Abstract
Caspase 8, the initiator caspase for death receptor induced apoptosis, functions as a negative regulator of receptor interacting protein kinase 3 (RIPK3), an essential factor for TNF-, TLR3- or TLR4-induced necroptosis. In certain situations, caspase 8 can also participate in pro-IL-1β processing. However, the biochemical complex that mediates caspase 8-mediated processing is not defined. Here, we show that RIPK3 is crucial for caspase 1- and caspase 8-mediated pro-IL-1β and pro-IL-18 processing in BMDCs in response to LPS stimulation. Caspase 8-mediated pro-IL-1β processing requires intact RIPK1, RIPK3, TRIF and FADD. In response to LPS, a complex that contains RIPK1, RIPK3, FADD and caspase 8 is formed. Surprisingly, RIPK3-specific kinase inhibitors strongly enhanced caspase 8 activation and pro-IL-1β processing in LPS-stimulated BMDCs. However, studies in BMDCs expressing the kinase inactive RIPK3-K51A mutant or RIPK1-K45A mutant showed that neither the kinase activity of RIPK1 nor RIPK3 is required for LPS-induced caspase 8 activation and IL-1β secretion. Hence, RIPK3 is an unexpected positive regulator of caspase 8 activity that promotes IL-1β maturation in BMDCs.
Introduction
IL-1β is an inflammatory cytokine that is closely associated with acute and chronic inflammation and has emerged as a therapeutic target for various systemic and local inflammatory diseases (1). In response to pattern recognition receptor stimulation, antigen-presenting cells such as dendritic cells (DCs) and macrophages produce pro-IL-1β through NF-κB dependent transcription. Full maturation and secretion of IL-1β requires caspase 1-mediated proteolytic processing. Caspase-1 is the enzymatic component of a macromolecular structure termed the inflammasome, which also contains the adaptor protein ASC and a sensor protein such as NLRP3 or AIM2.
Caspase 8 is the initiator caspase for death receptors in the TNF receptor family. It is recruited to the receptor through binding to its upstream adaptor FADD. Interestingly, recent studies show that caspase 8 can also regulate IL-1β expression by promoting de novo synthesis of pro-IL-1β and processing of pro-IL-1β into its cleaved mature form (2). For instance, in DCs, fungi and mycobacteria stimulate Dectin-1-mediated and caspase 8-dependent IL-1β secretion (3, 4). Infection of macrophages with Salmonella, Yersinia, Citrobacter rodentium, or E. coli also induced caspase 8-dependent IL-1β secretion (5–7). In addition, stimulation with Fas ligand, chemotherapeutic agents, or ER stress elicited IL-1β secretion in a caspase 8-dependent manner in LPS-primed macrophages or DCs (8–11). However, the biochemical mechanism that stimulates caspase 8-mediated pro-IL-1β processing is undefined.
RIPK3 is a serine/threonine kinase that is crucial for necroptosis in response to ligands binding to TNF receptor-like death receptors, TLR3 and TLR4 (12). RIPK3 interacts with its upstream activator RIPK1 via the “RIP homotypic interaction motif (RHIM)” to form an amyloid-like complex termed the “necrosome” to signal for necroptosis downstream of TNF receptor-like death receptors (13). The kinase activities of RIPK3 and RIPK1 are critical for stabilizing the necrosome and to promote necroptosis. On the other hand, RIPK3 interacts with another RHIM-containing adaptor TRIF to mediate TLR3- and TLR4-induced necroptosis (14, 15). Interestingly, both RIPK1 and RIPK3 are cleaved and inactivated by caspase 8 (16, 17). Hence, necroptosis is optimally induced when the FADD/caspase 8 complex is inactivated (18).
Although necroptosis is the most prominent function of RIPK3, several studies showed that RIPK3 could also promote non-necrotic signaling under certain conditions. For example, RIPK3 drives IL-1β secretion in LPS-primed macrophages or DCs when IAP proteins or caspase 8 are depleted (19–21). In addition, in cells that lack TAK1, RIPK3 promotes TNF-induced apoptosis (22). Mice genetically engineered to express the kinase-dead RIPK3 mutant D161N (Ripk3D161N-KD mice) died at mid-gestation because of hyperactivation of caspase 8 and excessive apoptosis (23). However, since the RIPK3-dependent effects on IL-1β and apoptosis were detected in highly manipulated experimental systems, it remains unclear whether RIPK3 has physiological functions beyond necroptosis. Here, we show that RIPK3 promotes IL-1β secretion through assembly of an alternative FADD-caspase 8 activating complex. This necroptosis-independent effect of RIPK3 also requires TRIF and RIPK1. In contrast to necroptosis, the kinase activities of RIPK1 and RIPK3 are dispensable for assembly of this complex and IL-1β secretion. In fact, RIPK3 kinase inhibitors facilitate conformational change that is conducive for assembly of this alternative caspase 8 activating complex. These results reveal RIPK3 as a positive regulator of non-apoptotitc caspase 8 activation and highlight the complex interplay between RIPK3 and caspase 8 in cell death-dependent and independent signaling.
