of MF Patients Studied
| Patient # | Gender | Age | Diagnosis | JAK2V617F Allele Burden (%)* |
Chromosomal Abnormalities (%)# |
Indication for Splenectomy |
|---|---|---|---|---|---|---|
| A | F | 70 | Post PV MF | 85 | None | Prior to Transplant |
| B | M | 64 | PMF | 0 | None | Cytopenias |
| C | M | 79 | PMF | 2.4 | del(20)(q11.1q13.3) (97) |
Cytopenias |
| D | M | 66 | PMF | 0 | None | Prior to Transplant |
| E | F | 45 | Post PV MF | 90 | del(20)(q11.1q13.3) (50) |
Cytopenias |
| F | F | 64 | Post PV MF | 78 | +der(9)t(1;9)(q12;q12) (35) del(20)(q11.2q13.1) (41) |
Portal hypertension |
Indicates the granulocytic JAK2V617F allele burden as detected by real-time allele specific PCR assay.
Indicates the percentage of primary MF spleen cells having the given chromosomal abnormality.
JAK2V617F, cytogenetic, and fluorescence in situ hybridization (FISH) analyses
The JAK2V617F status of the MF patients was determined by analyzing the PB granulocytes of patients utilizing a real-time allele-specific polymerase chain reaction (AS-PCR) assay as previously described. 16,17 Karyotypic and FISH analyses of MF cells to detect the presence of marker cytogenetic abnormalities were carried out as previously described. 18, 19 None of the patients had a mutation on the thrombopoietin receptor, MPL or calreticulin.
CD34+ cell homing assay
NOD/LtSz-Prkdcscid (NOD/SCID) mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). All experiments were approved by the Animal Care Committee of the Icahn School of Medicine at Mount Sinai (ISMMS). The mPB, splenic MF, or PB MF CD34+ cells (0.5×l06/mouse) were transplanted via the tail vein into 8- to 9-wk-old sublethally irradiated (320 cGy) NOD/SCID mice. Mice were sacrificed 24 hours after the transplantation and cells were recovered from the BM and the spleens of the recipient. The presence of human CD34+ cells in BM cells (BMC) and spleen cells was determined by mAb staining and flow cytometric analysis of 106 cells/sample. Cells obtained from mice not receiving transplants were stained with isotope control antibodies to exclude false positivity.
Flow cytometric analysis of splenic and PB CD34+ cells
To determine if the expression of chemokine receptors and adhesion molecules could account for the homing and/or location of MF CD34+ cells to the spleen, isolated splenic and PB MF CD34+ cells as well as mPB CD34+ cells were labeled with anti-human CD34 mAb-allophycocyanin (APC), anti-human CXCR4 mAb-phycoerythrin (PE), anti-human CD49d mAb-PE, anti-human CD47 mAb-fluorescein isothiocyanate (FITC), or anti-human CD44 mAb-PE. All mAbs were purchased from BD Biosciences. Each analysis was paired with corresponding matched isotype control. Immediately prior to flow cytometric analysis, 1 µg/mL propidium iodide (PI; Sigma-Aldrich) was added to exclude nonviable cells. Cells were analyzed flow cytometrically; at least 10,000 viable CD34+ cells were acquired from each sample (CellQuest software, BD).
Preparation of splenic and PB plasma, measurement of CD26, NE, MMP-2 and MMP-9 levels and determination of albumin concentration
To prepare normal and MF splenic plasma, spleen tissues were weighed, cut into pieces (1cm×1cm) and ground gently to produce splenic homogenates. Distilled water was added to the splenic homogenates (1 mL/g splenic tissue) and mixed immediately. In order to avoid the possibility that a small number of erythrocytes and platelets might have been lysed in preparation of the spleen plasmas which would affect the protein levels quantified in the splenic plasma, the tube containing the splenic homogenate was centrifuged immediately at 1,800 rpm (740 g) at room temperature for 30 min after the water was added. The supernatant was collected and was referred to as splenic plasma. The splenic plasma was filtered and aliquots were frozen at −80°C and stored. The cell pellets were re-suspended and more than 99% of cells were viable as assessed using trypan blue staining, making it unlikely that the release of intracellular proteins would affect the protein levels in splenic plasma. PB from MF patients was collected in tubes containing sodium heparin. These tubes were first centrifuged (as described above), and the platelet-free plasma was removed, divided into 1-mL aliquots, and immediately frozen at −80°C. The plasma levels of CD26, NE, MMP-2, total and active MMP-9 were measured using ELISAs as described previously. 8 Albumin concentrations were determined by the Clinical Pathology Laboratory of the ISMMS using standard laboratory procedures.
Mass spectrometric analysis of intact CXCL12 and individual truncates in MF splenic and PB plasma
Chemicals and reagents
Full-length human recombinant CXCL12 (1–67 amino acid length, carrier free) was purchased from EMD Millipore Co. (Billerica, MA). Synthetic truncates of CXCL12α were kindly provided by Dr. L. Pelus (Indiana University School of Medicine). Recombinant mouse EFG (internal standard, IS) was purchase from R&D systems Inc. (Minneapolis, MN). Ultrafiltration membrane filters (30 kDa cutoff) were purchased from EMD Millipore Co. (Billerica, MA). High performance liquid chromatography (HPLC) grade solvents were purchased from Thermo Fisher Scientific Inc. (Waltham, MA).
Instrumentation for combined liquid chromatography mass spectrometry
The combined high performance liquid chromatograph-mass spectrometer (HPLC-MS) system consisted of a binary HPLC pump (Model 1525µ, Waters Co, Milford, MA) connected to a triple quadruple mass spectrometer equipped with an electrospray ionization (ESI) source (Model QuattroLC, Waters Co., Milford, MA). Samples were introduced onto a reversed-phase C18 HPLC column (XSelect CSH™, 3 cm long, 2.1 mm diameter, Waters Co., Milford, MA). The column was connected between an autosampler (Model 717 Plus, Waters Co.) and an ESI source. The ESI source was operated in the positive ion mode under the following conditions: capillary voltage 3 kV, cone voltage 40 V, source temperature 80 °C, desolvation temperature 250 °C, nitrogen nebulizer gas flow 80 L/h, desolvation gas flow 800 L/h. Operating conditions during data acquisition: mass spectral scanning time 2.5 s, to cover a 750 – 1,450 Da mass range (300 mass unit/s) and 1,200 scans made.
