Abstract
Nicotine and chlorpyrifos are developmental neurotoxicants that target serotonin systems. We examined whether prenatal nicotine exposure alters the subsequent response to chlorpyrifos given postnatally. Pregnant rats received nicotine throughout gestation at 3 mg/kg/day, a regimen designed to achieve plasma levels seen in smokers; chlorpyrifos was given to pups on postnatal days (PN) 1–4 at 1 mg/kg, just above the detection threshold for brain cholinesterase inhibition. We assessed long-term effects from adolescence (PN30) through full adulthood (PN150), measuring the expression of serotonin receptors and serotonin turnover (index of presynaptic impulse activity) in cerebrocortical brain regions encompassing the projections that are known targets for nicotine and chlorpyrifos. Nicotine or chlorpyrifos individually increased the expression of serotonin receptors, with greater effects on males than on females and with distinct temporal and regional patterns indicative of adaptive synaptic changes rather than simply an extension of initial injury. This interpretation was confirmed by our finding an increase in serotonin turnover, connoting presynaptic serotonergic hyperactivity. Animals receiving the combined treatment showed a reduction in these adaptive effects on receptor binding and turnover relative to the individual agents, or even an effect in the opposite direction; further, normal sex differences in serotonin receptor concentrations were dissipated or reversed, an effect that was confirmed by behavioral evaluations in the Novel Objection Recognition Test. In addition to the known liabilities associated with maternal smoking during pregnancy, our results point to additional costs in the form of heightened vulnerability to neurotoxic chemicals encountered later in life.
Keywords: Chlorpyrifos, Nicotine, Novel Object Recognition, Organophosphate pesticides, Serotonin, Sex differences
INTRODUCTION
Although maternal smoking rates in U.S. have declined to 10–15%, more than 400,000 babies are born to active smokers each year, with a larger proportion exposed via second-hand smoke (U.S. Surgeon General, 2014). Exposure rates may actually be higher because of deception about self-reported tobacco use (Dietz et al., 2010b) and any progress in smoking cessation seen in economically advanced countries, is offset worldwide by the sharp increase in smoking among women in less economically-developed regions (World Health Organization, 1997). Thus, smoking remains the most preventable cause of perinatal morbidity and mortality (Dietz et al., 2010a). Beyond the immediate perinatal period, fetal tobacco exposure damages the developing brain, leading to a wide variety of neurodevelopmental disorders, including Sudden Infant Death Syndrome, attention deficit/hyperactivity disorder, learning disabilities and conduct disorders, all at enormous cost to individuals, families and society (Clifford et al., 2012; Cornelius and Day, 2009; DiFranza and Lew, 1995; Ernst et al., 2001; Herrmann et al., 2008; Pauly and Slotkin, 2008; Slotkin, 2008). Nicotine contributes in large measure to the developmental neurotoxicity of tobacco smoke exposure by interfering with trophic signals that control neuronal cell replication, differentiation, synaptic connectivity and neural circuits underlying behavioral function (Dwyer et al., 2008; Slotkin, 2004, 2008). Consequently, tobacco cessation strategies that utilize nicotine replacement may not avoid neurodevelopmental damage to the fetus (Ginzel et al., 2007; Pauly and Slotkin, 2008; Slotkin, 2008) and the increasing popularity of recreational nicotine delivery products, such as e-cigarettes, may soon reverse the recent progress in reducing fetal nicotine exposure (Ginzel et al., 2007).
In addition to its direct impact on fetal brain development, nicotine can alter the response to subsequently chemical exposures. We recently showed that, when prenatal nicotine exposure was followed by dexamethasone treatment as used in the management of preterm labor, there was greater net damage to cholinergic and serotonergic circuits and their dependent behaviors, than with either agent alone (Levin et al., 2014; Slotkin et al., 2013, 2014); many of the effects could be recapitulated with neuronotypic cells undergoing differentiation in culture, indicating a direct interaction on developmental processes rather than indirect effects on maternal-fetal physiology (Slotkin et al., 2012). Further, we found that nicotine also augmented the effects of postnatal exposure to chlorpyrifos directed toward acetylcholine systems (Slotkin and Seidler, 2015). Chlorpyrifos is a widely-used organophosphate pesticide, with virtually ubiquitous exposure of the human population (Casida and Quistad, 2004). In the adult, nicotine enhances chlorpyrifos catabolism, leading to lower levels and a smaller degree of cholinesterase inhibition by the pesticide (Lee et al., 2010); however, we found that prenatal nicotine treatment actually increased the ability of chlorpyrifos to inhibit brain cholinesterase in newborn rats (Slotkin and Seidler, 2015), pointing to a unique developmental sensitivity.
An interaction between nicotine and chlorpyrifos directed toward acetylcholine systems is logical, given that both agents enhance net cholinergic stimulation (Slotkin, 1999, 2004). However, the more general disruption of brain development by either agent also encompasses other neurotransmitters, notably serotonin (5-hydroxytryptamine, 5HT) (Aldridge et al., 2003. 2004. 2005a, 2005b; Muneoka et al., 1997; Slikker et al., 2005; Slotkin et al., 2006b, 2009a, 2009b; Slotkin and Seidler, 2005, 2010; Timofeeva et al., 2008; Xu et al., 2001). This may be an important contributor to the increased risk of depression associated with smoking or organophosphate exposure (Beseler et al., 2006, 2008; Chen et al., 2011; Goodman and Capitman, 2000; Jaga and Dharmani, 2007; Lee et al., 2007; Schuetze and Eiden, 2007; Upadhyaya et al., 2002; Wu and Anthony, 1999). Accordingly, in the current study, we examined whether prenatal exposure to nicotine alters the subsequent developmental neurotoxicity of chlorpyrifos directed toward 5HT synaptic function. As in our earlier study with this combination (Slotkin and Seidler, 2015), we used well-established treatment paradigms for both agents. Nicotine was given throughout gestation via implanted osmotic minipump, at a dose (3 mg/kg/day) that produces nicotine plasma levels similar to those in moderate smokers (Lichtensteiger et al., 1988; Murrin et al., 1987; Trauth et al., 2000). Chlorpyrifos was then given to pups daily on postnatal days (PN) 1–4 at 1 mg/kg, a regimen that produces just-detectable inhibition of brain cholinesterase and that disrupts neurobehavioral development, but that is not systemically toxic (Slotkin, 1999, 2004, 2005; Song et al., 1997). This exposure model successfully predicts both the neurobehavioral deficits and abnormalities of brain structure seen in children exposed prenatally to chlorpyrifos (Bouchard et al., 2011; Engel et al., 2011; Rauh et al., 2006, 2011, 2012). Our study thus encompassed four treatment groups: control, nicotine alone, chlorpyrifos alone, and nicotine followed by chlorpyrifos. In addition, we evaluated sex differences, which are known to be important in the developmental neurotoxicity of both agents.
Our evaluations were conducted in cerebrocortical regions possessing major 5HT projections (frontal/parietal cortex, temporal/occipital cortex), studied throughout development from adolescence, to young adulthood and then to full adulthood, as the persistent effects of both nicotine and chlorpyrifos on 5HT systems emerge over these stages (Aldridge et al., 2003, 2004, 2005a, 2005b; Slotkin et al., 2006c, 2007b; Slotkin and Seidler, 2005, 2010; Xu et al., 2001). First, we focused on 5HT1A and 5HT2 receptors, the subtypes that are known to be major targets for effects of nicotine and chlorpyrifos (Aldridge et al., 2003, 2004, 2005a, 2005b; Slotkin et al., 2006c 2007b; Slotkin and Seidler, 2005, 2010; Xu et al., 2001), and that play major roles in 5HT-related mental disorders, including depression (Arango et al., 2001; Fujita et al., 2000; Yatham et al., 1999, 2000). Then, at two stages in adulthood, we assessed cerebrocortical 5HT presynaptic activity by measuring concentrations of 5HT and its principal metabolite, 5-hydroxyindoleacetic acid (5HIAA), calculating transmitter utilization (turnover) as the 5HIAA/5HT ratio. Further, we established whether persistent effects on 5HT receptors extended to other regions containing 5HT projections (hippocampus, striatum) or 5HT cell bodies (midbrain, brainstem). Finally, we assessed the effect of these treatments on behavioral performance in the Novel Object Recognition Test. We chose this test because of its dependence on 5HT1A and 5HT2 receptor function (Meltzer et al., 2011) and because its sex-selectivity is highly dependent on 5HT systems (Sutcliffe et al., 2007).
