Abstract
Coinhibitory receptor blockade is a promising strategy to boost T-cell immunity against a variety of human cancers. However, many patients still do not benefit from this treatment, and responders often experience immune-related toxicities. These issues highlight the need for advanced mechanistic understanding to improve patient outcomes and uncover clinically relevant biomarkers of treatment efficacy. However, the T cell-intrinsic signaling pathways engaged during checkpoint blockade treatment are not well defined, particularly for combination approaches. Using a murine model to study how effector CD8+ T-cell responses to tumors may be enhanced in a tolerizing environment, we identified a critical role for the T-box transcription factor T-bet. Combination blockade of CTLA-4, PD-1, and LAG-3 induced T-bet expression in responding tumor/self-reactive CD8+ T cells. Eradication of established leukemia using this immunotherapy regimen depended on T-bet induction, which was required for IFNγ production and cytotoxicity by tumor-infiltrating T cells, and for efficient trafficking to disseminated tumor sites. These data provide new insight into the success of checkpoint blockade for cancer immunotherapy, revealing T-bet as a key transcriptional regulator of tumor-reactive CD8+ T-cell effector differentiation under otherwise tolerizing conditions.
Keywords: checkpoint blockade, adoptive immunotherapy, T cells, T-bet, Eomesodermin
INTRODUCTION
CD8+ T cells are important for tumor-surveillance and play a major role in controlling tumor growth (1, 2). However, mechanisms of immune evasion undermine T-cell activity and promote disease (3). The quality of CD8+ T-cell responses to cancer is often dictated by environmental cues received during priming and after encounter with tumor cells. These signals determine the amplitude of the CD8+ T-cell response, and can be either stimulatory or inhibitory in nature. While costimulatory signals induce T-cell differentiation and cytokine production associated with effective immunity, coinhibitory signals subvert costimulation to limit CD8+ T-cell function (4-6). These coinhibitory pathways are essential to maintain tolerance toward self-tissues and prevent autoimmunity, but also impede immune responses against cancer (7). Studies in animal models have demonstrated that attenuation of such tolerizing signals using blocking antibodies (i.e. checkpoint blockade) can boost antitumor immunity (8-10), and these strategies have been translated into clinical successes in human cancer patients (11, 12). Although checkpoint blockade is reshaping the landscape of cancer immunotherapy, little is known about the CD8+ T-cell intrinsic pathways required to achieve therapeutic benefits. Additional unknowns surround the cellular mechanisms that lead to adverse immune-related toxicities in patients treated with checkpoint blockade antibodies, which may intriguingly coincide with therapeutic efficacy (13).
The T-box transcription factors, Eomesodermin (Eomes) and T-bet, have important and well-described roles in CD8+ T-cell activation, differentiation, and memory formation (14-17). These T-box-mediated operations are undeniably valuable for the generation of antitumor immunity, which has been directly demonstrated in mouse models of cancer (18). More importantly, the induction of T-bet in tumor-infiltrating lymphocytes (TIL) has been correlated with positive outcomes in patients with colorectal cancer (19). However, there is only sparse evidence that coinhibitory receptor pathways influence T-bet and Eomes expression in T cells (20, 21), leaving the exact relationship between these transcriptional regulators and checkpoint blockade immunotherapy largely undefined.
We recently demonstrated that combination antibody blockade of CTLA-4, PD-1, and LAG-3 improved IFNγ production and cytotoxicity by transferred tumor/self-reactive CD8+ T cells during cancer immunotherapy (8). These effector responses hint at a possible link to T-box transcription factor-mediated CD8+ T-cell differentiation. We now report that low expression of T-bet and Eomes defines dysfunctional T cells rendered tolerant in vivo by encounter with tumor/self-antigen. Therapeutic intervention with combination checkpoint blockade (i.e. anti-CTLA-4, PD-1, and LAG-3) induced both T-bet and Eomes expression in responding T cells under these same tolerizing conditions, but only T-bet was required for restored effector function. T-bet was predictably important for expression of known T-bet target genes such as ifng and gzmb, but also for expression of other effector genes not previously associated with T-bet transcriptional regulation. Moreover, T-bet expression in transferred tumor/self-reactive T cells was necessary to achieve cure rates in better than 95% of leukemia-bearing mice treated with checkpoint blockade immunotherapy. These results provide a new understanding into the molecular mechanisms engaged during checkpoint blockade treatment that likely transform the immune response against cancer by facilitating direct cytolytic killing of tumors by infiltrating CD8+ T cells.
MATERIALS AND METHODS
Mice
Alb:Gag, and rag1−/− TCRGag transgenic mice on wild type and tbx21−/−, eomesf/f or T-bet-Tg backgrounds have been described (8, 17). C57BL/6 (B6) and CD90.1 (Thy1.1) congenic mice were purchased from The Jackson Laboratory. T-bet-ZsGreen reporter mice were obtained from Taconic and described previously (22), and were crossed with rag1−/− TCRGag transgenic mice. All mice were maintained under specific pathogen-free conditions, and used in accordance with our animal protocol approved by the Animal Care Committee of the Department of Comparative Medicine, Saint Louis University School of Medicine.