Mass spectrometric analysis
Aliquots of 100 µL of normal splenic, MF splenic and PB plasma were ultra-filtrated using 30 kDa cutoff membrane filter, followed by centrifugation for 30 min at 9000 g. To 50 µL of each ultrafiltrate, 25 µL of IS (10 µg/mL) was added. Ten µL aliquots were injected into the LC/MS system. Conditions for gradient elution: Gradients from 20% acetonitrile with 0.1% formic acid to 100% acetonitrile with 0.1% formic acid in 30 min were used. Molecular masses of intact CXCL12 and truncated forms of CXCL12, resulting from the action of individual proteolytic enzymes, were obtained and quantified from multiply charged ion profiles, using transformation software (Waters MassLynx™ 4.0): 980 for intact CXCL12, 952 for the truncated product due to CD26 (2 aa removed), 940 for the truncated product due to NE (3 aa removed), 929 for the truncated products due to MMP-2 and MMP-9 (4 aa removed in both cases), 915 for the truncated product due to cathepsin G (CG, 5 aa removed), Analyte concentrations were calculated using the method described previously. 20
Migration assay
The in vitro migratory behavior of MF splenic CD34+ cells towards CXCL12, as well as MF splenic, or PB plasma was assessed using 6.5-mm diameter, 5-µm pore transwell plates as previously described. 21 Briefly, transwell filters were coated overnight at 4°C with 10 ug/cm2 of fibronectin (Sigma). To block nonspecific binding sites, the coating solution was aspirated and replaced by a 1% bovine serum albumin (BSA) solution in PBS and allowed to incubate at 37°C for 30 min. The coated transwell filters were washed twice with migratory buffer (IMDM with 0.5% BSA), before cells were added to the upper compartment. 1- 2 × 105 CD34+ cells suspended in 100 µL of buffer were then placed in the upper chamber of the transwell. About 600 µL of diluted CXCL12 (1:2=corresponding concentrations of splenic and PB plasma from each patient: migratory buffer) or diluted MF splenic, PB or normal splenic plasma (1:2=various plasma: migratory buffer) were added to the lower compartment. Non-migrating and migrating cells were harvested from the upper and lower compartments, respectively, after incubation at 37°C for 4 h. Non-migrating cells were recovered following two washes, each consisting of a 5-min treatment with an enzyme-free cell dissociation buffer (Life Technologies, Grand Island, NY) at 37°C, followed by vigorous pipetting. The number of the harvested cells in the two fractions was enumerated using a hemocytometer. The percentage of migrating cells was calculated by determining the ratio of the number of cells recovered from the lower compartment to the total number of cells loaded in the upper compartment.
To determine if the migration of splenic MF CD34+ cells occurred in response to CXCL12 present within the respective plasmas, anti-CXCL12 neutralizing mAbs (100ug/ml) and isotype control mAbs (100ug/ml, R&D Systems) were added to the lower compartment containing MF splenic or PB plasma and to the upper compartment containing the cell suspension. The number of migrating cells was enumerated as described above.
Hematopoietic progenitor cell (HPC) assays
In order to determine the migratory ability of assayable HPC present in MF splenic cells towards PB or splenic plasma, input MF splenic CD34+ cells and MF splenic CD34+ cells that had migrated were assayed in semisolid medium as described previously 22. The number of hematopoietic colonies (HC) including CFU-GM, BFU-E, and CFU-Mix were enumerated after 12–14 days of incubation. Finally, the colonies were individually plucked and the percentage of JAK2V617F-positive colonies determined.22
Statistical analysis
Results are reported as the mean ± SD of data obtained from 3–4 individual experiments. Statistical significance was determined using two tailed Student’s t tests. All P values were two-sided, and P values < 0.05 were considered significant.
Results
Homing of both splenic and PB MF CD34+ cells to the spleens of NOD/SCID mice
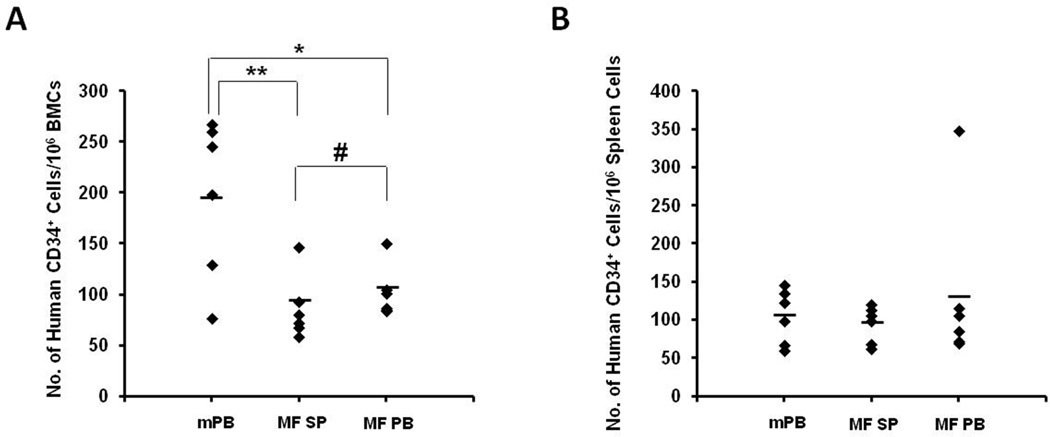
We have previously demonstrated that the homing of PB MF CD34+ cells to the marrow but not the spleen is altered. 7 G-CSF is thought to induce BM stem cell mobilization in normal individuals by promoting proteolytic degradation of BM CXCLl2 by NE and CG. 5 A similar mechanism has been shown to be partially responsible for the constitutive mobilization of MF HSCs/HPCs.8–10 In this study, we, therefore, utilized mPB CD34+ cells as a normal control in the studies of homing of splenic MF CD34+ cells. As shown in Figure 1A, following the infusion of paired splenic or PB MF CD34+ cells or normal mPB CD34+ cells (5×105/mouse), reduced numbers of splenic MF CD34+ cells (86±13/106 BMCs) and PB MF CD34+ cells (102±10/106 BMCs) were detected in the murine marrows as compared with mPB CD34+ cells (196±32/106 BMCs; splenic MF CD34+ cells vs mPB CD34+ cells: P<0.01; PB MF CD34+ cells vs mPB CD34+ cells: P<0.05). However, similar numbers of splenic and PB MF CD34+ cells homed to the marrow and spleen of recipient mice (P=0.36; Figure 1B). These findings suggest that intrinsic features of both splenic and PB MF CD34+ cells result in their limited ability to home to the marrow but not the spleens of NOD/SCID mice.