MATERIALS AND METHODS
Animal treatments
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague-Dawley rats were shipped on the second day of gestation by climate-controlled truck (total transit time < 1 h), housed individually and allowed free access to food and water. There were four treatment groups, each comprising 10–12 litters: controls (prenatal vehicle infusion + postnatal vehicle injections), nicotine treatment alone (prenatal nicotine infusion + postnatal vehicle injections), chlorpyrifos treatment alone (prenatal vehicle infusion + postnatal chlorpyrifos injections), and those receiving the combined treatment (prenatal nicotine infusion + postnatal chlorpyrifos injections). On gestational day 4, before implantation of the embryo in the uterine wall, each animal was quickly anesthetized with ether, a small area on the back was shaved, and an incision made to permit s.c. insertion of a Model 2ML2 Alzet minipump, after which the incision was closed with wound clips. The pumps contained nicotine bitartrate dissolved in bacteriostatic water so as to deliver 3 mg/kg/day of nicotine free base, with the dosage determined by the initial body weights of the dams (215 ± 2 g); control pumps contained bacteriostatic water and equivalent concentrations of sodium bitartrate. Because weights increased with gestation, the dose rate fell accordingly to 2 mg/kg/day by the end of the infusion period, but the dose rates remained well within the range that produces nicotine plasma levels similar to those in moderate smokers (Fewell et al., 2001; Trauth et al., 2000). It should be noted that the pump, marketed as a two week infusion device, actually takes approximately 17 days to be exhausted completely (information supplied by the manufacturer) and thus the nicotine infusion terminates on the 21st day of gestation; in earlier work, we confirmed the termination of nicotine delivery coinciding with the calculated values (Trauth et al., 2000). Parturition occurred during gestational day 22, which was also taken as PN0.
After birth, pups were randomized within treatment groups and litter sizes were culled to 10 (5 males and 5 females) to ensure standard nutrition. Control and nicotine-treated litters were then assigned to either the vehicle or chlorpyrifos postnatal treatment groups. Chlorpyrifos was dissolved in dimethylsulfoxide to provide consistent absorption (Whitney et al., 1995) and pups were injected subcutaneously at a dose of 1 mg/kg in a volume of 1 ml/kg once daily on postnatal days 1–4; control pups received equivalent injections of the dimethylsulfoxide vehicle. This regimen has been shown previously to produce developmental neurotoxicity, including robust effects on 5HT systems, without eliciting growth retardation or any other signs of systemic toxicity (Aldridge et al., 2003. 2004. 2005a, 2005b). Pups were weighed, litters were re-randomized within treatment groups and dams were rotated among litters within treatment groups every few days to distribute differential effects of maternal caretaking equally among all litters, making sure that all the pups in a given litter were from the same treatment group to avoid the possibility that the dams might distinguish among pups with different treatments; cross-fostering, by itself, has no discernible impact on pup development (Nyirenda et al., 2001). Offspring were weaned on PN21. Subsequently, on PN30, 60, 100 and 150, animals were decapitated and brain regions were dissected into frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum, midbrain and brainstem. The two cortical regions were sectioned at the midline and the right half used for the current determinations. The left halves of the cortical regions were reserved for other studies, along with the cerebellum, which is sparse in 5HT projections; likewise, the hippocampus, striatum, midbrain and brainstem samples obtained on PN30 and PN60 were committed to other studies, whereas those from PN100 and PN150 were used here. Tissues were frozen in liquid nitrogen and stored at −80°C until assayed. For every set of determinations, each treatment group comprised 12 animals at each age point, equally divided into males and females, with each final litter assignment contributing no more than one male and one female to any of the treatment groups. Assays were conducted on each individual tissue, so that each determination represented a value from the corresponding brain region of one animal.
5HT receptors
All of the ligand binding methodologies used in this study have appeared in previous papers (Aldridge et al., 2004; Slotkin et al., 2006a; Slotkin and Seidler, 2005, 2007), so only brief descriptions will be provided here. Tissues were thawed and homogenized (Polytron, Brinkmann Instruments, Westbury, NY) in ice-cold 50 mM Tris (pH 7.4), and the homogenates were sedimented at 40,000 × g for 15 min. The pellets were washed by resuspension (Polytron) in homogenization buffer followed by resedimentation, and were then dispersed with a homogenizer (smooth glass fitted with Teflon pestle) in the same buffer. An aliquot was assayed for measurement of membrane protein (Smith et al., 1985). Two radioligands were used to determine 5HT receptor binding: 1 nM [3H]8-hydroxy-2-(di-n-propylamino)tetralin for 5HT1A receptors, and 0.4 nM [3H]ketanserin for 5HT2 receptors. For 5HT1A receptors, specific binding was displaced by addition of 100 μM 5HT; for 5HT2 receptors, we used 10 μM methylsergide for displacement.
5HT and 5HIAA
Tissues were thawed and homogenized in ice-cold 0.1 M perchloric acid and sedimented for 20 min at 40,000 × g. The supernatant solution was collected and aliquots were used for analysis of 5HT and 5HIAA by high-performance liquid chromatography with electrochemical detection (Xu et al., 2001). Concurrently-run standards were used to calculate the regional concentrations. Transmitter turnover was calculated as the 5HIAA/5HT ratio.
Novel Object Recognition Test
During the ninth postnatal week, 8–9 animals of each sex from each treatment group were assessed. First, the animals were habituated to the apparatus for a 10 min session on two consecutive days. Testing began on the third day with a 10 min information session with identical objects, followed by a 15 min, 1 hr, or 6 hr delay period. This was followed by a subsequent 10 min session with dissimilar objects (one novel object and one object that had been used in the information session). Time spent with either object was recorded.
Data analysis
The initial statistical comparisons were conducted by a global ANOVA (data log-transformed because of heterogeneous variance among regions, measures and ages) incorporating all the variables and measurements in a single test so as to avoid an increased probability of type 1 errors that might otherwise result from multiple tests of the same data set. The variables in the global test were prenatal treatment (vehicle, nicotine), postnatal treatment (DMSO vehicle, chlorpyrifos), brain region, age and sex, with multiple dependent measures (5HT1A receptor and 5HT2 receptor; 5HT and 5HIAA for the HPLC determinations); in both cases, the dependent measures were treated as repeated measures, since multiple determinations were derived from the same sample. Where we identified interactions of treatment with the other variables, data were then subdivided for lower-order ANOVAs to evaluate treatments that differed from the corresponding control. As permitted by the interaction terms, individual treatments that differed from control were identified with Fisher’s Protected Least Significant Difference Test. Where treatment effects were not interactive with other variables, we report only the main treatment effects without performing lower-order analyses of individual values. Significance was assumed at the level of p < 0.05.
Data were compiled as means and standard errors. To enable ready visualization of treatment effects across different regions, ages and measures, the results are given as the percent change from control values. Statistical procedures were always conducted on the original data, with log transforms because of heterogeneous variance as noted above. In addition, the log-transform evaluates the treatment differences as a proportion to control values, rather than as an arithmetic difference. This was important because of technical limitations: on any single day, we could conduct assays for all treatment groups and both sexes, but only for one region at one age point. Accordingly, representing the data as proportional differences (percent control) enables a full comparison of treatment effects and treatment interactions with all the other variables, even though absolute values for the controls cannot be compared across regions and ages. Graphs were scaled to encompass the different dynamic ranges of the changes in the various parameters. The absolute values for each set of determinations appear in the Supplemental Tables.