Material and Methods
Mice
Ripk3−/− (V. Dixit, Genentech), Trif−/−, Myd88−/− (E. Lien, UMMS), Casp1−/−Casp11−/−(K. Rock, UMMS), RIPK1-K45A (Ripk1K45A-KD) and RIPK3-K51A kinase dead (Ripk3K51A-KD) knock-in mice (GlaxoSmithKline (GSK)) were used (24, 25). Faddfl/fl:Cd11c-Cre (dcFadd−/−) mice were kindly provided by A. Winoto (UC Berkeley) (26). Wild type mice from Ripk3+/− intercross were used. All mice were housed in specific pathogen-free facility at UMMS. All animal experiments were approved by the institutional animal care and use committee.
Reagents
LPS (Invivogen), z-VAD-fmk, z-YVAD-fmk, z-IETD-fmk, Necrostain-1 (Nec-1), MG-132 (Enzo Life Sciences), cycloheximide (CHX) (Sigma), and N-acetyl-L-cysteine (NAC) (Calbiochem) were used. RIPK3 kinase inhibitors GSK’840, ‘872, and ‘843 were kindly provided by GSK and have been described in previous publications (15, 25).
Generation and stimulation of BMDCs
BM cells were cultured for a week with 10 ng/ml GM-CSF and 5 ng/ml IL-4 to generate BMDCs. Faddfl/fl and Faddfl/fl:Cd11c-Cre (dcFadd−/−) BM cells were differentiated into BMDCs by GM-CSF alone. Ripk1+/− and Ripk1−/− newborn liver cells were differentiated to DCs with GM-CSF and IL-4. After stimulation, culture media and cells were used for ELISA, protein, and cell death analyses (CellTiter-Glo Luminescent Cell Viability Assay, Promega). The untreated sample is defined as having a value of 0% cell death. IL-1β and IL-18 were measured by ELISA sets from BD Biosciences and R&D systems, respectively.
Western blotting and immunoprecipitation
Whole cell extracts (WCEs) were prepared in RIPA buffer. Protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Sigma) were included in lysis buffer. Secreted proteins in culture media were precipitated by trichloroacetic acid. For immunoprecipitation, anti-RIPK3 (Prosci) or anti-caspase 8 (Enzo Life Sciences) antibodies were used. Western blotting was performed with anti-RIPK1 (BD Biosciences), RIPK3 (Prosci), FADD (kindly provided by A. Winoto), Caspase 8 (Enzo Life Sciences), MyD88 (eBioscienes), IL-1β, cIAP1/2 (R&D systems), GFP (Roche), Caspase-1 (Santa Cruz), and IL-18 (BioVision) antibodies. Anti-HSP90 antibody (BD Biosciences) was used as a loading control.
Lentivirus transfection
Various mutants of mouse RIPK3 were cloned into pTRIPZ vector (Open Biosystems). 293T cells were transfected by LV-GFP pLKO.1 (addgene 25999), LV-Cre pLKO.1 (addgene 25997), or mRIPK3/pTRIPZ in combination with pMD2.G and psPAX2 to generate lentivirus. On day 4 during BMDC differentiation, floating cells were collected, plated in media containing lentivirus, 10 µg/ml polybrene, GM-CSF, and IL-4, and incubated for additional 3 days prior to stimulation. Lentivirus carrying RIPK3 or its mutant genes was transduced to Ripk3−/− 3T3 cells and then cells were selected for puromycin resistance. To induce RIPK3 expression, cells were treated with 1 µg/ml doxycycline.
Statistical analysis
P values were calculated using unpaired t test with Welch’s correction. P values lower than 0.05 were considered statistically significant.
Results
LPS induces RIPK3-dependent caspase 8 activation and IL-1β secretion in BMDCs
IL-1β production generally requires two distinct signals: a first signal that elicits pro-IL-1β expression through NF-κB pathway and a second signal that causes inflammasome activation and caspase 1-dependent processing of pro-IL-1β. However, LPS alone was sufficient to induce a low level of IL-1β secretion in DCs (27). Surprisingly, we found that LPS-induced IL-1β secretion was strongly suppressed in Ripk3−/− BMDCs (Fig. 1A, Supplemental Fig. 1A and B) (28). LPS alone did not lead to secretion of the related cytokine IL-18 (Supplemental Fig.1B and 1C), although pro-IL-18 was constitutively expressed in BMDCs (Supplemental Fig. 1D). Stimulation of the inflammasome with ATP led to greater level of secreted IL-1β and detectable IL-18 secretion that were also dependent on intact RIPK3 (Supplemental Fig. 1C). Genetic deletion of caspase 1, Nlrp3, Asc (27), or inhibition of caspase 1 with the inhibitor z-YVAD-fmk only partially suppressed LPS-induced IL-1β secretion (Fig. 1B). This suggests that RIPK3 is responsible for caspase 1-dependent and independent pathways of pro-IL-1β processing.