Figure 1. Homing of mPB, splenic and PB MF CD34+ cells to the BM and spleens of NOD/SCID mice.
The mPB, splenic MF, or PB MF CD34+ cells (0.5×l06/mouse) were transplanted into NOD/SCID mice and the homing of these CD34+ cells to the marrow and spleens of the recipient mice was evaluated 24 hours after the transplantation. (A): The numbers of splenic and PB MF CD34+ cells detected in murine marrow were reduced as compared with mPB CD34+ cells. (B): The number of splenic and PB MF CD34+ cells and mPB CD34+ cells detected in the murine spleens, however, were comparable. Black diamonds show the number of human CD34+ cells per 106 cells that migrated to the BM and spleen of each individual mouse. Horizontal bars indicate the mean of the number of human CD34+ cells per 106 cells that migrated to the BM and spleen of mice. *, P < 0.05; **, P < 0.01; #, P=0.36. mPB: G-CSF mobilized peripheral blood CD34+ cells; MF SP: myelofibrosis splenic CD34+ cells; MF PB: myelofibrosis peripheral blood CD34+ cells. BMCs: bone marrow cells.
Expression of chemokine receptors and adhesion molecules by splenic and PB MF CD34+ cells
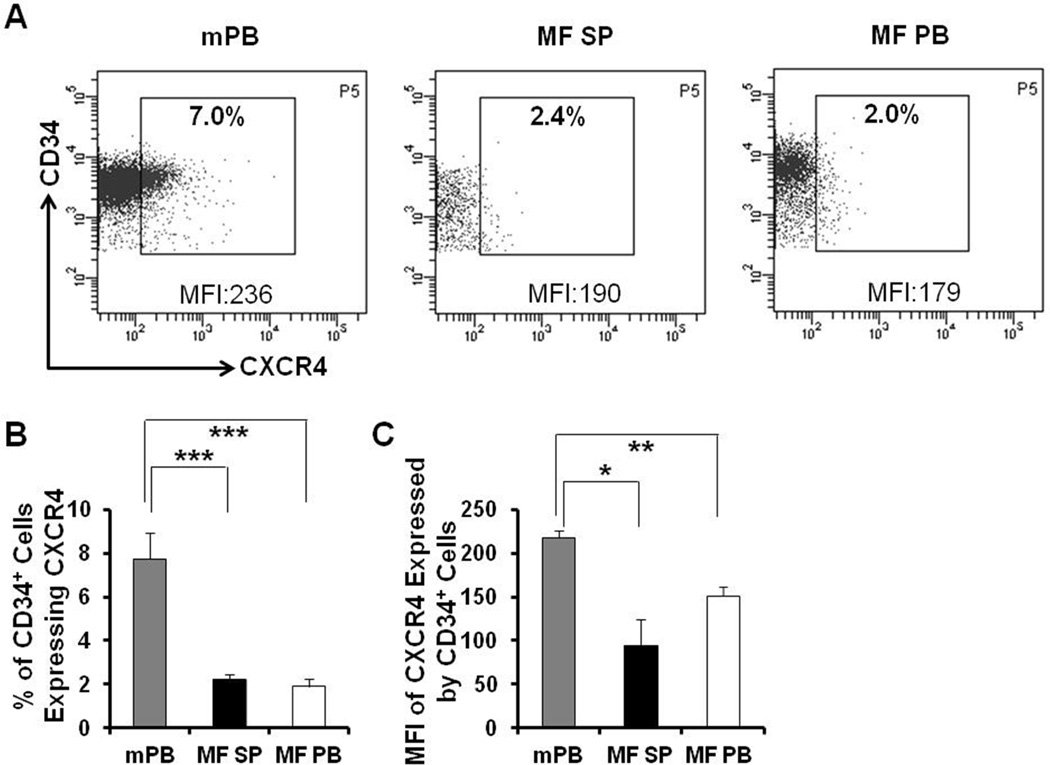
Recently, we have documented that a higher frequency of MF-SCs are present in MF spleens as compared to the corresponding PB of the same patient. Splenic MF-SCs also have a unique differentiation program which distinguishes them from their PB counterparts.23 We next investigated if the expression of chemokine receptors and adhesion molecules could account for the homing and location of MF CD34+ cells to the spleen and their impaired homing to the marrow. The expression of CXCR4, CD49d 24 and CD44 25–27 by mPB, splenic and PB MF CD34+ cells was evaluated flow cytometrically. As shown in Figure 2, the proportion of both splenic and PB MF CD34+ cells expressing CXCR4 and the level of CXCR4 expression as indicated by mean fluorescence intensity (MFI) of both splenic and PB MF CD34+ cells were significantly decreased as compared with mPB CD34+ cells. By contrast, as shown in Table 2, splenic and PB MF CD34+ cells expressed CD49d and CD44 to a similar degree as mPB CD34+ cells. Furthermore, we evaluated the expression of the integrin associated protein, CD47 28–29 which impairs the phagocytosis of hematopoietic cells by macrophages as an alternative mechanism of MF EMH. A difference in CD47 expression by splenic and PB CD34+ cells was not observed.
Figure 2. Down-regulated expression of CXCR4 by MF splenic and PB CD34+ cells.
(A) A representative flow cytometric plot demonstrating the proportion of mPB, splenic and PB MF CD34+ cells expressing CXCR4 and mean fluorescence intensity (MFI) of CXCR4 on these CD34+ cells. Data of Patient E are shown. (B-C) The proportion of both splenic and PB MF CD34+ cells expressing CXCR4 (B) and the MFI of CXCR4 expression on both splenic and PB MF CD34+ cells were significantly decreased as compared with mPB CD34+ cells. N=6. mPB: G-CSF mobilized peripheral blood; MF SP: myelofibrosis spleen; MF PB: myelofibrosis peripheral blood. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Table 2.