There were two separate issues addressed in the data analysis. First, we determined whether the effects of nicotine, chlorpyrifos, or the combination, differed from control values and from each other. Second, we wanted to assess whether the effects of the combination were additive (sum of individual effects of nicotine and chlorpyrifos), or non-additive (synergistic, less-than-additive or antagonistic). The first issue required an ANOVA regarding the four treatment groups as one factor (“treatment”), followed by post-hoc comparisons for intergroup differences. The second issue required that the nicotine and chlorpyrifos treatments be considered as two separate factors, with the interaction term (nicotine × chlorpyrifos) thus testing for additivity (not significant if additive, significant if non-additive).
Materials
Animals were purchased from Charles River Laboratories (Raleigh, NC, USA) and chlorpyrifos was obtained from Chem Service (West Chester, PA, USA). PerkinElmer Life Sciences (Boston, MA, USA) was the source for [3H]8-hydroxy-2-(di-n-propylamino)tetralin (specific activity, 135 Ci/mmol) and [3H]ketanserin (63 Ci/mmol). Methylsergide was obtained from Sandoz Pharmaceuticals (E. Hanover, NJ, USA) and all other chemicals came from Sigma Chemical Co. (St. Louis, MO, USA).
RESULTS
Maternal, litter and growth effects
The animals used in this study were littermates of those used for our earlier study of acetylcholine systems and the effects on litter characteristics and growth were presented previously (Slotkin and Seidler, 2015). Prenatal nicotine treatment reduced body weights in the pregnant dams by about 3% but there were no significant effects on the proportion of dams giving birth, litter size, birth weight or sex ratio. None of the treatment groups showed any growth impairment, nor were there deficits in brain region weights.
Cerebrocortical 5HT receptors
We first conducted a global ANOVA encompassing the factors of treatment, age, brain region and sex, and the dependent measures of the two receptor subtypes (considered as a repeated measure, since both were evaluated from the same samples). There was a main effect of treatment (p < 0.0001), reflecting significant differences between the control and nicotine groups (p < 0.0001), between the control and chlorpyrifos groups (p < 0.0001), between the nicotine group and the group receiving nicotine + chlorpyrifos (p < 0.005), and between the group receiving chlorpyrifos and the group receiving nicotine + chlorpyrifos (p < 0.05). The treatment effects interacted with the other factors (p < 0.0003 for treatment × age, p < 0.02 for treatment × sex, p < 0.02 for treatment × age × sex, p < 0.003 for treatment × age × region) and also were distinct for the two different receptor measures (p < 0.05 for treatment × measure, p < 0.0001 for treatment × age × measure, p < 0.04 for treatment × age × measure, p < 0.05 for treatment × age × sex × measure, p < 0.002 for treatment × age × region × measure). In light of these interactions, the data were subdivided so as to evaluate the two receptor subtypes individually for each treatment group, evaluating main treatment effects and interactions of treatment with the remaining variables (age, region, sex).
In addition to analyses where the treatment was considered as a single ANOVA variable with four categories (control, nicotine, chlorpyrifos, nicotine + chlorpyrifos), we also examined a global test where the treatments were regarded as two separate factors (prenatal treatment, postnatal treatment) so as to detect interactions between nicotine and chlorpyrifos treatments that would indicate synergistic, less-than additive or antagonistic effects. We found a significant interaction of nicotine × chlorpyrifos (p < 0.0001), reflecting the fact that the group receiving combined treatment had lower values across both receptor subtypes than would have been expected from summation of the individual effects of nicotine and chlorpyrifos. This interaction also depended on the other variables: nicotine × chlorpyrifos × age (p < 0.03), nicotine ×chlorpyrifos × measure (p < 0.03). Accordingly, we performed similar analyses for each of the lower-order groupings to look for interactions between the nicotine and chlorpyrifos treatments.
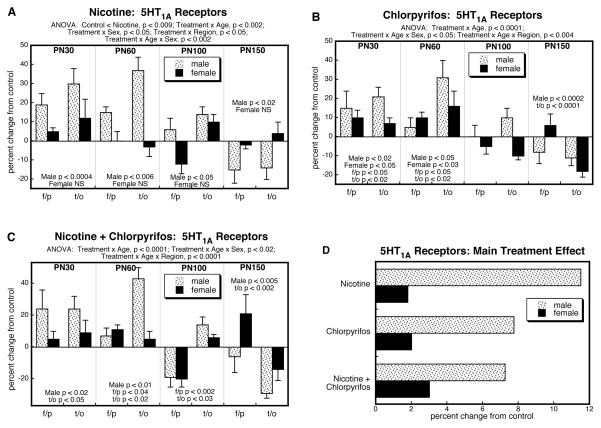
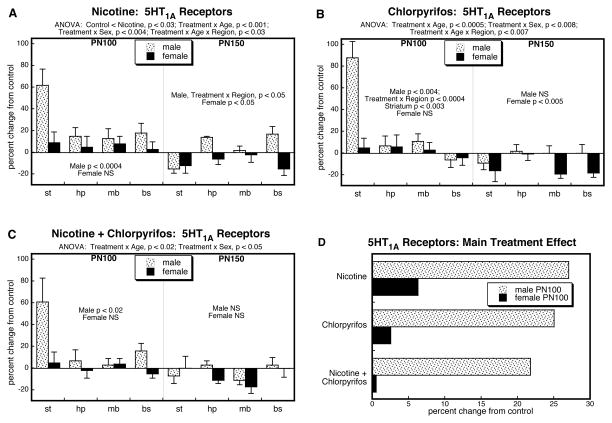
For 5HT1A receptors, ANOVA encompassing all four treatment groups identified a main effect of treatment (p < 0.05) and interactions of treatment × age (p < 0.0001), treatment × age ×sex (p < 0.02), and treatment × age × region (p < 0.0001). Regarding the prenatal and postnatal treatments as two separate factors in the analysis, we also found a significant nicotine ×chlorpyrifos interaction (p < 0.05), indicative of effects that could not be accounted for by simple summation of the individual effects of each agent; the two treatment factors also interacted with age (nicotine × chlorpyrifos × age, p < 0.009) but not region or sex.
By itself, prenatal nicotine exposure elicited upregulation of cerebrocortical 5HT1A receptors that was selective for males (Figure 1A). Superimposed on this main treatment effect, there were age and regional differences (interactions of treatment × age and treatment × region). Values were elevated in adolescence and early adulthood but eventually reversed, showing deficits by PN150. The initial increases were greater and more persistent in the temporal/occipital cortex compared to the frontal/parietal cortex. A similar pattern was seen in the group receiving just postnatal chlorpyrifos (Figure 1B), with initial increases in 5HT1A receptors, evolving to eventual deficits. Likewise, chlorpyrifos showed more rapid reversion in the frontal/parietal cortex as compared to temporal/occipital cortex. However, unlike nicotine, the effects of chlorpyrifos extended to females, albeit that the effects in males were still substantially greater. The group receiving both nicotine and chlorpyrifos showed a pattern qualitatively similar to those of the individual agents (Figure 1C). 5HT1A receptor binding was significantly elevated in males on PN30 and PN60, becoming subnormal in later adulthood, and with the decline emerging first in the frontal/parietal cortex.
Figure 1.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on 5HT1A receptor binding in frontal/parietal cortex (f/p) and temporal/occipital cortex (t/o), shown as the percent change from control values (see original values in Supplementary Table 1). Multivariate ANOVA for each treatment appears at the top of the panels. Where there were interactions of treatment with age, region and/or sex, the corresponding lower order tests are presented within the panels; in the absence of interactions, only the main treatment effects are given. Panel (D) shows the simple main treatment effects for each sex, collapsed across regions and ages. Abbreviation: NS, not significant.