Cell lines, peptides, and antibodies
FBL is a tumor line that expresses an immunogenic virus-derived H-2b-restricted Gag epitope as described (8) and was a gift from Dr. Philip Greenberg (University of Washington, Seattle WA). FBL have not been authenticated. Gag (CCLCLTVFL) and control ovalbumin (SIINFEKL) peptides were purchased from Pi Proteomics. Cell culture was performed in complete high glucose DMEM with 10% fetal bovine serum (Sigma, St. Louis MO). Flow cytometry, staining and ex vivo stimulations were performed as previously described (8), and nuclear staining for transcription factors was performed according to manufacturer's protocol (eBioscience). Antibodies used here are described in Supplemental Methods.
Adoptive T-cell transfer
Gag-specific T cells were isolated from spleens and lymph nodes (LN) of indicated rag1−/− TCRGag donors. Cell suspensions containing 3×106 Vα3-TCR+ CD8+ were intravenously (i.v.) injected into sex- and (6-12 week old) age-matched recipients. Mice treated with checkpoint blockade received 100μg each of anti-CTLA-4 and anti-PD-1 and anti-LAG-3 (blockade) intraperitoneally (i.p.). In vivo killing assays were performed as previously described (8).
Immunotherapy assay
Disseminated FBL leukemia was established in Alb:Gag mice by intravenous injection with 5×104 viable FBL tumor cells. One week later, tumor-bearing mice received blockade antibodies and adoptive transfers of 1×106 Gag-reactive CD8+ T cells. Recipient survival was tracked out to 75 days with daily health monitoring.
Microarray
Naive Gag-specific T cells were transferred into B6 mice with established FBL tumor (immune), or into Alb:Gag mice (tolerant). Two days later, transferred T cells were sorted based on CD8+ CD90.1+ CD69hi expression to >96% purity using a FACSAria III (BD Biosciences), and RNAs were isolated from sorted cells using RNeasy Plus Mini Kit (QIAGEN). Samples were hybridized to a GeneChip® Mouse Genome 430 2.0 Array and scanned using a GeneChip scanner 3000 7G (Affymetrix). Results were obtained from 3 biological replicates per condition. All data have been deposited in the Gene Expression Omnibus (GEO) with accession code GSE58722.
Real-time quantitative PCR
T cells were sorted to >95% purity and total RNA isolated using an RNeasy Plus Mini Kit (QIAGEN) and cDNA synthesized using SuperScript® III RT (Life Technologies). Quantitative real-time PCR (qRT-PCR) was performed with SYBR® Select Master Mix (Life Technologies) on a 7500 Fast Real-Time PCR System (Applied Biosystems). Beta-actin was used as the endogenous amplification control. Primer sequences are listed in the Supplemental Methods.
Statistical analysis
The Kruskal-Wallis test was used for all cell frequency comparisons. Survival data were analyzed with the log-rank test. P values of less than 0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism 4.
RESULTS & DISCUSSION
To uncover the intrinsic mechanisms that dictate whether CD8+ T cells become tolerant or differentiate into effector cells after priming, we compared the gene expression profiles of T cells shortly after in vivo encounter with antigen (Gag) expressed in distinct contexts. Specifically, naive Gag-specific CD8+ T cells were transferred into B6 mice with an established immunogenic Gag-positive FBL leukemia (immune), or into Alb:Gag mice that express the same Gag protein as a tolerizing self-antigen in healthy hepatocytes (tolerant). To be clear, T-cell tolerance in this model system is due to self-antigen encounter, regardless of the presence of FBL tumor (8, 23). Two days after transfer, genes encoding the negative regulatory receptors PD-1 (pdcd1), CTLA-4, and LAG-3 were induced under immune and tolerizing conditions, but expression was uniquely high in tolerant T cells (Fig. 1A and Supplemental Fig. S1). Expression of the gene encoding the lymphoid homing molecule L-selectin (sell) remained high under tolerizing conditions, whereas cd44 expression was limited mostly to immunized T cells (Fig. 1A). These results confirmed our previous protein expression analysis (8), providing confidence that this transcriptional profile accurately reflects the biology of CD8+ T cells actively receiving tolerizing signals in vivo.