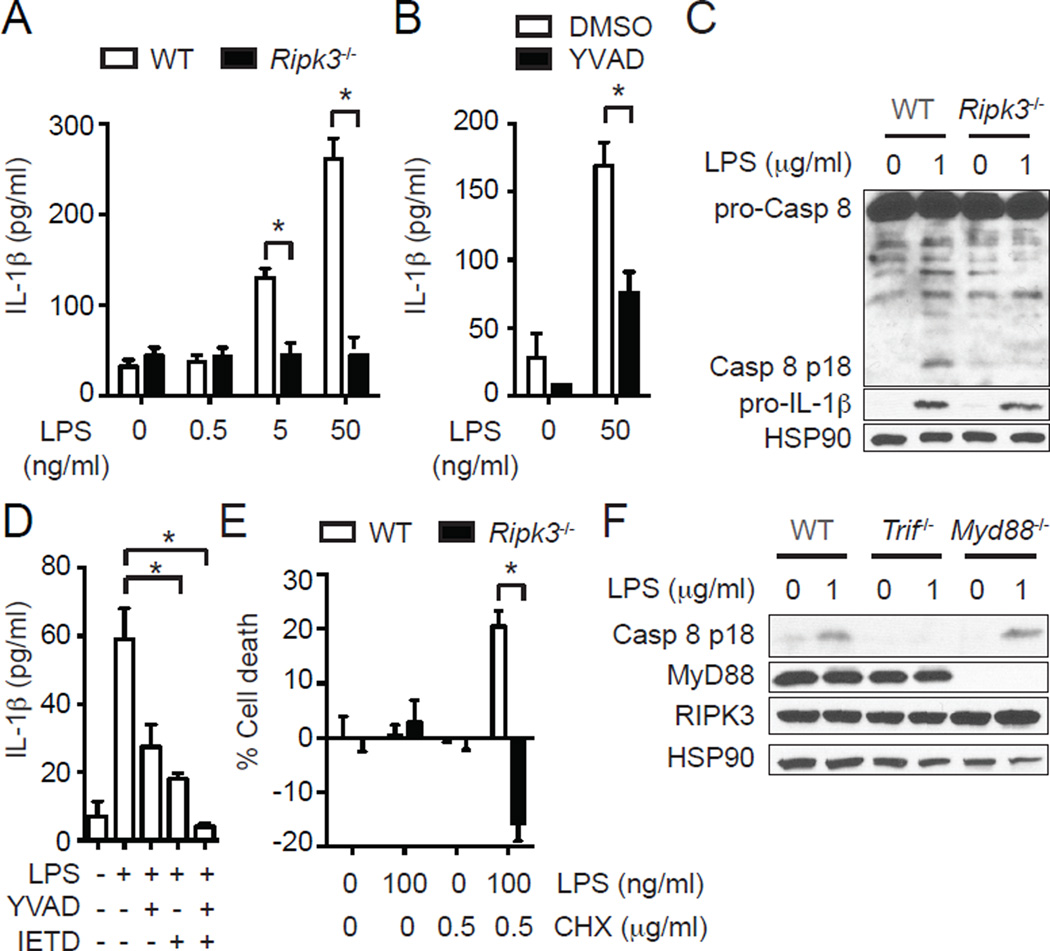
FIGURE 1.
RIPK3 promotes caspase 1- and caspase 8-dependent IL-1β secretion in BMDCs. (A) RIPK3 is required for LPS-induced IL-1β secretion in BMDCs. IL-1β secretion by WT and Ripk3−/− BMDCs stimulated with LPS for 6 hours was determined by ELISA (n=4). (B) Caspase 1 is partially responsible for RIPK3-dependent IL-1β secretion. WT BMDCs were pretreated with 10 µM z-YVAD-fmk (YVAD) for 1 hour and stimulated with LPS for 4 hours (n=4). IL-1β secretion was determined by ELISA. (C) RIPK3 is critical for LPS-induced caspase 8 activation. Cell lysates from WT and Ripk3−/− BMDCs stimulated with LPS for 1 hour were subjected to western blot analysis. (D) Caspase 1 and caspase 8 cooperate to mediate optimal IL-1β secretion. WT BMDCs pretreated with 10 µM YVAD and/or 10 µM z-IETD-fmk (IETD) for 1 hour were stimulated with 50 ng/ml LPS for 6 hours (n=4). IL-1β secretion was determined by ELISA. (E) Inhibition of protein synthesis converts the LPS-induced RIPK3 signal to one that causes apoptosis. WT and Ripk3−/− BMDCs pretreated with CHX for 1 hour were stimulated with LPS for 14 hours. Cell death was determined by measuring intracellular ATP level (n=3). (F) RIPK3-dependent caspase 8 activation requires an intact TRIF. Cell lysates from BMDCs of the indicated genotypes stimulated with LPS for 1 hour were subjected to western blot analysis. Results shown are mean ± SEM. Asterisks: p < 0.05.