To illustrate the differences in the main treatment effects among the three groups, we calculated the mean values for 5HT1A receptor binding, collapsed across the interactive variables (Fig. 1D). This simplified picture dilutes the effects seen for specific regions or ages by averaging them with data points for which there was no effect or an opposite effect, so that the absolute magnitude becomes smaller. Despite these limitations, there was an obvious overall pattern: the group receiving the combined exposure had lower values than those predicted from summation of the individual effects of nicotine and chlorpyrifos. This was verified by ANOVA treating nicotine and chlorpyrifos as two separate factors (nicotine × chlorpyrifos interaction, p < 0.05), indicative of an outcome that differed significantly from simple summation of the individual effects.
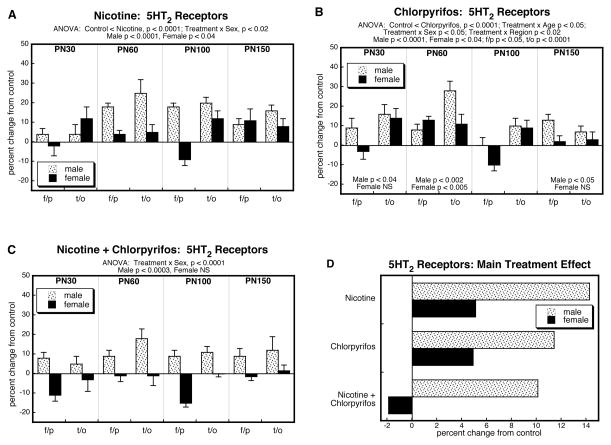
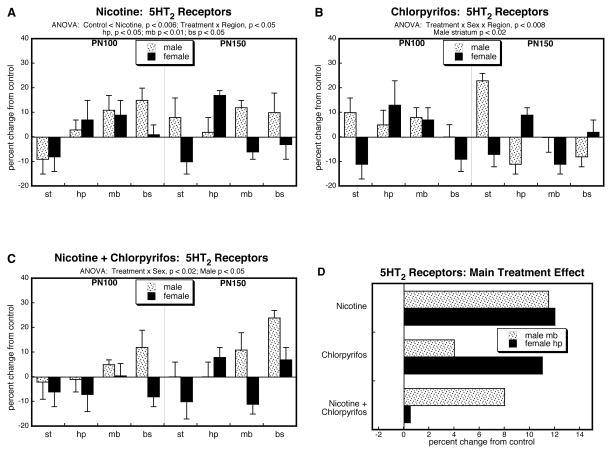
For 5HT2 receptors, ANOVA encompassing all four treatment groups again identified a main effect of treatment and interactions of treatment with the other variables: p < 0.0001 for treatment, p < 0.009 for treatment × age, p < 0.0002 for treatment × sex, p < 0.05 for treatment × region. Regarding the prenatal and postnatal treatments as two separate factors, we also identified a significant nicotine × chlorpyrifos interaction (p < 0.0001), pointing to less-than-additive effects of the two agents; this was interactive with region (nicotine × chlorpyrifos × region, p < 0.05), although both cerebrocortical regions maintained a significant interaction after subdivision of the data (nicotine × chlorpyrifos, p < 0.0001 for both frontal/parietal cortex and temporal/occipital cortex).
The group receiving just prenatal nicotine showed significant elevations in cerebrocortical 5HT2 receptors, with a greater effect in males than in females (Figure 2A); unlike the situation seen for 5HT1A receptors, the increase in 5HT2 receptors did not reverse in late adulthood. Again, the main treatment effects of chlorpyrifos were similar to those of nicotine, with significant increases in 5HT2 receptors that were preferentially greater in males (Figure 2B). When prenatal nicotine treatment was followed by postnatal chlorpyrifos, there were the same overall increases in males but in contrast, the effects were no longer significant in females (Figure 2C). These differences were further apparent when the treatment effects were collapsed across ages and regions (Figure 2D). In males, the group receiving combined exposure exhibited smaller increases than those seen with either agent alone, and clearly much less than would have been expected from summation of the individual effects; in females, there were increases with either agent alone, smaller than those seen in males, but the combined treatment actually produced a slight reduction in 5HT2 receptors instead of the larger increase that would have been predicted from additive effects. These differences were reflected in a significant nicotine × chlorpyrifos interaction (p < 0.0001).
Figure 2.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on 5HT2 receptor binding in frontal/parietal cortex (f/p) and temporal/occipital cortex (t/o), shown as the percent change from control values (see original values in Supplementary Table 2). Multivariate ANOVA for each treatment appears at the top of the panels. Where there were interactions of treatment with age, region and/or sex, the corresponding lower order tests are presented within the panels; in the absence of interactions, only the main treatment effects are given. Panel (D) shows the simple main treatment effects for each sex, collapsed across regions and ages. Abbreviation: NS, not significant.
Finally, we noted that the sex-selective effects of the various treatments were superimposed on underlying, normal sex differences in 5HT receptors, which showed a characteristically higher value in females than in males (Supplementary Tables 1 and 2). Accordingly, we compared the sex differences under each of the treatment conditions (ANOVA separated by treatment, with factors of sex, region and age). The controls showed a significant main effect of sex for both 5HT1A and 5HT2 receptors (female > male, p < 0.05 and p < 0.0001, respectively). These sex differences were eliminated (no main effect of sex) by nicotine treatment, chlorpyrifos treatment, or the combined exposure.
Cerebrocortical 5HT and 5HIAA levels
The global ANOVA, encompassing all the factors (treatment, age, brain region, sex) with the two dependent measures of 5HT and 5HIAA (repeated measures) identified a main effect of treatment (p < 0.004) and an interaction of treatment × measure (p < 0.005). Accordingly, we separated the data for the two measures and performed lower-order tests for treatment effects and interactions with the remaining variables. Regarding the treatments as two separate factors, we found a significant interaction of nicotine × chlorpyrifos × measure (p < 0.002) and therefore made the same comparisons separately for 5HT and 5HIAA.
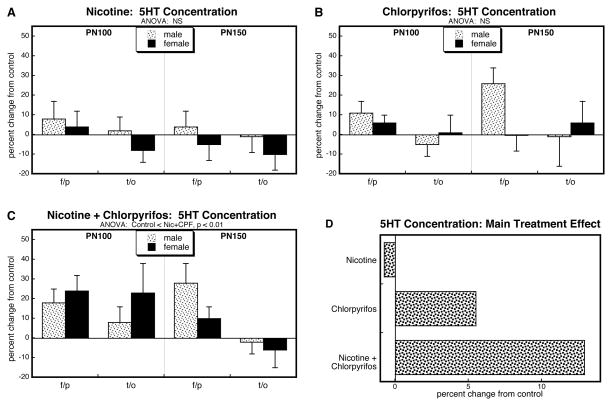
Analysis of 5HT levels for all four treatments identified a significant main treatment effect (p < 0.02) but no interactions of treatment with other variables, so values were separated for the individual treatments and reexamined for main treatment effects individually. We did not see any statistically significant changes in cerebrocortical 5HT levels with either prenatal nicotine (Figure 3A) or postnatal chlorpyrifos (Figure 3B), although there was a trend toward increased values in the latter group. Notably, though, the group receiving the combined treatment uniquely showed significant overall increases (Figure 3C). These differences were more apparent when the values were collapsed across sex, age and region to illustrate the main treatment effects (Figure 3D). Nicotine had little or no overall effect, chlorpyrifos caused a small increase, but the combination caused a much larger increase, significantly greater than would have been expected from the summation of the individual effects (nicotine × chlorpyrifos, p < 0.05).