Adhesion Molecule Expression by mPB, Splenic MF and PB MF CD34+ Cells
| Molecules | mPB CD34+ Cells (%) |
SP MF CD34+ Cells (%) |
PB MF CD34+ Cells (%) |
|---|---|---|---|
| CD49d | 52.8±19.5 | 35.7±12.8 | 25.0±11.2 |
| CD44 | 95.0±2.1 | 85.6±5.1 | 81.3±11.2 |
| CD47 | 20.1±2.9 | 27.2±7.7 | 23.9±10.5 |
mPB: G-CSF mobilized peripheral blood; SP: splenic; PB: peripheral blood ; MF: myelofibrosis.
Increased levels of intact CXCL12 are present in splenic MF plasma
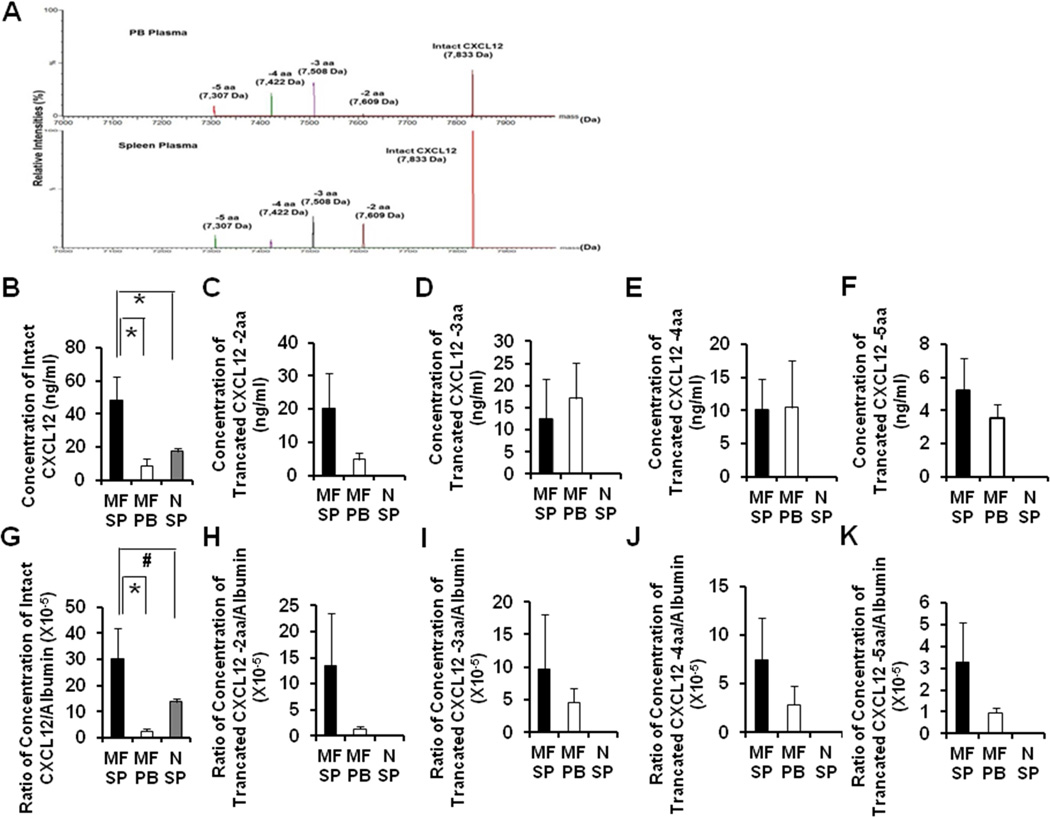
Unlike intact CXCL12, truncated forms of CXCL12 either lack the ability to act as a chemo-attractant for CD34+ cells or act as an antagonist to the action of CXCL12.20 To assess whether the splenic microenvironment of MF patients was altered and could contribute to the abnormal trafficking of MF-SC to the spleens of patients with MF, the intact and truncated forms (loss of 2 aa, 3 aa, 4 aa, and 5 aa) of CXCL12 in paired splenic and PB MF plasma as well as normal splenic plasma were quantified using mass spectrometry. The representative relative intensity of intact CXCL12 and its four truncated products in splenic and PB plasma of Patient D is shown in Figure 3A. The concentration of intact CXCL12 in splenic MF plasma from each of the six patients studied was 48.4 ±14.4 ng/mL, which was markedly higher than that detected in PB MF plasma (9.0 ± 4.1 ng/mL) (P<0.05) or normal splenic plasma (17.4 ± 1.0 ng/mL, P<0.05) (Figure 3B). The concentration of each of the 4 truncated forms of CXC12 were comparable in splenic and PB MF plasma (Figure 3C–F), whereas, solely intact CXCL12 but none of the truncated forms of CXCL12 were detected in normal splenic plasma (Figure 3C–F).
Figure 3. Concentrations of the intact CXCL12 and 4 truncated forms of CXC12 in splenic MF, PB MF and normal splenic plasma.
(A): The representative relative intensities of intact CXCL12 and four truncated forms in splenic (bottom) and PB plasma (top) of patient D. (B-F): Concentrations of intact CXCL12 (B) and four truncated forms (loss of 2 aa, 3 aa, 4 aa, and 5 aa) (C-F) of CXCL12 in paired splenic and PB MF plasma of all 6 patients and normal splenic plasma (n=7) were quantified using mass spectrometry. A higher level of intact CXCL12 is present in splenic MF plasma as compared with PB MF plasma or normal splenic plasma. The concentrations of the 4 truncated forms of CXCL12 were comparable in splenic and PB MF plasma, while none of the truncated forms of CXCL12 was detected in normal splenic plasma. (G-K): CXCL12 levels relative to albumin contained in corresponding splenic, PB MF and normal splenic plasma. The normalized level of intact CXCL12 in splenic MF plasma was again significantly higher than that of PB MF plasma (G). However, normal splenic plasma had marginally lower levels of intact CXCL12 than MF splenic plasma (G). The normalized levels of the 4 truncated forms of CXCL12 were found again similar in both splenic and PB MF plasma (H-K). *, P<0.05; #, P=0.08. aa: amino acid; MF SP: myelofibrosis splenic plasma; MF PB: myelofibrosis peripheral blood plasma; N SP: normal splenic plasma.