Besides caspase 1 activation, LPS also triggered caspase 8 activation in wild type BMDCs (Fig. 1C and Supplemental Fig. 1D). Strikingly, caspase 8 activation was also abolished in Ripk3−/− BMDCs (Fig. 1C and Supplemental Fig. 1D) (28). RIPK3 did not affect the priming of BMDCs by LPS, since pro-IL-1β induction 1 hour after LPS treatment was normal in Ripk3−/− BMDCs (Fig. 1C and Supplemental Fig. 1D). However, the level of intracellular pro-IL-1β was higher in Ripk3−/− BMDCs than in WT BMDCs 6 hours after LPS treatment (Supplemental Fig. 1E). These results are most consistent with impaired cleavage of pro-IL-1β in Ripk3−/− BMDCs. Consistent with a deficiency in cleavage of pro-IL-1β, the caspase 8 specific inhibitor z-IETD-fmk partially suppressed LPS-induced IL-1β secretion, but not pro-IL-1β induction (Fig. 1D and Supplemental Fig. 1F). Co-administration of caspase 1 and caspase 8 inhibitors further abolished the residual IL-1β secretion (Fig. 1D), indicating that both caspases contributed to IL-1β secretion by LPS-treated BMDCs.
IAP proteins are natural inhibitors of RIPK3-dependent IL-1β secretion (19, 20). However, LPS treatment did not alter cIAP1/2 expression in BMDCs (Supplemental Fig. 1G), indicating that RIPK3-dependent IL-1β secretion was not due to loss of cIAP expression. Although it is an initiator caspase for apoptosis, caspase 8 activation did not cause cell death in LPS-treated wild type BMDCs. However, inhibition of de novo synthesis of pro-survival molecules with cycloheximide (CHX) led to LPS-induced cell death that was dependent on RIPK3 (Fig. 1E). These results indicate that besides caspase 1, RIPK3 also stimulates caspase 8-mediated pro-IL-1β processing in LPS-treated BMDCs. Moreover, in contrast to death receptor-induced apoptosis, LPS-induced caspase 8 activation did not result in cell death.
RIPK3 mediates assembly of an alternative FADD-caspase 8 complex in a TRIF-dependent manner
MyD88 is required for signaling by all TLR family receptors except TLR3 while TRIF is a unique signal adaptor for TLR3 and TLR4. LPS-induced caspase 8 activation was abolished in Trif−/− BMDCs (Fig. 1F). In contrast, Myd88−/− BMDCs exhibited normal caspase 8 activation. These results are consistent with a previous report that in macrophages, TRIF is required for caspase 8-dependent IL-1β secretion downstream of TLR3 or TLR4 and CHX stimulation (2). Moreover, these results also indicate that TRIF acts upstream of RIPK3 to promote caspase 8 activation.
Our recent work shows that RIPK3 promotes caspase 1 activation in BMDCs through reactive oxygen species (ROS) production (28). However, unlike caspase 1, the ROS scavenger N-acetyl cysteine (NAC) did not block LPS-induced caspase 8 activation (Fig. 2A). This indicates that RIPK3 controls caspase 1 and caspase 8 activity through different mechanisms. Binding to the adaptor FADD is a requisite step for autocleavage and activation of caspase 8 in response to death receptor stimulation (29). Interestingly, we detected constitutive interaction between caspase 8 and FADD in BMDCs (Fig. 2B, Supplemental Fig. 2A). The interaction between FADD and caspase 8 was not changed upon LPS treatment (Fig. 2B), suggesting that LPS causes a qualitative, but not quantitative change in FADD-caspase 8 interaction. This is in contrast to TNF and CHX-stimulated 3T3 fibroblasts, which showed inducible interaction between FADD and caspase 8 (Supplemental Fig. 2A).