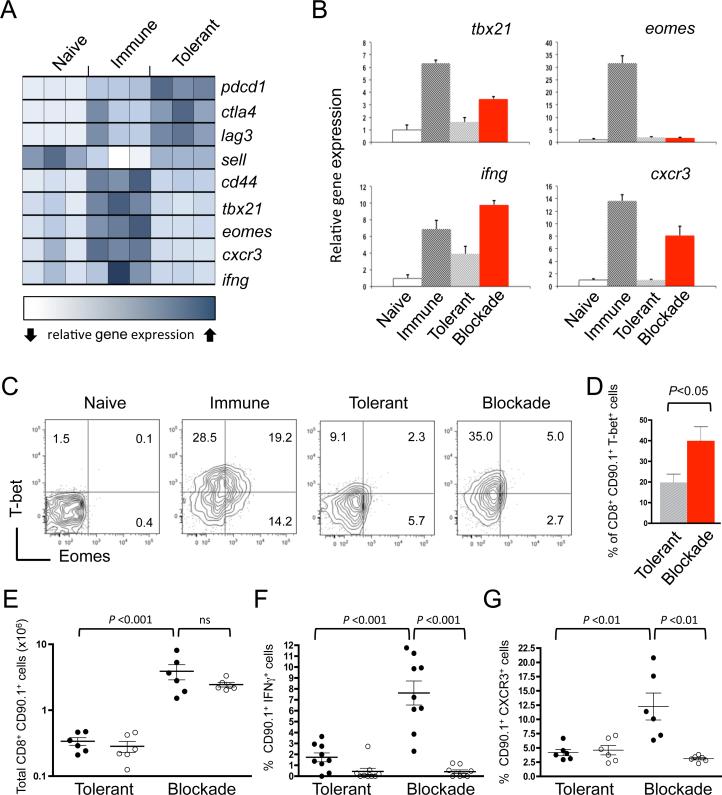
Figure 1. T-bet is induced by combination checkpoint blockade.
Naive Gag-specific CD8+ T cells (CD90.1+) were transferred into B6 mice bearing an immunogenic FBL tumor (immune), Alb:Gag mice (tolerant), or Alb:Gag mice treated with checkpoint blockade antibodies (blockade). (A) Two days after transfer, T cells were FACS purified and RNA isolated for gene expression analysis by microarray. Each square represents one biological triplicate for each experimental condition. (B) Transferred CD8+ T cells from the indicated environments were FACS purified after 3 days in vivo, and relative gene expression assessed by qRT-PCR normalized to actin. Error bars depict SD. (C) T-bet and Eomes intracellular protein expression was determined in Gag-reactive T cells 3 days after transfer into the indicated recipients. Quadrants were set based on T-bet and Eomes expression in naive T cells from B6 mice (left). Inset numbers within contour plots are the percent of CD90.1+ CD8+ cells in the quadrant fields. (D) Pooled data from 3 independent experiments showing the frequency of T-bet+ CD8+ T cells in recipient spleens 3 days after transfer. (E) Naive WT (●) or tbx21−/− (○) T cells were transferred into the tolerant or blockade environment and the total number of transferred T cells (CD8+ CD90.1+) in spleens was determined 4 days after infusion. Graph shows pooled data from 2 separate experiments. (F) Splenocytes were stimulated overnight and the frequency of CD8+ CD90.1+ T cells producing IFNγ was determined by intracellular flow cytometry; graphs are pooled from 3 experiments. (G) The percent of CD8+ CD90.1+ T cells expressing CXCR3 is graphed from 2 pooled experiments. Each circle represents individual recipient mice, horizontal lines depict the mean, and error bars indicate SEM, unless otherwise noted. P-values are indicated for the bracketed groups (ns=not significant).
In contrast to immunized T cells, those within the tolerizing environment lacked expression of the T-box transcription factors T-bet (tbx21) and Eomesodermin (eomes), and the reported T-box target genes, cxcr3 and ifng (Fig. 1A). Because T-bet and Eomes are important for CD8+ T-cell differentiation into cytolytic effector cells (14-16), the failure to express these transcription factors may explain why T cells proliferate but do not acquire effector functions under tolerizing conditions. An intriguing extrapolation of this hypothesis is that simultaneous blockade of CTLA-4, PD-1 and LAG-3, which promotes effector T cell differentiation and function under otherwise tolerizing conditions (8), does so by promoting T-box transcription factor activity and subsequent expression of effector target genes. Defining such a relationship would provide novel mechanistic insight into the success of combination checkpoint blockade immunotherapy for patients with cancer (11, 12).
Checkpoint blockade induces T-bet expression in tumor/self-reactive CD8+ T cells
To determine if checkpoint blockade influenced T-box transcription factor expression, T cells were again transferred into immunizing and tolerizing environments. An additional “blockade” condition was also examined in which tolerizing Alb:Gag recipients were treated with anti-CTLA-4/PD-1/LAG-3. Three days after transfer, tbx21, eomes and the T-box target genes ifng and cxcr3 were all induced in immunized CD8+ T cells relative to those in naive T cells (Fig. 1B). These genes were not highly expressed in tolerant T cells, but checkpoint blockade treatment of Alb:Gag recipients resulted in increased expression of tbx21, ifng and cxcr3. Unexpectedly, eomes was not affected by this same blockade treatment. The induction of T-bet, but not Eomes at this time point, was further confirmed by intracellular protein detection (Fig. 1C & 1D). Subsequent experiments using T-bet-deficient T cells (tbx21−/−) showed that T-bet was dispensable for expansion and survival of transferred T cells following checkpoint blockade (Fig. 1E), but was required to induce expression of the known T-bet targets IFNγ and CXCR3 within the tolerizing environment (Fig. 1F & 1G). These results suggest that T-bet may serve a more prominent role than Eomes in dictating CD8+ T-cell effector responses during checkpoint blockade immunotherapy.