To account for the differences in preparation of the splenic and PB plasmas, as well as microenvironmental differences in the components of MF and normal spleens, the albumin concentrations of the various plasmas were determined. As shown in supplemental Figure 1, the concentration of albumin was higher in MF PB plasma than MF splenic plasma (P=0.058) and normal splenic plasma (P<0.001).This was anticipated since the splenic plasma was diluted with water. The concentration of CXCL12 was then normalized based on the albumin concentration of the corresponding plasma to exclude the influence of the differences in preparation. The normalized concentration of intact CXCL12 in splenic MF plasma of all six patients studied (30.1±11.9×10−5) was again significantly higher than that of PB MF plasma (2.5±1.1×10−5, Figure 3G, P<0.05). Normalized MF splenic plasma had higher levels of intact CXCL12 than normal splenic plasma (13.7±1.5×10−5, Figure 3G, P=0.08). The morphology of the majority of cells that were labeled positive for CXCL12 using immunohistochemical staining of the red pulp of normal and MF spleen included vascular cells lining splenic sinusoids and endothelial cells lining capillaries. There were also a limited number of additional cells that stained positive for CXCL12 which could represent reticular cells/mesenchymal cells/fibroblasts (Supplemental Figure 2). The normalized levels of the 4 truncated forms of CXCL12 was found again to be similar in both splenic and PB MF plasma (Figure 3H–K). These findings confirm that splenic MF plasma is characterized by higher levels of intact CXCL12 than PB MF plasma which likely contributes to the preferential homing of MF CD34+ cells to the spleens of MF patients.
Concentrations of serine proteases CD26, NE, MMP-2, MMP-9 in splenic and PB MF plasma
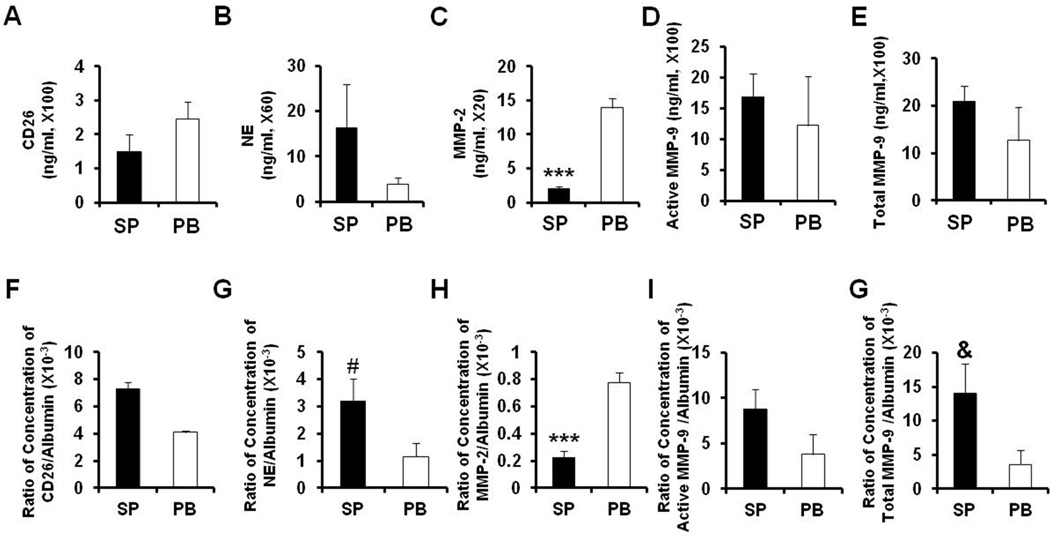
A variety of serine proteases, including NE and MMP-9 are capable of degrading a number of marrow matrix proteins, including VCAM-1 and CXCL12. 8 We measured levels of NE, CD26, MMP-2, total and active MMP-9 in splenic and PB MF plasma. As shown in Figure 4A–B and D-E, comparable concentrations of NE, CD26, both total and active MMP-9, were detected in splenic and PB MF plasma. However, the levels of MMP-2 were significantly lower in splenic MF plasma than PB MF plasma (P<0.001, Figure 4C). Furthermore, the normalized concentrations of NE, CD26, both total and active MMP-9 based on the albumin concentration of the corresponding plasma were similar in splenic and PB MF plasma (Figure 4F–G, I–J), while, the normalized concentration of MMP-2 was found again to be significantly lower in splenic MF plasma than in PB MF plasma than (P<0.001, Figure 4H).
Figure 4. MF plasma concentrations of various proteases that are involved in CXCL12 degradation.
(A-E): Concentrations of CD26 (A), NE (B), MMP-2 (C), total (D) and active MMP-9 (E) in splenic and PB MF plasma were measured by ELISAs. Comparable levels of CD26, NE, both total and active MMP-9 were detected in splenic and PB MF plasma, however, the level of MMP-2 was significantly higher in the PB MF plasma than splenic MF plasma. (F-J): Normalized concentrations of CD26 (F), NE (G), MMP-2 (H), total (I) and active MMP-9 (J) in splenic and PB MF plasma relative to corresponding albumin concentration. The normalized level of MMP-2 was again found to be significantly greater in PB MF plasma than splenic MF plasma, which might contribute to the higher levels of intact CXCL12 being present in splenic MF plasma. ***, P<0.001; #, P=0.057; &, P=0.053. NE: neutrophil elastase; MMP: matrix metalloproteinase; SP: splenic myelofibrosis plasma; PB: peripheral blood myelofibrosis plasma.