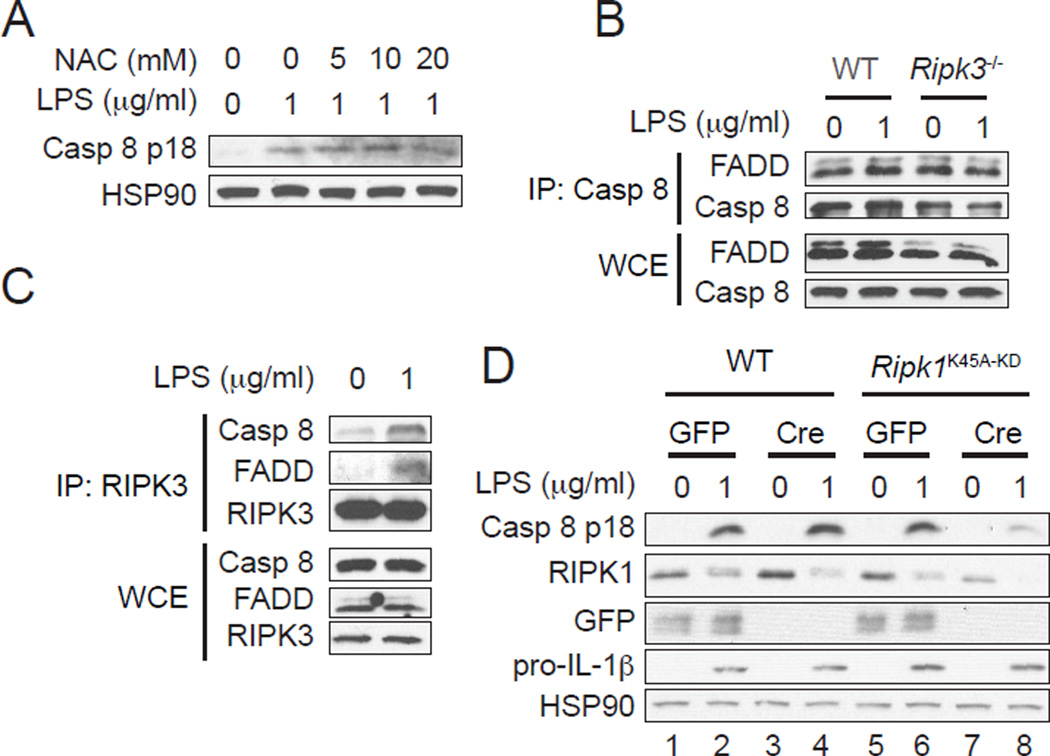
FIGURE 2.
RIPK3 promotes formation of an alternative caspase 8 activating complex. (A) RIPK3 mediates caspase 8 activation independent of ROS production. WT BMDCs pretreated with NAC for 1 hour were stimulated with LPS for 1 hour. Cell lysates were subjected to western blot analysis. (B and C) LPS induces assembly of a complex between RIPK3, FADD and caspase 8. Cell lysates from WT and Ripk3−/− BMDCs stimulated with LPS for 1 hour were subjected to immunoprecipitation with (B) anti-caspase 8 or (C) anti-RIPK3 antibodies followed by western blot analysis. WCE: whole cell extract. IP: immunoprecipitate. (D) An intact RIPK1, but not its kinase activity, is required for LPS-induced caspase 8 activation. WT and Ripk1K45A-KD BMDCs were transduced with lentivirus expressing GFP or Cre and subsequently stimulated with LPS for 1 hour. Cell lysates were subjected to western blot analysis.
Since RIPK3 was required for LPS-induced caspase 8 activation (Fig. 1C), we examined RIPK3 recruitment to the caspase 8 complex. Indeed, LPS significantly enhanced the interaction of RIPK3 with caspase 8 and FADD (Fig. 2C). RIPK1 is a RHIM- and death domain (DD)-containing adaptor that facilitates death receptor-induced apoptosis and necroptosis. We reasoned that RIPK1 might interact with RIPK3 via the RHIM and FADD or caspase 8 via the DD to mediate caspase 8 activation. To ascertain this possibility, we took advantage of Ripk1K45A-KD mice, which express a “knock-in” version of kinase inactive RIPK1 (24). Because exon 3 of the Ripk1K45A-KD allele is flanked by loxP sites, we can specifically delete Ripk1 by Cre-mediated recombination in BMDC cultures. We transduced WT or Ripk1K45A-KD BMDCs with lentivirus expressing Cre or GFP as control and observed significant reduction of RIPK1 expression in Ripk1K45A-KD, but not WT BMDCs transduced with Cre-expressing lentivirus (Fig. 2D, compare lanes 3 and 7). In these cells, LPS-induced caspase 8 activation and IL-1β secretion were suppressed compared to the cells transduced with GFP vector (Fig. 2D, compare lanes 6 and 8 and Supplemental Fig. 2B). In contrast, WT and Ripk1K45A-KD BMDCs transduced with control GFP lentivirus exhibited equal caspase 8 activation and IL-1β secretion (Fig. 2D, compare lanes 2 and 6, and Supplemental Fig. 2B). Consistent with results from Ripk1K45A-KD BMDCs, the RIPK1 kinase inhibitor Nec-1 also did not affect TLR4-induced caspase 8 activation (Supplemental Fig. 2C). These results indicate that while an intact RIPK1 is essential for TLR4-induced caspase 8 activation, its kinase activity is dispensable. Moreover, our results suggest that LPS triggers formation of a RIPK1-RIPK3-FADD-caspase 8 complex. This complex is reminiscent of the apoptosis complex driven by the kinase inactive RIPK3 mutant D161N (23). However, recruitment of RIPK1 to the RIPK3-FADD-caspase 8 complex in LPS-treated BMDCs was weak (see below), in part because of a reduction in overall RIPK1 expression (Fig. 2D). The reduction in cellular RIPK1 was not due to caspase-mediated cleavage or proteasome-dependent degradation (Supplemental Fig. 2D), but might be caused by translocation to detergent-insoluble compartment (30).