Figure 3.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on 5HT levels in frontal/parietal cortex (f/p) and temporal/occipital cortex (t/o), shown as the percent change from control values (see original values in Supplementary Table 3). Multivariate ANOVA for each treatment appears at the top of the panels. Because treatment did not interact with the other variables, only the main treatment effects are given. Panel (D) shows the simple main treatment effects, collapsed across regions and ages, and combined for males and females because of the absence of a treatment × sex interaction. Abbreviation: NS, not significant.
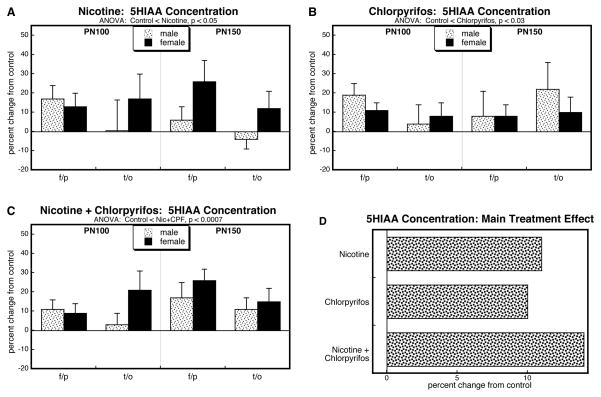
Just as for 5HT, cerebrocortical 5HIAA levels evaluated for all four treatment groups showed a main effect of treatment (p < 0.02) without interactions of treatment with the other variables. For the group receiving nicotine alone, there was a significant overall elevation in 5HIAA (Figure 4A). By itself, chlorpyrifos had very similar effects (Figure 4B) as did the group receiving combined treatment (Figure 4C). Analysis of the main treatment effects collapsed across sex, region and age showed the increases from each individual agent, and an effect of the combination that was not substantially greater than the individual treatments, but that was significantly smaller than would have been expected if the effects had been additive (nicotine × chlorpyrifos, p < 0.05).
Figure 4.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on 5HIAA levels in frontal/parietal cortex (f/p) and temporal/occipital cortex (t/o), shown as the percent change from control values (see original values in Supplementary Table 3). Multivariate ANOVA for each treatment appears at the top of the panels. Because treatment did not interact with the other variables, only the main treatment effects are given. Panel (D) shows the simple main treatment effects, collapsed across regions and ages, and combined for males and females because of the absence of a treatment × sex interaction.
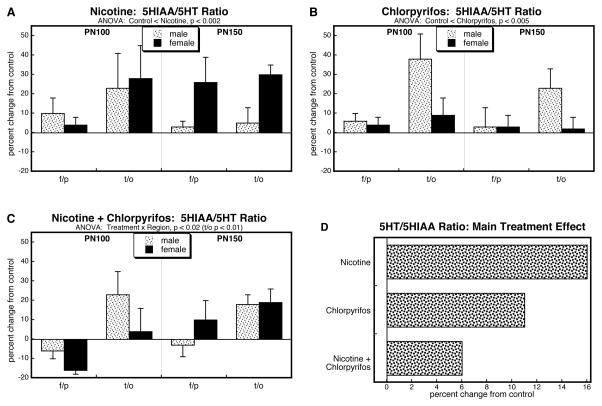
In light of the opposite directions obtained for nicotine × chlorpyrifos interactions for 5HT (synergistic) and 5HIAA (less than additive), we used the 5HT and 5HIAA values to calculate cerebrocortical 5HT turnover, determined by the 5HT/5HIAA ratio. The global ANOVA for turnover found a main treatment effect (p < 0.005). The turnover ratio was elevated by either nicotine (Figure 5A) or chlorpyrifos (Figure 5B) alone but the effect was less evident in the group receiving the combined treatment (Figure 5C). The values collapsed across sex, age and region (Figure 5D) showed a substantial main effect of nicotine, a slightly smaller effect of chlorpyrifos, and an even smaller effect in the group given both nicotine and chlorpyrifos that was clearly distinguishable from summation of the individual effects (nicotine × chlorpyrifos, p < 0.002).
Figure 5.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on 5HT turnover (5HIAA/5HT ratio) in frontal/parietal cortex (f/p) and temporal/occipital cortex (t/o), shown as the percent change from control values (see original values in Supplementary Table 3). Multivariate ANOVA for each treatment appears at the top of the panels. In A and B, treatment did not interact with the other variables, so only the main treatment effects are given; in C, the significant treatment × region interaction triggered a lower-order analysis of individual regions, shown at the top of the panel. Panel (D) shows the simple main treatment effects, collapsed across regions and ages, and combined for males and females because of the absence of a treatment × sex interaction.
5HT receptors in other regions
To determine whether the treatment effects seen in the cerebral cortex were shared by other brain regions, we conducted additional studies on PN100 and PN150, encompassing regions with 5HT nerve terminals (striatum, hippocampus) and cell bodies (midbrain, brainstem). Global ANOVA encompassing all factors (all treatments, age, brain region, sex) and both dependent measures (5HT1A and 5HT2 receptors; repeated measure) identified treatment interactions with all the variables: p < 0.04 for treatment × age, p < 0.05 for treatment × sex, p < 0.03 for treatment × region, p < 0.05 for treatment × sex × region, p < 0.001 for treatment × age × measure, p < 0.0001 for treatment × age × region × measure, and p < 0.05 for treatment × age × sex × region × measure. Regarding the nicotine and chlorpyrifos treatments as two separate factors, we found significant interactions of the two treatments with the other measures: p < 0.02 for nicotine × chlorpyrifos × age, p < 0.02 for nicotine × chlorpyrifos × age × measure, p < 0.05 for nicotine × chlorpyrifos × sex × measure, p < 0.0001 for nicotine × chlorpyrifos × age × region × measure, and p < 0.03 for nicotine × chlorpyrifos × age × sex × region × measure. Accordingly, results were separated into the individual treatments and receptor subtypes for further analyses.
Analysis of treatment effects on 5HT1A receptors in the four brain regions showed significant interactions of treatment with the other variables: p < 0.002 for treatment × age, p < 0.02 for treatment × sex, and p < 0.05 for treatment × age × region. By itself, prenatal nicotine treatment evoked significant upregulation of 5HT1A receptors that was preferential for males, was largest in the striatum and waned between PN100 and PN150 (Figure 6A). Postnatal chlorpyrifos exposure evoked a similar pattern (Figure 6B), as did the combined exposure to both nicotine and chlorpyrifos (Figure 6C). Importantly, the effects in the latter group were no larger than those seen with nicotine alone, so that the net effect was significantly less than would have been expected from additivity of the two individual agents. Accordingly, when nicotine and chlorpyrifos were treated as two separate factors, there was: (1) a nicotine × chlorpyrifos × age interaction (p < 0.004), reflecting the fact that the less-than-additive response involved primarily the PN100 age point (nicotine × chlorpyrifos, p < 0.005); (2) a nicotine × chlorpyrifos × sex interaction, reflecting the greater impact on males (nicotine × chlorpyrifos, p < 0.006) than females (not significant); and (3) a nicotine × chlorpyrifos × age × region interaction (p < 0.008), reflecting the fact that the predominant effect was in the striatum, with a more prominent interaction on PN100 (nicotine × chlorpyrifos, p < 0.009) than on PN150 (nicotine × chlorpyrifos, p < 0.05). To illustrate these interactions, we collapsed the main treatment effects across regions for PN100, the age point showing the significant overall nicotine × chlorpyrifos interaction (Figure 6D). Both nicotine and chlorpyrifos by themselves evoked robust overall increases in 5HT1A receptors in males, with a much smaller effect in females. The group receiving the combination treatment showed lower values than with either agent alone, well below the values expected from additive effects of nicotine and chlorpyrifos.