T-bet is required for T cell effector function during checkpoint blockade immunotherapy
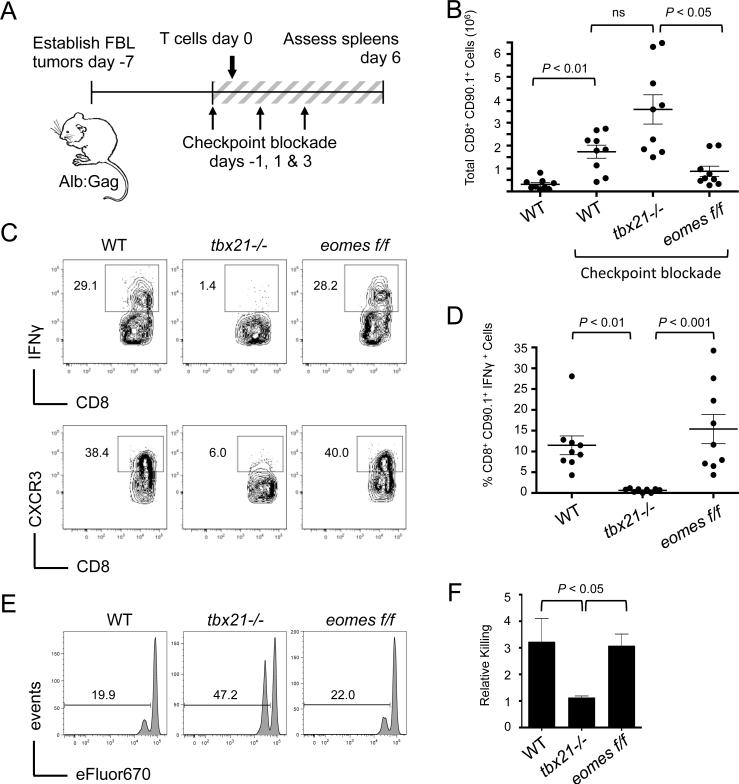
Previous reports have demonstrated the redundant roles of T-bet and Eomes in CD8+ T cells, showing that expression of either one is sufficient for IFNγ production, cytolytic function, and antitumor responses (15-18, 24). Thus, the loss of T-bet alone was not expected to grossly alter T-cell function here, but did so likely because Eomes was not adequately induced by checkpoint blockade within the tolerizing Alb:Gag environment (Fig. 1B and 1C). To specifically address the individual contributions of T-bet and Eomes with respect to cancer immunotherapy, WT, tbx21−/− or Eomes-deficient (eomesf/f) tumor/self-reactive CD8+ T cells were independently transferred into Alb:Gag recipients with disseminated FBL leukemia and treated with anti-CTLA-4/PD-1/LAG-3 (Fig. 2A). After 6 days, WT T cells were nearly undetectable in the absence of blockade treatment due to peripheral deletion of most tumor/self-reactive T cells at this time point (Fig. 2B). T cells deficient for tbx21 or eomes were similarly deleted in untreated Alb:Gag recipients (data not shown), preventing reliable assessment of T-cell function in untreated hosts. However, in recipients treated with checkpoint blockade, transferred T cells were detected in spleens regardless of genotype, with tbx21−/− T cells expanding to slightly higher numbers compared to WT and eomesf/f T cells (Fig. 2B). Despite this expansion, tbx21−/− T cells did not produce IFNγ in response to checkpoint blockade, whereas WT and eomes−/− T cells mounted similarly robust IFNγ responses under the same conditions (Fig. 2C and 2D). Likewise, tbx21−/− T cells again failed to express the chemokine receptor CXCR3 (Fig. 2C), a phenotype consistent with poor effector function and compromised tumor immunity (18, 25). Indeed, WT and eomesf/f T cells were capable of efficient in vivo cytolytic activity, but tbx21−/− T cells failed to demonstrate this same ability (Fig. 2E and 2F). These results imply that T-bet was required for checkpoint blockade to promote effector differentiation of responding tumor/self-reactive CD8+ T cells.
Figure 2. T-bet is required for blockade-mediated T-cell effector function.
FBL tumor-bearing Alb:Gag mice were infused with of WT, tbx21−/−, or eomesf/f Gag-reactive CD8+ T cells and treated on days −1, 1 and 3 with combination checkpoint blockade. (A) Diagram of experimental setup. (B) Six days after T-cell infusion, the total number of transferred CD90.1+ CD8+ T cells in recipient spleens was assessed. Data are pooled from 3 independent experiments. (C) IFNγ production by transferred T cells was assessed after overnight restimulation with Gag peptide. Expression of CXCR3 on transferred T cells was determined directly ex vivo. Inset numbers represent the percent of CD90.1+ CD8+ cells within the designated region. (D) The frequency of IFNγ-producing CD90.1+ CD8+ T cells from 3 individual experiments is shown graphically. Circles represent individual recipients and horizontal lines show the mean of each group with p-values indicated for the bracketed groups. (E) Five days after T-cell transfer, recipients were infused with a 1:1 ratio of Gag (eFluorlow) and control (eFluorhigh) peptide-pulsed target cells. Target-cell frequency in spleens was assessed one day later, with representative histograms shown. Inset numbers are the percent of total target cells under the indicated region. (F) The ratio of eFluorlow to eFluorhigh target cells is graphically displayed from 2 pooled experiments (n=6 for each group). Error bars depict the SEM and p-values are indicated for the bracketed groups.