RIPK3 kinase inhibitor enhanced LPS-induced caspase 8 activation and IL-1β secretion
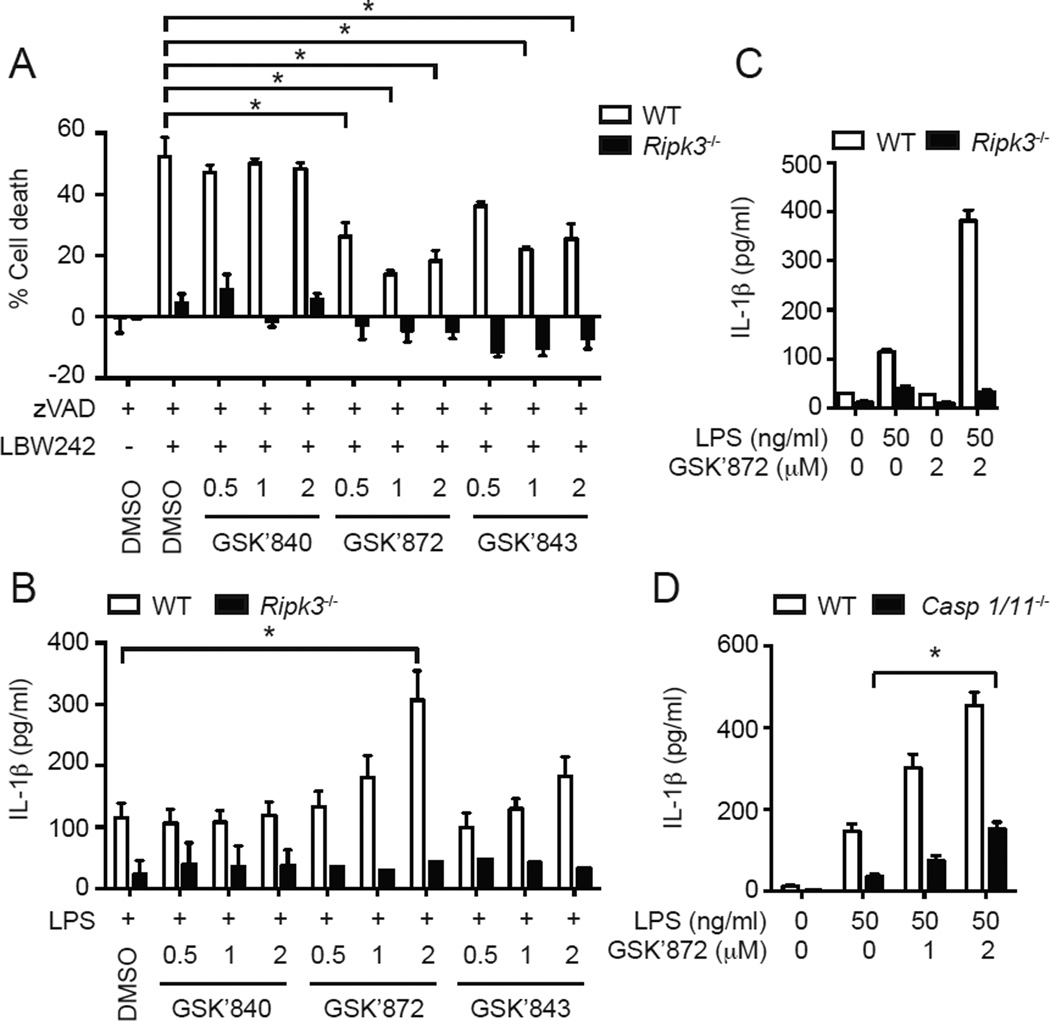
The results shown above indicate that RIPK3 licenses activation of the FADD-caspase 8 complex. In necroptosis, RIPK3 kinase activity is essential for assembly of a signaling complex termed the necrosome (31). To address whether RIPK3 kinase activity is similarly required for LPS-induced caspase 8 activation, we used the recently described RIPK3-specific kinase inhibitors GSK’872 and GSK’843 (15, 25). Treatment with the SMAC mimetic LBW242 and the pan-caspase inhibitor z-VAD-fmk induced autocrine TNF production and RIPK3-dependent necroptosis in BMDCs (Fig. 3A) (32). This necrotic cell death was suppressed by GSK’872 and GSK’843, but not by the human RIPK3-specific inhibitor GSK’840 (25). Surprisingly, GSK’872 and GSK’843 significantly increased LPS-induced IL-1β secretion in a dose-dependent manner (Fig. 3B). The enhanced IL-1β secretion requires TLR4 signaling, since the inhibitors did not induce IL-1β secretion without LPS (Fig. 3C). The effect of the inhibitors was RIPK3-specific, since neither GSK’872 nor GSK’843 increased IL-1β secretion in Ripk3−/− BMDCs (Fig. 3B). The RIPK3 kinase inhibitor-induced increase in IL-1β secretion was not driven by caspase 1, since enhanced LPS-induced IL-1β secretion was also detected in RIPK3 inhibitor-treated Casp1−/−Casp11−/− BMDCs (Fig. 3D, filled bars).