Increased migration of splenic MF CD34+ cells towards splenic MF plasma
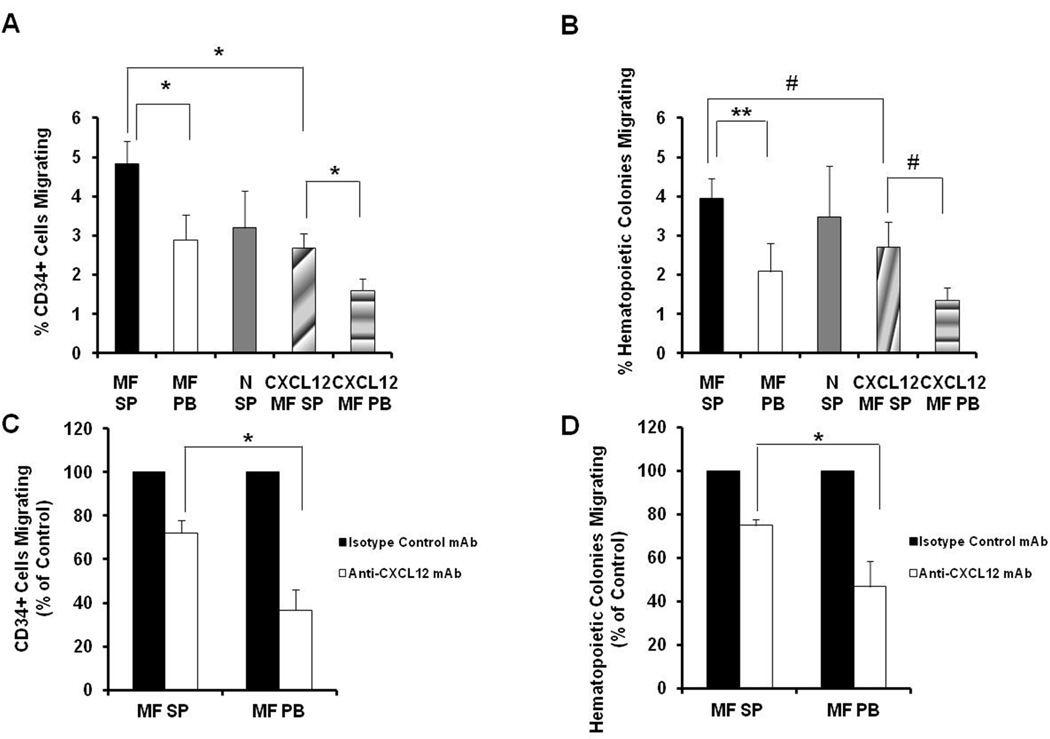
In order to investigate whether intact CXCL12 plays a crucial role in the migration of MF CD34+ cells to the spleens of MF patients, we initially assessed the chemo-attractant response of splenic MF CD34+ cells to purified, intact CXCL12 in vitro using a transwell assay. As shown in Figure 5A and B, 2.69±0.36% of splenic MF CD34+ cells and 2.70±0.65% HCs migrated in response to intact CXCL12 at a concentration corresponding to that determined to be present in the splenic plasma from each patient, while 1.61±0.29% of splenic MF CD34+ cells and 1.35±0.33% HCs migrated in response to the concentration of intact CXCL12 calculated to be present in the corresponding PB plasma from each patient, indicating that the migratory capacity of splenic MF CD34+ cells towards intact CXCL12 is positively related to the dose of intact CXCL12 (CD34+ cells: P<0.05; HCs: P=0.06). Next, the migratory behavior of splenic MF CD34+ cells towards splenic and PB MF plasma as well as normal splenic plasma was evaluated. The number of splenic MF CD34+ cells and HCs that migrated towards splenic MF plasma was significantly greater than the number that migrated towards PB MF plasma (Figure 5A and B, CD34+ cells: P<0.05; HCs: P<0.01). Surprisingly, the number of splenic MF CD34+ cells and HCs that migrated towards splenic MF plasma was even greater than the number which migrated towards the dose of intact CXCL12 known to be present in the MF splenic plasma of each corresponding patient (Figure 5A and B, CD34+ cells: P<0.05, HCs: P=0.06). However, similar numbers of MF splenic CD34+ cells and HCs migrated towards normal and MF splenic plasma (Figure 5A and B, P both >0.05), which is likely due to intact CXCL12 being present in both normal and MF splenic plasma and an absence of CXCL12 truncates in normal splenic plasma.
Figure 5. Migratory capacity of splenic MF CD34+ cells.
(A, B) The migratory behavior of splenic MF CD34+ cells towards splenic and PB MF plasma, normal splenic plasma as well as intact CXCL12 was determined as described in the Materials and Methods. The number of both splenic MF CD34+ cells (A) and hematopoietic colonies (B) that migrated towards splenic MF plasma was significantly greater than the number that migrated towards PB MF plasma. N=6. (C, D)MF splenic and PB plasma was treated with anti-CXCL12 neutralizing monoclonal antibodies (mAb) and the migratory behavior of MF splenic CD34+ cells towards these 2 plasmas were assessed. Equal concentration of anti-CXCL12 mAbs resulted in greater reductions in the numbers of splenic MF CD34+ cells and hematopoietic colonies that migrated towards PB MF plasmas as compared with MF splenic plasma. N=5. *, P<0.05; **, P<0.01; #, P=0.06. MF SP: myelofibrosis splenic plasma; MF PB: myelofibrosis peripheral blood plasma; N SP: normal splenic plasma; CXCL12 MF spleen: intact CXCL12 at the concentration assayed in MF splenic plasma of each patient; CXCL12 MF PB: intact CXCL12 at the concentration assayed in MF PB plasma of each patient.
In order to confirm that increased concentration of intact CXCL12 present in MF splenic plasma contributes to the homing of MF CD34+ cells to the spleens, MF splenic or PB plasma were treated with anti-CXCL12 neutralizing mAbs and the migratory behavior of splenic MF CD34+ cells assessed. As shown in Figure 5C and D, the number of splenic MF CD34+ cells and HCs that migrated towards splenic MF plasma were reduced by 27.8±5.7% and by 25.1±2.8%, respectively following the treatment with anti-CXCL12 neutralizing mAb (100ug/ml). An equal concentration of anti-CXCL12 mAb resulted in greater reduction in the numbers of splenic MF CD34+ cells and HCs that migrated towards PB MF plasma (CD34+ cell: 63.4±9.7%; HC: 53.3±11.9%). The differences in the ability of equal concentrations of the neutralizing antibody to reduce the chemo-attractant capacity of the two sources of MF plasma is likely due to the differences in the concentration of CXCL12. These data further validate that increased concentration of intact, functional CXCL2 plays a crucial role in the preferential homing of MF CD34+ cells to the spleens of MF patients.
The percentage of JAK2V617F + colonies that migrated towards splenic plasmas was similar to that observed with PB plasmas from 2 JAK2V617F + patients (Patient A and E, data not shown) indicating that malignant HPCs do not have a unique migratory pattern as compared to HPC lacking mutated JAK2.