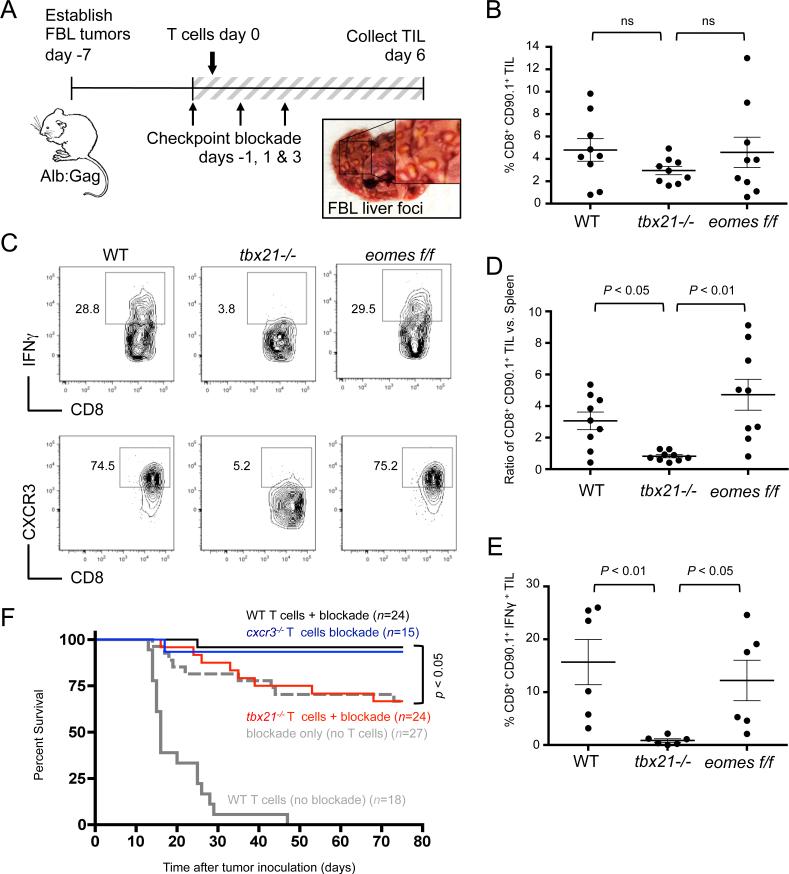
In a departure from our results, CD8+ T cells that lack both T-bet and Eomes have been shown to carry out essentially normal effector activity in a non-tolerant tumor-vaccine model of murine melanoma (18). However, antitumor responses were still compromised, which was attributed to poor T-cell migration due to low expression of the chemokine receptor CXCR3. Likewise, our results correlate with low CXCR3 expression in the absence of T-bet (Figs. 1 and 2). To determine if T-bet-dependent CXCR3 expression was required for T-cell trafficking to disseminated tumor sites, tumor-infiltrating lymphocytes (TIL) were examined within leukemic foci that form in recipient livers (Fig. 3A). WT, tbx21−/−, or eomesf/f T cells were transferred into Alb:Gag mice with disseminated FBL leukemia and treated with anti-CTLA-4/PD-1/LAG-3 antibodies. Six days later, the frequency of infused T cells within tumor foci was similar regardless of genotype (Fig. 3B). Tumor infiltration did not appear to rely on CXCR3, which was very low on tbx21−/− T cells compared to WT and eomesf/f T cells (Fig. 3C). However, taking into consideration the higher frequency of tbx21−/− T cells in the spleens of these same animals (Fig. 2B), a somewhat lower frequency of tbx21−/− TILs indicates at least a minor defect in migration (Fig. 3D). Separate studies using cxcr3−/− Gag-specific T cells confirmed that CXCR3 was not required for checkpoint blockade to promote the persistence, function, or migration of CD8+ T cells within tumor-bearing Alb:Gag recipients (Supplemental Fig. S2). Expression of T-bet however was necessary for effector differentiation and IFNγ production by tumor/self-reactive TILs, whereas Eomesodermin appeared completely dispensable in this role (Fig. 3C and 3E).
Figure 3. T-bet is required for TIL effector function and immunotherapy but not for tumor infiltration.