FIGURE 3.
RIPK3 kinase inhibitor enhances LPS-induced IL-1β secretion. (A) RIPK3 kinase inhibitors protect BMDCs against necroptosis. WT and Ripk3−/− BMDCs pretreated with indicated concentration (µM) of RIPK3 kinase inhibitors for 1 hour were stimulated with 10 µM z-VAD-fmk (zVAD) and 0.1 µM LBW242 for 14 hours to induce necroptosis (n=3). (B and C) RIPK3 kinase inhibitors increase LPS-induced IL-1β secretion. WT or Ripk3−/− BMDCs pretreated with the indicated RIPK3 inhibitors (µM) for 1 hour were stimulated with 50 ng/ml LPS for 6 hours (n=3). IL-1β secretion was determined by ELISA. (D) The effect of RIPK3 inhibitor on IL-1β secretion is not mediated by caspase 1 or caspase 11. WT and Casp1−/−Casp11−/− BMDCs pretreated with GSK’872 for 1 hour were stimulated with LPS for 6 hours (n=4). IL-1β secretion was determined by ELISA. Results shown are mean ± SEM. Asterisks: p < 0.05.
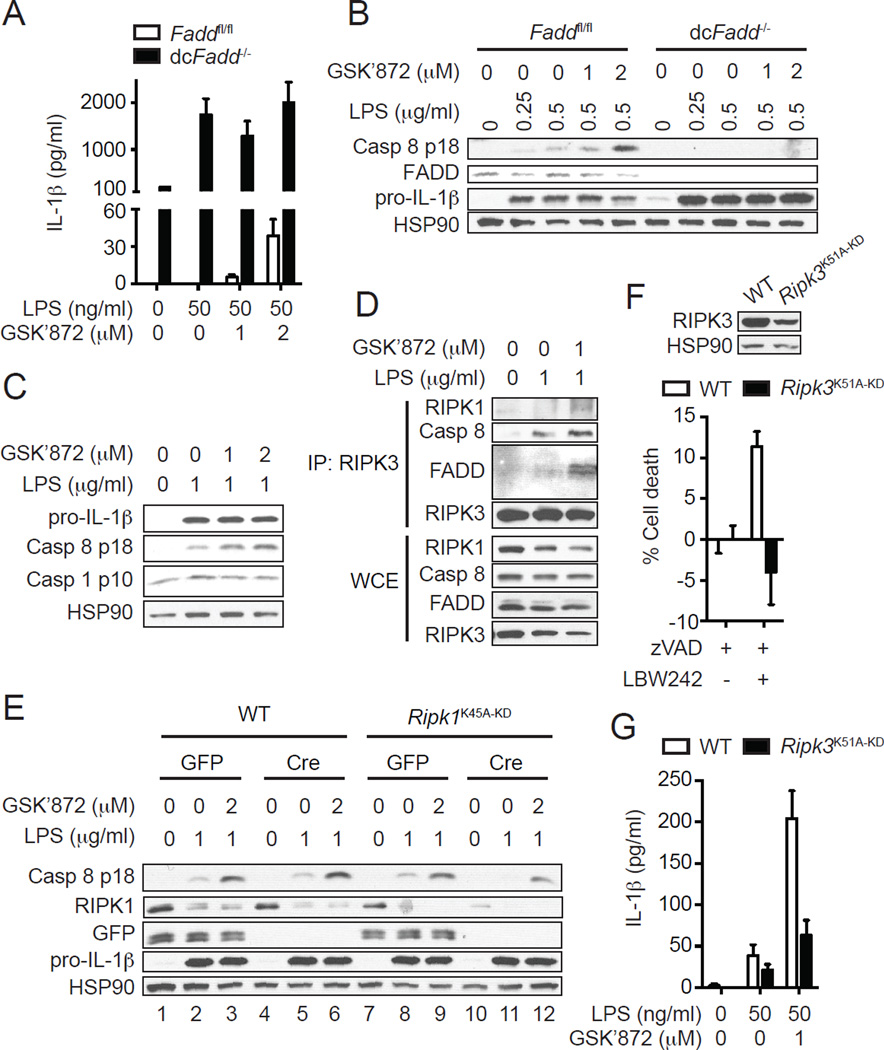
FADD is an essential adaptor of caspase 8 activation. DC-specific Fadd-deficient (dcFadd−/−) mice developed systemic inflammation associated with elevated proinflammatory cytokines including IL-1β (26). BMDCs generated from dcFadd−/− mice showed significantly increased IL-1β secretion upon treatment with LPS (Fig. 4A) (26). The enhanced IL-1β secretion was due to increased pro-IL-1β induction and caspase 1, but not caspase 8, activation, since caspase 8 activation was abolished in dcFadd−/− BMDCs (Fig. 4B)(21). Moreover, the RIPK3 inhibitor GSK’872 did not enhance IL-1β secretion or caspase 8 activation in dcFadd−/− BMDCs (Fig. 4A–B). Since GSK’872 only enhanced caspase 8 activation, but not pro-IL-1β expression and caspase 1 activation (Fig. 4C), these results indicate that the RIPK3 inhibitor enhanced pro-IL-1β processing through caspase 8, but not caspase 1. In addition to FADD, RIPK1, and RIPK3, LPS-induced caspase 8 activation also requires an intact TRIF, but not MyD88 (Supplemental Fig. 2E).