Discussion
Abnormal CD34+ cell trafficking and EMH are integral components of the patho-biology of PMF. 13–14, 30 We have previously reported that the constitutive mobilization of MF CD34+ cells could be accounted for by the down-regulation of CXCR4 on MF CD34+ cells and a reduction of the amount of intact CXCL12 which serves as a chemo-attractant for CD34+ cells. 7, 20 We have attempted to determine the mechanism by which MF CD34+ cells lodge in the spleens of MF patients and develop EMH. We have provided data here which indicates an increased concentration of intact fully functional CXCL12 within MF spleen but not PB and presumably marrow likely contributes to the homing of MF CD34+ cells to the spleens rather than the marrows of MF patients, ultimately leading to EMH in the spleen. The initial establishment of EMH in MF patients is likely in part due to the presence of intact CXCL12 in normal spleens, the production of which has been localized in this report to cells lining vessels. The importance of CXCL12 produced by endothelial cells in the hematopoietic stem cell niches has previously been reported in murine models by Ding and Morrison.31 As MF progresses, the marrow is progressively depleted of CD34+ cells but CD34+ cells are present in the PB which likely is due to CD34+ cell trafficking between the MF spleen, PB and marrow as previously proposed by Massa and co-workers.32
Initially, we established that the homing of both splenic and PB MF CD34+ cells to the marrow was defective, while these cells retained their ability to home to the spleens of NOD/SCID mice. We next evaluated the expression of chemokines and integrins by splenic and PB MF CD34+ cells which contribute to the homing and retention of HSCs/HPCs to their microenvironment. We speculate that the reduced homing of MF CD34+ cells to the marrow is likely due to the epigenetic down-regulation of CXCR4. These studies indicated that the expression of other adhesion molecules were not differentially expressed by splenic and PB CD34+ cells. Furthermore, the anti-phagocytosis signaling molecule CD47 was equally expressed by PB and splenic CD34+ cells indicating that a blockade of phagocytosis by macrophages could not account for MF EMH.
Chemokine truncation can result in inactivation of chemokines or changing the chemokine into an effective receptor antagonist. 33–35 We have previously reported a reduction in the amount of intact marrow CXCL12 and the predominance of CXCL12 truncates within the PB of MPN patients.20 By contrast, we have demonstrated that the MF splenic microenvironment contains higher levels of intact CXCL12 than that present in MF PB plasma. The truncated forms of CXCL12 lack chemo-attractant capabilities and/or act as receptor antagonists, suggesting that CXCL12 status plays a role in determining the sites of hematopoiesis in MF. In the present study, comparable concentrations of the four truncated forms of CXCL12 were detected in the splenic and PB MF plasmas, which are due to proteolytic degradation by serine proteases including CD26, NE, MMP-2 and MMP-9. Recently, Richter and coworkers have demonstrated that truncates of CXCL12 might also affect HSC/HPC trafficking by altering the binding of CXCL12 to glycosaminoglycans which leads to increased oligomerization, and increased local concentrations of CXCL12 leading to the generation of a chemokine gradient. 36 One can speculate that such a scenario might occur within the red pulp of the spleen which would further enhance the chemo-attractant potential of splenic CXCL12. MMP-2 is known to cleave and inactivate CXCL12. 37 MMP-2 protein expression has been shown to be inversely related to CXCL12 protein levels and reduced chemotactic support.37 Splenic MF plasma contained relatively reduced levels of MMP-2 which could account for the increased intact CXCL12 present in splenic MF plasma. Dramatically increased numbers of splenic MF CD34+ cells migrated in response to splenic MF plasma, as compared with PB MF plasma, which might be due in part to the increased concentrations of intact CXCL12. Splenic MF CD34+ cells, however, exhibited an even greater migratory capacity towards splenic MF plasma than the quantity of intact CXCL12 documented to be present in the splenic MF plasmas, raising the possibility that additional chemo-attractants may also contribute to the homing of MF CD34+ cells to the spleens of MF patients.
In conclusion, our data indicate that splenic MF plasma is characterized by increased concentrations of intact CXCL2, which we conjecture, results in the initiation and development of EMH in MF patients. The splenic cells responsible for the excessive production of CXCL12 appear to be endothelial cells.38 We would like to directly compare the fraction of intact CXCL12 and its truncated forms in paired spleens and marrows of MF patients. Unfortunately, in advanced forms of MF, marrow plasma could not be obtained due to the marrows being inaspirable. Furthermore, none of the patients were willing to undergo an additional marrow biopsy for such studies. However, previously, we reported that the degradation patterns of CXCL12 in the marrow and PB of patients with PV were similar. 20 Based on these observations, we have concluded that the CXCL12 degradation patterns observed in PB resembles that present in marrow. The present studies indicate that the microenvironment within the marrow and spleen differ in MF in part due to the increased levels of intact CXCL12 present in the spleen. These different microenvironments within the marrow and spleen likely contribute to MF phenotype and disease progression.
Supplementary Material
Highlights.
Splenic and PB MF CD34+ cells home normally to the spleens of NOD/SCID mice.
Splenic and PB MF CD34+ cells have similar degrees of down-regulation of CXCR4.
Increased levels of intact, active forms of CXCL12 are present in MF splenic plasma as compared to PB plasma.
Increased levels of intact CXCL12 contributes to the development of splenic hematopoiesis.