FBL tumor-bearing Alb:Gag mice were infused with of WT, tbx21−/−, or eomesf/f Gag-reactive CD8+ T cells and treated on days −1, 1 and 3 with combination checkpoint blockade. (A) Diagram of experimental setup for panels B-E, and example of tumor foci (inset box) on a representative liver at day 6. (B) The frequency of transferred CD90.1+ CD8+ T cells among all TILs was assessed 6 days after T-cell infusion, and data pooled from 3 separate experiments and displayed graphically. (C) IFNγ production by transferred T cells after overnight restimulation with Gag peptide and CXCR3 surface expression directly ex vivo were assessed. (D) The ratio of CD90.1+ CD8+ T-cell frequency in TILs versus in spleens from the same recipients in Fig. 2B is shown. (E) Pooled data from 3 individual experiments showing the frequency of CD90.1+ CD8+ TILs producing IFNγ. Circles within all graphs represent individual recipient mice and horizontal lines show the mean of each group with p-values indicated for the bracketed groups; all error bars represent SEM. (F) Survival of tumor-bearing Alb:Gag recipients was assessed following treatment with WT T cells only (gray line), checkpoint blockade (anti-CTLA-4/PD-1/LAG-3) only (dashed gray line), or checkpoint blockade and adoptive transfer of WT T cells (black line), cxcr3−/− T cells (blue) or tbx21−/− T cells (red). The graph displays pooled data from 5 independent experiments, showing percent survival (y-axis) over time in days (x-axis). The n-values depict the number of total mice per treatment group and the p-value is indicated for the bracketed groups.
T-bet expression by transferred T cells is required for checkpoint blockade immunotherapy of disseminated leukemia
Administration of anti-CTLA-4/PD-1/LAG-3 blockade antibodies engages endogenous immune cells and is sufficient to cure approximately 65% of Alb:Gag mice with an established and disseminated FBL leukemia (8). This approaches 100% when checkpoint blockade is combined with the transfer of Gag-specific CD8+ T cells. Because T-bet was required for effector differentiation of transferred tumor-reactive T cells (Fig. 2), we examined whether loss of T-bet impacted the efficacy of adoptive T-cell immunotherapy. Consistent with our previous study, mice receiving WT T cells alone (no blockade) had a median survival of only 16 days due to induction of T-cell tolerance (Fig. 3F). The addition of combination checkpoint blockade overcame these tolerizing influences and resulted in a 96% survival rate 75 days after tumor inoculation. This was not the case for mice administered tbx21−/− T cells with checkpoint blockade, which gained no survival benefit compared to those receiving checkpoint blockade alone. Despite a modest reduction in tumor infiltration by tbx21−/− T cells expressing low levels of CXCR3 (Fig. 3D), adoptive transfer of Gag-specific cxcr3−/− T cells with checkpoint blockade led to recipient survival rates nearly identical to WT T cell recipients (Fig. 3F). Together, these data affirm that the success of this combination checkpoint blockade immunotherapy relies on the induction of T-bet in responding tumor-reactive CD8+ T cells, and does not require expression of the T-bet target molecule, CXCR3.
Induction of T-bet drives effector gene expression in tumor-reactive CD8+ T cells responding to checkpoint blockade
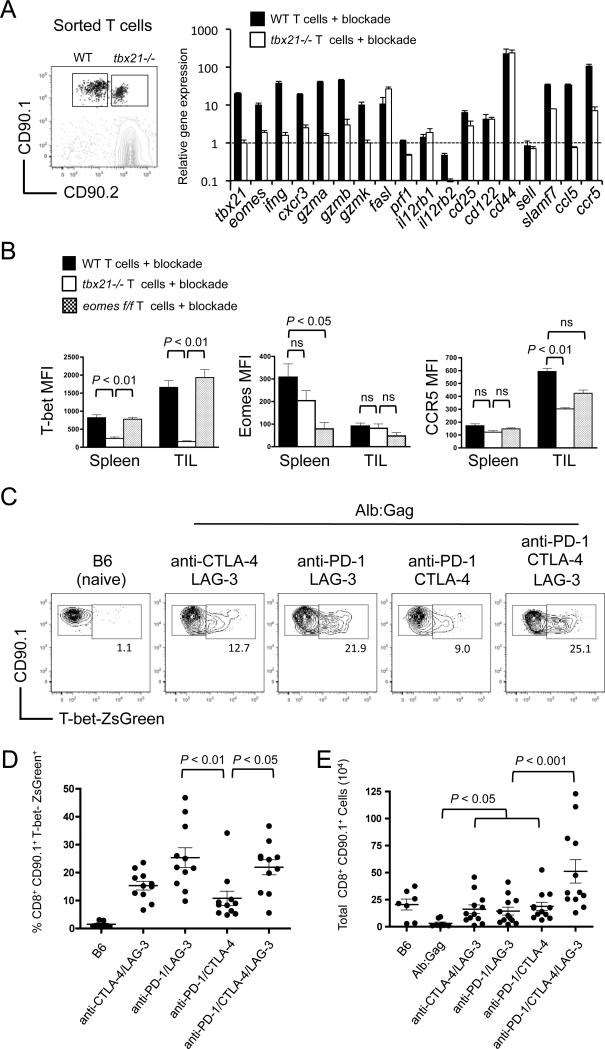
To define how T-bet influences responding CD8+ T cells during immunotherapy, gene expression was compared in WT and tbx21−/− T cells co-transferred together into the same tumor-bearing Alb:Gag recipients treated with combination checkpoint blockade. Transferred T cells were sorted 6 days after infusion and subjected to gene expression analysis by qRT-PCR (Fig. 4A). Specific genes were chosen based on uniquely low expression in tolerant CD8+ T cells as determined by prior gene array studies (Supplemental Fig. S1), indicating a possible link to the dysfunctional phenotype. In line with our data to this point, tbx21, ifng and cxcr3 were all induced with checkpoint blockade in WT but not tbx21−/− T cells. Similarly, the well-described T-bet target gene, gzmb was also induced in a T-bet-dependent manner. Two other granzyme genes, gzma and gzmk, which have not been directly associated with T-bet transcriptional regulation, were nevertheless reliant on T-bet. In contrast, the reported T-bet target gene fasl was induced independently of T-bet after checkpoint blockade, whereas prf1 (Perforin) and the IL12 receptor genes (il12rb1 and il12rb2) were not induced at all (Fig. 4A).