Figure 6.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on 5HT1A receptor binding in striatum (st), hippocampus (hp), midbrain (mb) and brainstem (bs), shown as the percent change from control values (see original values in Supplementary Table 1). Multivariate ANOVA for each treatment appears at the top of the panels. Where there were interactions of treatment with age, region and/or sex, the corresponding lower order tests are presented within the panels; in the absence of interactions, only the main treatment effects are given. Panel (D) shows the simple main treatment effects for each sex, collapsed across regions, for PN100, the age at which there was a significant nicotine × chlorpyrifos interaction (p < 0.005). Abbreviation: NS, not significant.
For 5HT2 receptors, the overall ANOVA found that treatment effects depended on the factors of region and sex, but not age: p < 0.007 for treatment × region, and p < 0.02 for treatment × region × sex. For nicotine treatment, there was an overall upregulation of 5HT2 receptors (main treatment effect) that was regionally-dependent, with significant increases in hippocampus, midbrain and brainstem (Figure 7A); we did not detect any sex-preference (no significant treatment × sex interaction). In contrast, chlorpyrifos specifically affected the male striatum, whereas effects in females or in other regions were more variable (Figure 7B). For the group receiving combined treatment, we found a significant sex difference (treatment × sex, p < 0.02), with preferential upregulation in males (Figure 7C); again, though, the upregulation was no larger than that seen for the individual agents, pointing to less-than-additive effects. Treating nicotine and chlorpyrifos as two separate treatment factors, we found a significant interaction that depended on sex and region (nicotine × chlorpyrifos × sex × region, p < 0.03). After subdivision of the data by those two variables, we found a nicotine × chlorpyrifos interaction in the male midbrain (p < 0.05) and the female hippocampus (p < 0.02). As shown in Figure 7D, these interactions once again reflected clearly less-than-additive effect of the combination treatment and indeed, for females, the combination had a far lower effect than for either individual agent.
Figure 7.
Effects of nicotine (A), chlorpyrifos (B), and combined treatment (C) on 5HT2 receptor binding in striatum (st), hippocampus (hp), midbrain (mb) and brainstem (bs), shown as the percent change from control values (see original values in Supplementary Table 2). Multivariate ANOVA for each treatment appears at the top of the panels, along with lower order tests for individual regions or sexes as permitted by the appropriate interaction terms.. Panel (D) shows the simple main treatment effects for each sex, collapsed across age, for the individual regions showing a significant nicotine × chlorpyrifos interaction (male midbrain, p < 0.05; female hippocampus, p < 0.02). Abbreviation: NS, not significant.
Like the cerebrocortical regions, control values for 5HT receptors in the other four regions showed higher values in females than males (main effect of sex, p < 0.006 for 5HT1A, p < 0.03 for 5HT2; Supplementary Tables 1 and 2). Again, these sex differences were no longer apparent in any of the three treatment groups.
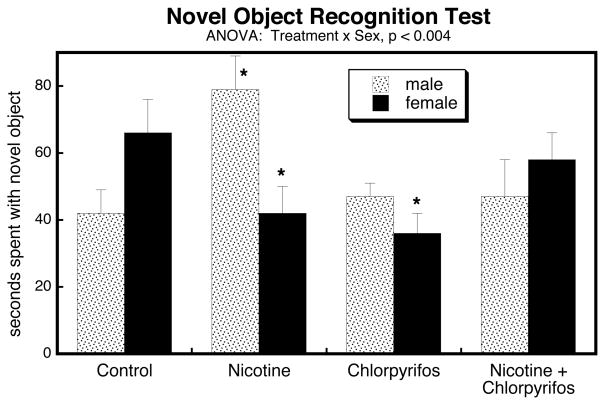
Novel Object Recognition Test
Treatment effects on performance in the novel object recognition test were highly sex-dependent (Figure 8). Whereas controls showed the typical pattern of females spending more time spent with the novel object than males, this preference was reversed in the groups exposed either to nicotine or chlorpyrifos alone (treatment × sex interaction, p < 0.002 and p < 0.02, respectively). Nicotine raised the value in males while suppressing it in females; chlorpyrifos reduced the value in females while leaving males unchanged. The group receiving combined exposure showed a slight (nonsignificant) increase in time spent with the novel object in males, and a small (nonsignificant) decrease in females; nevertheless, these trends reduced the sex difference normally seen for this test, and the effects were significantly non-additive for each sex (nicotine × chlorpyrifos × sex, p < 0.0009) and in the opposite direction from the effects of nicotine alone (treatment × sex, p < 0.02).
Figure 8.
Effects of nicotine, chlorpyrifos and combined treatment on performance in the Novel Object Recognition Test. ANOVA appears at the top of the panel and asterisks denote values that differ significantly from the corresponding control.
DISCUSSION
In our earlier work, we showed that postnatal chlorpyrifos exposure leads to upregulation of 5HT receptors preferentially in males, comprising both direct effects on 5HT synaptic development and adaptive responses to injury (Aldridge et al., 2004, 2005b; Slotkin et al., 2014; Slotkin and Seidler, 2005). In particular, 5HT hyperactivity served to replace lost cholinergic function, leading to situations where responses normally mediated by acetylcholine instead became dependent on 5HT (Aldridge et al., 2005a). In the current study, we found that prenatal nicotine exposure likewise led to later-life upregulation of 5HT receptors, again, preferentially in males; for both nicotine and chlorpyrifos individually, the effects on receptor expression showed distinct temporal and regional patterns, compatible with adaptive change rather than just an extension of the initial injury. It was thus surprising that when the two exposure paradigms were combined, we did not find an augmented effect and instead, actually observed a much smaller degree of upregulation than would be expected from summation, and often less than with either agent alone. There are two possibilities: either nicotine is protecting the developing nervous system from the subsequent effects of chlorpyrifos, or the combination is suppressing the emergence of adaptive responses to the initial injury. As shall be presented below, the latter is more likely.
Nicotine can provide protection against a variety of neurotoxic insults, in part by promoting neurotrophic activity (Belluardo et al., 2000; Ferrea and Winterer, 2009; Kawamata and Shimohama, 2011). Indeed, we previously found that nicotine can protect neuronotypic cells in culture from the direct antimitotic and oxidative effects of chlorpyrifos (Qiao et al., 2003, 2005; Slotkin et al., 2007a). Nevertheless, using the same in vivo treatment model as in the present study, we found that prolonged prenatal nicotine exposure actually worsened the impact of chlorpyrifos on the development of cholinergic synaptic function (Slotkin and Seidler, 2015), reflecting the fact that nicotine itself is a developmental neurotoxicant (Slotkin, 2008). Thus, the question is whether the present results, a smaller-than-expected upregulation of cerebrocortical 5HT receptors from combined exposure relative to the predicted, additive actions of the two agents, is a reflection of neuroprotection. If nicotine is serving solely as a neuroprotectant, then the net effect of combined exposure should be no different from that of nicotine alone. Instead, though, we found that receptor expression in animals given both nicotine and chlorpyrifos was lower than that of nicotine, and typically lower than that of chlorpyrifos as well. Indeed, for females, the combined exposure produced a net reduction in cerebrocortical 5HT2 receptor expression, whereas each agent alone elicited an increase. This pattern of smaller upregulation repeated itself in the other brain regions as well, showing that the unexpected interaction is occurring in 5HT projections throughout the brain.