Acknowledgements
We thank Virginia Gillespie for reviewing immunohistochemical staining sections. This study was support by grants from National Cancer Institute (1P01CA108671) to R. Hoffman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
Contributions: X.W. designed the experiments, performed the experiments, analyzed the data, and wrote the paper. S.Y. C. performed mass spectrometric analysis and analyzed the data. C. H, and D. C. performed some of the experiments. J. R. interpreted the data and reviewed the paper. R.H. designed the experiments, interpreted the data, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Dor FJ, Ramirez ML, Parmar K, Altman EL, Huang CA, Down JD, Cooper DK. Primitive hematopoietic cell populations reside in the spleen: Studies in the pig, baboon, and human. Exp Hematol. 2006;34:1573–1582. doi: 10.1016/j.exphem.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 5.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 6.Loetscher M, Geiser T, OReilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 7.Wang X, Zhang W, Ishii T, Sozer S, Wang J, Xu M, Hoffman R. Correction of the Abnormal Trafficking of Primary Myelofibrosis CD34+ Cells by Treatment with Chromatin-Modifying Agents. Cancer Res. 2009;69(19):7612–7618. doi: 10.1158/0008-5472.CAN-09-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M, Bruno E, Chao J, et al. Constitutive mobilization of CD34+ cells into the peripheral blood in idiopathic myelofibrosis may be due to the action of a number of proteases. Blood. 2005;105:4508–4515. doi: 10.1182/blood-2004-08-3238. [DOI] [PubMed] [Google Scholar]
- 9.Migliaccio AR, Martelli F, Verrucci M, et al. Altered SDF-1/CXCR4 axis in patients with primary myelofibrosis and in the Gata1 low mouse model of the disease. Exp Hematol. 2008;36:158–171. doi: 10.1016/j.exphem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lataillade JJ, Pierre-Louis O, Hasselbalch HC, et al. French INSERM and the European EUMNET networks on myelofibrosis. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood. 2008;112:3026–3035. doi: 10.1182/blood-2008-06-158386. [DOI] [PubMed] [Google Scholar]
- 11.Rosti V, Massa M, Vannucchi AM, et al. The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol Dis. 2007;38:280–286. doi: 10.1016/j.bcmd.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Bogani C, Ponziani V, Guglielmelli P, et al. Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells. 2008;26:1920–1930. doi: 10.1634/stemcells.2008-0377. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Lewis SM. The splenomegaly of myeloproliferative and lymphoproliferative disorders: splenic cellularity and vascularity. Eur J Haematol. 1989;43(1):63–66. doi: 10.1111/j.1600-0609.1989.tb01253.x. [DOI] [PubMed] [Google Scholar]
- 14.Thiele J, Pierre R, Imbert M, Vardiman JW, Brunning RD, Glandrin G. World Health Organization Of Tumors Of Hematopoietic And Lymphoid Tissues. In: Chronic idiopathic myelofibrosis. Jaffe ES, Harris N, Stan N, Vardiman JW, editors. Washington, DC, USA: IARC Press; 2001. pp. 35–38. [Google Scholar]
- 15.Barosi G, Rosti V, Massa M, et al. Spleen neoangiogenesis in patients with myelofibrosis with myeloid metaplasia. Br J Haematol. 2004;124(5):618–625. doi: 10.1111/j.1365-2141.2004.04829.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Zhang W, Tripodi J, et al. Sequential treatment of CD34+ cells from patients with primary myelofibrosis with chromatin-modifying agents eliminate JAK2V617F–positive NOD/SCID marrow repopulating cells. Blood. 2010;116(26):5972–5982. doi: 10.1182/blood-2010-02-269696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nussenzveig RH, Swierczek SI, Jelinek J, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35(1):32–38. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, LeBlanc A, Gruenstein S, et al. Clonal analyses define the relationships between chromosomal abnormalities and JAK2V617F in patients with Ph-negative myeloproliferative neoplasms. Exp Hematol. 2009;37(10):1194–1200. doi: 10.1016/j.exphem.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Najfeld V, Coyle T, Berk PD. Transformation of polycythemia vera to acute nonlymphocytic leukemia accompanied by t (1;3)(p36;q21) karyotype. Cancer Genet Cytogenet. 1988;33(2):193–200. doi: 10.1016/0165-4608(88)90029-5. [DOI] [PubMed] [Google Scholar]
- 20.Cho SY, Xu M, Roboz J, Lu M, Mascarenhas J, Hoffman R. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70(8):3402–3410. doi: 10.1158/0008-5472.CAN-09-3977. [DOI] [PubMed] [Google Scholar]
- 21.Shi J, Zhao Y, Ishii T, et al. Effects of chromatin-modifying agents on CD34+cells from patients with idiopathic myelofibrosis. Cancer Res. 2007;67:6417–6424. doi: 10.1158/0008-5472.CAN-07-0572. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Xing S, Ho WT, et al. Erlotinib effectively inhibits JAK2V617F activity and polycythemia vera cell growth. J Biol Chem. 2007;282(6):3428–3432. doi: 10.1074/jbc.C600277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Prakash S, Lu M, et al. Spleens of myelofibrosis patients contain malignant hematopoietic stem cells. J Clin Invest. 2012;122(11):3888–3899. doi: 10.1172/JCI64397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmo-Cabañas M, Bartolomé RA, Wright N, Hidalgo A, Drager AM, Teixidó J. Integrin α4β1 involvement in stromal cell-derived factor-1α-promoted myeloma cell transendothelial migration and adhesion: role of cAMP and the actin cytoskeleton in adhesion. Exp Cell Res. 2004;294:571–580. doi: 10.1016/j.yexcr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Bourguignon LY, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3,8-10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43(4):269–287. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12(10):1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 27.Ghaffari S, Dougherty GJ, Lansdorp PM, Eaves AC, Eaves CJ. Differentiation-associated changes in CD44 isoform expression during normal hematopoiesis and their alteration in chronic myeloid leukemia. Blood. 1995;86(8):2976–2985. [PubMed] [Google Scholar]
- 28.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okazawa H, Motegi S, Ohyama N. Negative Regulation of Phagocytosis in Macrophages by the CD47-SHPS-1 System. J Immunol. 2005;174:2004–2011. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 30.Barosi G, Viarengo G, Pecci A, et al. Diagnostic and clinical relevance of the number of circulating CD34+ cells in myelofibrosis with myeloid metaplasia. Blood. 2001;98:3249–3255. doi: 10.1182/blood.v98.12.3249. [DOI] [PubMed] [Google Scholar]
- 31.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massa M, Campanelli R, Lupo L, et al. Splenectomy produces a rapid but transient decrease of the frequency of circulating CD34+ haematopoietic progenitor cells in primary myelofibrosis. Br J Haematol. 2011;152(5):665–667. doi: 10.1111/j.1365-2141.2010.08527.x. [DOI] [PubMed] [Google Scholar]
- 33.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 34.Christopherson KW, II, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 α-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 35.Proost P, Struyf S, Schols D, et al. Processing by CD26/dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell-derived factor-1α. FEBS Lett. 1998;432:73–76. doi: 10.1016/s0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- 36.Richter R, Jochheim-Richter A, Ciuculescu F, et al. Identification and Characterization of Circulating Variants of CXCL12 from Human Plasma: Effects on Chemotaxis and Mobilization of Hematopoietic Stem and Progenitor Cells. Stem Cells Dev. 2014 May 27; doi: 10.1089/scd.2013.0524. ahead of print. doi:10.1089/scd.2013.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clutter SD, Fortney J, Gibson LF. MMP-2 is required for bone marrow stromal cell support of pro-B-cell chemotaxis. Exp Hematol. 2005;33(10):1192–1200. doi: 10.1016/j.exphem.2005.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miwa Y, Hayashi T, Suzuki S, Abe S, Onishi I, Kirimura S, et al. Upregulated expression of CXCl-12 in human spleens with extramdullary hematpoiesis. Pathology. 2013;45(4):408–416. doi: 10.1097/PAT.0b013e3283613dbf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.