Figure 4. T-bet regulates expression of T-cell effector genes.
FBL tumor-bearing Alb:Gag mice received a co-transfer of WT (CD90.1) and tbx21−/− (CD90.1/CD90.2) Gag-reactive CD8+ T cells and were treated with combination checkpoint blockade on days −1, 1 and 3. (A) Six days after T-cell infusion, transferred T cells were isolated from the spleen and RNA isolated for analysis by qRT-PCR. Expression of the indicated genes from WT (closed bars) and tbx21−/− (open bars) T cells is shown relative to the level of the same gene expressed in naive Gag-reactive CD8+ T cells, which was arbitrarily set at a value of 1 and indicated by the dashed horizontal line. Results are representative of 2 independent experiments, and error bars represent SD among triplicate samples. (B) MFI of T-bet, Eomes, and CCR5 from WT (closed bars), tbx21−/− (open bars) and eomesf/f (shaded bars) Gag-reactive CD8+ T cells from spleens and TILs 6 days after infusion into FBL tumor-bearing Alb:Gag mice treated with checkpoint blockade. Graphs displays data from 2 pooled experiments and error bars indicate SEM with p-values indicated for the bracketed groups. (C) B6 or FBL tumor-bearing Alb:Gag mice were infused with T-bet-ZsGreen Gag-reactive CD8+ T cells and treated with checkpoint blockade antibodies on days −1, 1, and 3. Representative FACS plots display T-bet-ZsGreen expression on splenic CD8+ CD90.1+ cells 6 days after transfer. Inset numbers are the frequency of cells within the above region. (D) Pooled data from 4 independent experiments shows the frequency of CD8+ CD90.1+ T-bet-ZsGreen+ cells from indicated recipient mice. (E) Total CD8+ CD90.1+ T-bet-ZsGreen+ cell numbers pooled from 4 separate experiments are displayed graphically. Each circle represents an individual mouse and p-values are indicated for the bracketed groups.
Conceivably, any or all of the T-bet-dependent genes identified here have the potential to influence antitumor immunity. This is particularly true for granzymes and IFNγ, which have established roles in T-cell effector activity. For other genes like those encoding the chemokine CCL5 (RANTES) and its receptor CCR5, the contribution may be geared more toward T-cell migration, differentiation or the recruitment of other effector cells rather than direct tumor killing. Indeed, increased motility of melanoma-specific T cells was recently attributed to CTLA-4 blockade (26). To support these gene expression results, we analyzed T-bet, Eomes and CCR5 protein expression in transferred T cells from tumor-bearing Alb:Gag mice receiving checkpoint blockade. Here, mice received a co-transfer of either WT and tbx21−/− T cells or WT and eomesf/f T cells and analysis was performed 6 days later. Intracellular T-bet protein was expressed equivalently in both WT and eomesf/f T cells in spleens and TILs, but not in tbx21−/− T cells (Fig. 4B; left panel). While expression of intracellular Eomes was detected in WT and tbx21−/− T cells from the spleens, Eomes protein was essentially absent in CD8+ TILs regardless of genotype (Fig. 4B; middle panel). These data further marginalize the possible contributions of Eomes in driving CD8+ T cells effector mechanisms within the tumor. In contrast, CCR5 surface protein was markedly enriched on CD8+ T cells from tumors compared to the spleens. As in transcript analysis (Fig. 4A), CCR5 expression was reduced on tbx21−/− TILs relative to WT TILs (Fig. 4B; right panel), indicating a potentially important role for T-bet-mediated CCR5 expression in CD8+ T-cell migration to tumors during checkpoint blockade immunotherapy. This is supported by limited tbx21−/− T cell infiltration into disseminated tumor sites (Fig. 3D). It is worth noting that induction of a gene or protein in a T-bet-dependent manner here does not necessarily predict its requirement during immunotherapy, and CXCR3 is a good example of this. Clearly, further study is needed to fully discern the precise impact these genes have on responding T cells.
One of the complicating factors in promoting combination blockade therapies is identifying which components provide a given effect. To determine which blockade antibodies induced T-bet expression in responding tumor/self-reactive T cells, we utilized a sensitive fluorescent (ZsGreen) T-bet reporter system (22). Our previous work showed that double blockade of PD-1 and CTLA-4 produced only mild effector T-cell responses, which could be accentuated with the addition of anti-LAG-3 (8). Given the importance of T-bet for such effector mechanisms, we anticipated that this triple antibody combination would induce more T-bet-expressing T cells relative to any double blockade combinations, and this was true when compared to anti-PD-1 and CTLA-4 (Fig 4C and 4D). Unexpectedly though, the alternative combination of anti-PD-1 and LAG-3 induced equivalent T-bet expression as that induced by the triple blockade, suggesting the PD-1 and LAG-3 receptors may be most effective at limiting T-bet expression and effector responses in tolerant T cells. However, the number of persisting T cells was significantly higher in the triple blockade-treated group relative to any double combinations, indicative of a more effective overall immunotherapeutic strategy (Fig. 4E).