Sequential exposure to nicotine followed by chlorpyrifos thus produces a unique suppression of 5HT receptor expression relative to that achieved by the individual agents. Since many of the effects of chlorpyrifos on 5HT systems represent adaptations to the initial injury (Aldridge et al., 2004, 2005a, 2005b; Slotkin et al., 2014; Slotkin and Seidler, 2005), we next pursued the issue of whether a reduced effect on 5HT receptor expression was a reaction to enhanced 5HT presynaptic activity, which would be expected to downregulate 5HT receptors; if this were the explanation for the lowered effect of combined treatment, then we would expect to see a greater activation of 5HT presynaptic input, reflected in higher transmitter turnover, but in fact, we found the opposite (reduced turnover). The group receiving combined exposure did have higher 5HT concentrations than seen with just nicotine or chlorpyrifos individually, which could reflect either an increased density of innervation, or a reduction in 5HT turnover, leading to accumulation of 5HT in presynaptic terminals. The latter conclusion was substantiated: there was no corresponding increase in 5HIAA in the combined exposure group compared to individual treatments, and the turnover (5HIAA/5HT ratio) was substantially lower than with nicotine or chlorpyrifos alone, indicating a reduction in the amount of 5HT actually entering the synaptic cleft. The combined treatment thus produced a situation where presynaptic activity was lowered, and instead of evoking a further compensatory increase in 5HT receptor expression, the receptors were likewise reduced relative to the values of the individual agents. The net effect is thus an impairment of the adaptive responses of 5HT synapses to chlorpyrifos-induced injury. This conclusion is reinforced by similar findings in our previous work with the combination of prenatal dexamethasone and postnatal chlorpyrifos, which likewise found interference with compensatory adaptations, thus worsening the outcomes for synaptic and behavioral performance (Levin et al., 2014; Slotkin et al., 2013, 2014).
The Novel Object Recognition Test provided an opportunity to test the conclusion that nicotine did not simply protect the developing brain from the adverse effects of chlorpyrifos. This test normally shows distinct sex differences that are dependent on 5HT systems, with females showing greater time with the novel object compared to males (Meltzer et al., 2011; Sutcliffe et al., 2007). Accordingly, we made our assessment during the period of peak effects on 5HT receptors. Nicotine alone reversed the sex preference by increasing performance in males and decreasing it in females; in contrast, chlorpyrifos had little effect on males but lowered values substantially in females, thus eliminating the sex difference. The group receiving both agents showed slightly higher values in males and slightly lower values in females, with neither effect individually significant, but the combined effects still lowered the sex difference to the point of nonsignificance. There was a mechanistic connection of these behavioral findings to the 5HT receptor measurements: normal sex differences in receptor expression were eliminated by the same treatments. Although the Results section provides the statistical outcomes supporting the loss of sex differences for each receptor subtype separately for cerebrocortical and other regions, to illustrate this conclusion more clearly, we collapsed the values across all brain regions, all time points and both receptor subtypes (Figure 9); in the control group, receptor binding was highly significantly greater in females than in males (p < 0.003), whereas there were no significant differences in the groups receiving nicotine, chlorpyrifos, or the combined treatment.
Figure 9.
Overall sex differences in 5HT receptor binding, collapsed across all brain regions, all age points and both receptor subtypes. Data are geometric means derived from the data in Supplemental Tables 1 and 2. The sex difference is statistically significant for controls (p < 0.003 for main effect of sex), but not for any of the other treatment groups.
Finally, our results, showing an evolving pattern of changes in 5HT function over the lifespan, reinforce the concept that neurogenesis and formation of synaptic circuits, ordinarily considered to be primarily “developmental” events, actually continue into adulthood and senescence; in the present study, this was evident in the progressive changes in 5HT receptors and 5HT turnover occurring between adolescence, young adulthood, and full adulthood. In general, 5HT function and associated neurochemical markers achieve adult values by three months of age in the rat, but then show marked declines beginning at about six months, just past the final age point that we evaluated here (Nyakas et al., 1997; Slotkin et al., 2007b). It would be extremely important to evaluate in future studies, whether nicotine, chlorpyrifos, or the combined exposure accelerates the age-related decline in 5HT function. We have already shown such an effect after early-life exposure to another organophosphate pesticide, parathion (Levin et al., 2010).
The present results thus show that prenatal nicotine treatment changes the response of 5HT systems to subsequent chlorpyrifos exposure, specifically leading to impairment of adaptive increases in 5HT receptors and presynaptic activity; hence the combined treatment produces a global interference with 5HT synaptic function not seen with either agent alone. Because the unique effect is superimposed on the direct actions of each toxicant, the smaller upregulation of 5HT receptors could be mistaken for partial neuroprotection by nicotine, but evaluation of presynaptic activity and of the temporal, regional and sex-dependent patterns indicates otherwise. This mirrors our conclusions for the effects of nicotine on the developmental neurotoxicity of chlorpyrifos directed toward cholinergic pathways (Slotkin and Seidler, 2015) and resembles our findings for prenatal dexamethasone treatment, which likewise interfered with adaptive responses to chlorpyrifos, thus augmenting the deterioration of synaptic function and behavioral performance (Levin et al., 2014; Slotkin et al., 2013, 2014). In addition to the known liabilities associated with maternal smoking during pregnancy, our results point to additional costs in the form of heightened vulnerability to neurotoxic chemicals encountered later in life.
Supplementary Material
Nicotine (maternal smoking) and organophosphate pesticide coexposures are common
We gave nicotine to rats prenatally, followed by neonatal chlorpyrifos
Combined exposure uniquely suppressed indices of serotonin synaptic function
Behavior (Novel Object Recognition Test) paralleled neurochemical effects
Nicotine exposure may create a subpopulation that is vulnerable to neurotoxicants
Acknowledgments
Research was supported by NIH ES010356. The authors thank Jennifer Card, Marty Cauley, Dennis Burke and Brandon J. Hall for technical assistance.
Abbreviations
- 5HIAA
5-hydroxydindoleacetic acid
- 5HT
5-hydroxytryptamine, serotonin
- ANOVA
analysis of variance
- GD
gestational day
- PN
postnatal day
Footnotes
Disclaimer: TAS has received consultant income in the past three years from the following firms: Acorda Therapeutics (Ardsley NY), The Calwell Practice (Charleston WV), Carter Law (Peoria IL), Gutglass Erickson Bonville & Larson (Madison WI), Alexander Hawes (San Jose, CA), Pardieck Law (Seymour, IN), Tummel & Casso (Edinburg, TX), and Chaperone Therapeutics (Research Triangle Park, NC).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005a;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005b;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin-1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Blum M, Fuxe K. Central nicotinic receptors, neurotrophic factors and neuroprotection. Behav Brain Res. 2000;113:21–34. doi: 10.1016/s0166-4328(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Beseler C, Stallones L, Hoppin JA, Alavanja MCR, Blair A, Keefe T, Kamel F. Depression and pesticide exposures in female spouses of licensed pesticide applicators in the Agricultural Health Study Cohort. J Occup Environ Med. 2006;48:1005–1013. doi: 10.1097/01.jom.0000235938.70212.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beseler CL, Stallones L, Hoppin JA, Alavanja MCR, Blair A, Keefe T, Kamel F. Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study. Environ Health Perspect. 2008;116:1713–1719. doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Chen WQ, Yuan L, Xue R, Li YF, Su RB, Zhang YZ, Li J. Repeated exposure to chlorpyrifos alters the performance of adolescent male rats in animal models of depression and anxiety. Neurotoxicology. 2011;32:355–361. doi: 10.1016/j.neuro.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Clifford A, Lang L, Chen R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol Teratol. 2012;34:560–570. doi: 10.1016/j.ntt.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Day NL. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol. 2009;22:121–125. doi: 10.1097/WCO.0b013e328326f6dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prevent Med. 2010a;39:45–52. doi: 10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Homa D, England LJ, Burley K, Tong VT, Dube SR, Bernert JT. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2010b;173:355–359. doi: 10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and Sudden Infant Death Syndrome. J Family Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiat. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Ferrea S, Winterer G. Neuroprotective and neurotoxic effects of nicotine. Pharmacopsychiatry. 2009;42:255–265. doi: 10.1055/s-0029-1224138. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VKY. Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. J Appl Physiol. 2001;90:1968–1976. doi: 10.1152/jappl.2001.90.5.1968. [DOI] [PubMed] [Google Scholar]
- Fujita M, Charney DS, Innis RB. Imaging serotonergic neurotransmission in depression: hippocampal pathophysiology may mirror global brain alterations. Biol Psychiat. 2000;48:801–812. doi: 10.1016/s0006-3223(00)00960-4. [DOI] [PubMed] [Google Scholar]
- Ginzel KH, Maritz GS, Marks DF, Neuberger M, Pauly JR, Polito JR, Schulte-Hermann R, Slotkin TA. Nicotine for the fetus, the infant and the adolescent? J Health Psychol. 2007;12:215–224. doi: 10.1177/1359105307074240. [DOI] [PubMed] [Google Scholar]
- Goodman E, Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106:748–755. doi: 10.1542/peds.106.4.748. [DOI] [PubMed] [Google Scholar]
- Herrmann M, King K, Weitzman M. Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment. Curr Opinion Pediatr. 2008;20:184–190. doi: 10.1097/MOP.0b013e3282f56165. [DOI] [PubMed] [Google Scholar]
- Jaga K, Dharmani C. The interrelation between organophosphate toxicity and the epidemiology of depression and suicide. Rev Environ Health. 2007;22:57–73. doi: 10.1515/reveh.2007.22.1.57. [DOI] [PubMed] [Google Scholar]
- Kawamata J, Shimohama S. Stimulating nicotinic receptors trigger multiple pathways attenuating cytotoxicity in models of Alzheimer’s and Parkinson’s diseases. J Alzheimer Dis. 2011;24(Suppl 2):95–109. doi: 10.3233/JAD-2011-110173. [DOI] [PubMed] [Google Scholar]
- Lee S, Poet TS, Smith JN, Busby-Hjerpe AL, Timchalk C. Effect of in vivo nicotine exposure on chlorpyrifos pharmacokinetics and pharmacodynamics in rats. Chem Biol Interact. 2010;184:449–457. doi: 10.1016/j.cbi.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Alavanja MCR, Hoppin JA, Rusiecki JA, Kamel F, Blair A, Sandler DP. Mortality among pesticide applicators exposed to chlorpyrifos in the agricultural health study. Environ Health Perspect. 2007;115:528–534. doi: 10.1289/ehp.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Cauley M, Johnson J, Cooper EM, Stapleton HM, Ferguson PL, Seidler FJ, Slotkin TA. Prenatal dexamethasone augments the neurobehavioral teratology of chlorpyrifos: significance for maternal stress and preterm labor. Neurotoxicol Teratol. 2014;41:35–42. doi: 10.1016/j.ntt.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Timofeeva OA, Yang L, Ryde IT, Wrench N, Seidler FJ, Slotkin TA. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav Brain Res. 2010;208:319–327. doi: 10.1016/j.bbr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–157. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Horiguchi M, Massey BW. The role of serotonin in the NMDA receptor antagonist models of pychosis and cognitive impairment. Psychopharmacology. 2011;213:289–305. doi: 10.1007/s00213-010-2137-8. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, Kato H, Suzuki MR, Takigawa M. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Dev Brain Res. 1997;102:117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Ferrer JR, Wanyun Z, Haley NJ. Nicotine administration to rats: methodological considerations. Life Sci. 1987;40:1699–1708. doi: 10.1016/0024-3205(87)90020-8. [DOI] [PubMed] [Google Scholar]
- Nyakas C, Oosterink BJ, Keijser J, Felszeghy K, Dejong GI, Korf J, Luiten PGM. Selective decline of 5-HT1a receptor binding sites in rat cortex, hippocampus and cholinergic basal forebrain nuclei during aging. J Chem Neuroanat. 1997;13:53–61. doi: 10.1016/s0891-0618(97)00025-2. [DOI] [PubMed] [Google Scholar]
- Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids: fetal effect or maternal influence? J Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Pædiatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. 7-Year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera R, Andrews H, Hoepner L, Barr D, Whitehead D, Tang D, Whyatt RM. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, et al. Brain anomalies in children exposed to a common organophosphate pesticide. Proc Natl Acad Sci. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD. The association between prenatal exposure to cigarettes and infant and maternal negative affect. Infant Behav Dev. 2007;30:387–398. doi: 10.1016/j.infbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction — developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Toxicity of Organophosphate and Carbamate Pesticides. San Diego: Elsevier Academic Press; 2005. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos; pp. 293–314. [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Infante A, Seidler FJ. Prenatal dexamethasone augments the sex-selective developmental neurotoxicity of chlorpyrifos: implications for vulnerability after pharmacotherapy for preterm labor. Neurotoxicol Teratol. 2013;37:1–12. doi: 10.1016/j.ntt.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Seidler FJ. Chlorpyrifos developmental neurotoxicity: interaction with glucocorticoids in PC12 cells. Neurotoxicol Teratol. 2012;34:505–512. doi: 10.1016/j.ntt.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Seidler FJ. Prenatal dexamethasone, as used in preterm labor, worsens the impact of postnatal chlorpyrifos exposure on serotonergic pathways. Brain Res Bull. 2014;100:44–54. doi: 10.1016/j.brainresbull.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006a;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Developmental neurotoxicity of parathion: progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol Teratol. 2009a;31:11–17. doi: 10.1016/j.ntt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect. 2007a;115:1306–1313. doi: 10.1289/ehp.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Tate CA, Seidler FJ. Alterations of serotonin synaptic proteins in brain regions of neonatal Rhesus monkeys exposed to perinatal environmental tobacco smoke. Brain Res. 2006b;1111:30–35. doi: 10.1016/j.brainres.2006.06.094. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Tate CA, Seidler FJ. Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: separate or sequential exposure during fetal development and adulthood. Brain Res Bull. 2007b;73:259–272. doi: 10.1016/j.brainresbull.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res Bull. 2007;73:301–309. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Mimicking maternal smoking and pharmacotherapy of preterm labor: interactions of fetal nicotine and dexamethasone on serotonin and dopamine synaptic function in adolescence and adulthood. Brain Res Bull. 2010;82:124–134. doi: 10.1016/j.brainresbull.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal nicotine alters the developmental neurotoxicity of postnatal chlorpyrifos directed toward cholinergic systems: better, worse, or just “different? Brain Res Bull. 2015;110:54–67. doi: 10.1016/j.brainresbull.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the response to subsequent nicotine administration and withdrawal in adolescence: serotonin receptors and cell signaling. Neuropsychopharmacology. 2006c;31:2462–2475. doi: 10.1038/sj.npp.1300988. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Wrench N, Ryde IT, Lassiter TL, Levin ED, Seidler FJ. Neonatal parathion exposure disrupts serotonin and dopamine synaptic function in rat brain regions: modulation by a high-fat diet in adulthood. Neurotoxicol Teratol. 2009b;31:390–399. doi: 10.1016/j.ntt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177:117–125. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, et al. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res Bull. 2008;77:404–411. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- U.S. Surgeon General. The Health Consequences of Smoking - 50 Years of Progress. Rockville: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiat. 2002;41:1294–1305. doi: 10.1097/00004583-200211000-00010. [DOI] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Tobacco or Health: A Global Status Report. Geneva: World Health Organization; 1997. [Google Scholar]
- Wu LT, Anthony JC. Tobacco smoking and depressed mood in late childhood and early adolescence. Am J Public Health. 1999;89:1837–1840. doi: 10.2105/ajph.89.12.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Dennie J, Shiah IS, Adam MJ, Lane CJ, Lam RW, Ruth TJ. Decrease in brain serotonin-2 receptor binding in patients with major depression following desipramine treatment: a positron emission tomography study with fluorine-18-labeled setoperone. Arch Gen Psychiat. 1999;56:705–711. doi: 10.1001/archpsyc.56.8.705. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ. Brain serotonin-2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiat. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.