Several mechanisms have been proposed to explain exactly how checkpoint blockade antibodies boost T-cell immunity, including depletion of regulatory cells (27,28) and attenuation of negative regulatory signaling (29). However, these and other mechanisms are not mutually exclusive, and may cooperate to achieve enhanced antitumor responses. The current study was not designed to identify these mechanisms, but rather to define T-cell intrinsic pathways engaged during checkpoint blockade immunotherapy. To this end, our results support a requirement for T-bet in promoting blockade-induced CD8+ T-cell effector responses sufficient to eradicate disseminated and progressive leukemia. It is difficult to predict how closely the animal model examined here reflects the nuances of human checkpoint blockade immunotherapy. It makes sense though that boosted T-cell responses in patients may arise from increased T-bet expression. This could distinguish T-bet as a potential biomarker to indicate responsiveness to therapy. Such a sentinel molecule would allow clinicians to gauge whether individual patients are likely to benefit from continued treatment or those more at risk of treatment-related toxicities. It is also possible that checkpoint blockade induces T-bet expression in a variety of other cells including CD4+ T cells, NK cells, or even innate lymphoid cells (ILC1) that could contribute to antitumor immunity, or to autoimmunity in human patients (30). Identifying which cells are engaged by different checkpoint blockade regimens could provide translational insight toward higher fidelity treatment, thereby leading to better outcomes in cancer patients receiving immunotherapy.
Supplementary Material
Acknowledgments
Financial Support: Research reported in this publication was supported by the National Institute of Allergy and Infectious Disease (R01AI087764) to RMT, by a Cancer Research Institute Investigator Award to RMT, and by a National Cancer Institute fellowship (F30CA180375) to SRJ. The content is solely the interpretation of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 3.Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer. 2013;1:13. doi: 10.1186/2051-1426-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumortolerance systems. J Clin Invest. 2007;117:3383–92. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson SR, Yuan J, Teague RM. Targeting CD8(+) T-cell tolerance for cancer immunotherapy. Immunotherapy. 2014;6:833–52. doi: 10.2217/imt.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berrien-Elliott MM, Jackson SR, Meyer JM, Rouskey CJ, Nguyen TL, Yagita H, et al. Durable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T-cell tolerance. Cancer Res. 2013;73:605–16. doi: 10.1158/0008-5472.CAN-12-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–80. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11:91–9. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 14.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–3. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 15.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–23. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson SR, Yuan J, Berrien-Elliott MM, Chen CL, Meyer JM, Donlin MJ, et al. Inflammation programs self-reactive CD8+ T cells to acquire T-box-mediated effector function but does not prevent deletional tolerance. J Leukoc Biol. 2014;96:397–410. doi: 10.1189/jlb.1A0913-500RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, Ju S, Chen E, Dai S, Li C, Morel P, et al. T-bet and eomesodermin are required for T cell-mediated antitumor immune responses. J Immunol. 2010;185:3174–83. doi: 10.4049/jimmunol.1000749. [DOI] [PubMed] [Google Scholar]
- 19.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 20.Hegel JK, Knieke K, Kolar P, Reiner SL, Brunner-Weinzierl MC. CD152 (CTLA-4) regulates effector functions of CD8+ T lymphocytes by repressing Eomesodermin. Eur J Immunol. 2009;39:883–93. doi: 10.1002/eji.200838770. [DOI] [PubMed] [Google Scholar]
- 21.Kitano S, Tsuji T, Liu C, Hirschhorn-Cymerman D, Kyi C, Mu Z, et al. Enhancement of tumor-reactive cytotoxic CD4 T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res. 2013;1:235–44. doi: 10.1158/2326-6066.CIR-13-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012;37:660–73. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto J, Tan X, Teague RM, Ohlen C, Greenberg PD. Induction of tolerance in CD8+ T cells to a transgenic autoantigen expressed in the liver does not require crosspresentation. Journal of immunology. 2007;178:6849–60. doi: 10.4049/jimmunol.178.11.6849. [DOI] [PubMed] [Google Scholar]
- 24.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–11. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurachi M, Kurachi J, Suenaga F, Tsukui T, Abe J, Ueha S, et al. Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med. 2011;208:1605–20. doi: 10.1084/jem.20102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W, Allison JP. Cytotoxic T Lymphocyte Antigen-4 Blockade Enhances Antitumor Immunity by Stimulating Melanoma-Specific T-cell Motility. Cancer Immunol Res. 2014;2:970–80. doi: 10.1158/2326-6066.CIR-14-0104. [DOI] [PubMed] [Google Scholar]
- 27.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fcdependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti- CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 29.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–81. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.