FIGURE 4.
RIPK3 kinase activity is dispensable for LPS-induced caspase 8 activation. (A and B) FADD is required for RIPK3-dependent caspase 8 activation. BMDCs generated from Faddfl/fl and Faddfl/fl:Cd11c-Cre (dcFadd−/−) BM cells were pretreated with GSK’872 for 1 hour and then stimulated with LPS for (A) 6 hours or (B) 1 hour. (A) IL-1β secretion was determined by ELISA (n=2). (B) Cell lysates were subjected to western blot analysis. (C and D) RIPK3 kinase inhibitor specifically enhances caspase 8, but not caspase 1 activation. WT BMDCs pretreated with GSK’872 for 1 hour were stimulated with LPS for 1 hour. Cell lysates were subjected to (C) western blot analysis or (D) immunoprecipitation with anti-RIPK3 antibody followed by western blot analysis. WCE: whole cell extract. IP: immunoprecipitate. (E) RIPK3 kinase inhibitor enhances caspase 8 activation through RIPK1. WT and Ripk1K45A-KD BMDCs were transduced with lentivirus expressing GFP or Cre and subsequently treated with GSK’872 for 1 hour followed by LPS for 1 hour. Cell lysates were subjected to western blot analysis. (F–G) RIPK3 kinase activity is essential for necroptosis, but not IL-1β secretion. (F) WT and Ripk3K51A-KD BMDCs were treated with 10 µM z-VAD-fmk (zVAD) and 0.1 µM LBW242 for 14 hours to induce necroptosis (n=3). Cell death was determined by measuring intracellular ATP level. The upper panel shows protein expression level of RIPK3. (G) WT and Ripk3K51A-KD BMDCs pretreated with GSK’872 for 1 hour were stimulated with LPS for 6 hours (n=3). IL-1β secretion was determined by ELISA. Results shown are mean ± SEM.
Consistent with the specific stimulation of caspase 8-mediated pro-IL-1β processing, GSK’872 augmented LPS-induced interaction of RIPK3 with FADD and caspase 8 (Fig. 4D). Specifically, GSK’872 significantly enhanced LPS-induced RIPK1 recruitment to RIPK3 (Fig. 4D). Moreover, deletion of RIPK1 by Cre-mediated recombination in Ripk1K45A-KD BMDCs repressed the GSK’872-mediated enhancement of caspase 8 activation (Fig. 4E). The residual caspase 8 activation by GSK’872 was likely due to incomplete deletion of RIPK1 (Fig. 4E, lane 10, second panel). Indeed, GSK’872-mediated enhancement of caspase 8 activation was completely abrogated in Ripk1−/− DCs generated from Ripk1−/− newborn liver cells (Supplemental Fig. 2F). The RIPK1 kinase inhibitor Nec-1 failed to reverse the enhanced caspase 8 activation by the RIPK3 inhibitor (Supplemental Fig. 2G). Hence, the enhanced RIPK3-dependent caspase 8 activation and pro-IL-1β processing by RIPK3 kinase inhibitors require an intact RIPK1, but not its kinase activity.
RIPK3 kinase activity is not required for LPS-induced caspase 8 activation
The results with the RIPK3 inhibitors are surprising given the positive role of RIPK3 in pro-IL-1β processing. One possible explanation for these results is that RIPK3 phosphorylates and inhibits an unknown substrate that is required for switching on caspase 8 activity. In support of this model, mice expressing the kinase inactive RIPK3 mutant D161N (Ripk3D161N-KD) were recently reported to suffer from embryonic lethality due to massive caspase 8-mediated apoptosis in the yolk sac (23). However, mice expressing another kinase inactive RIPK3 mutant K51A (Ripk3K51A-KD) were viable (25), suggesting that inhibition of RIPK3 kinase activity per se does not necessarily induce caspase 8 activation. To further test the role of RIPK3 kinase activity in LPS-induced caspase 8 activation, we generated BMDCs from Ripk3K51A-KD mice. Treatment of DCs or macrophages with SMAC mimetics in the presence of caspase inhibition causes autocrine TNF production and necroptosis (32). Consistent with the essential role of RIPK3 kinase activity in necroptosis, Ripk3K51A-KD BMDCs were protected from SMAC mimetic and zVAD-induced necroptosis (Fig. 4F). In contrast to the RIPK3 inhibitors, LPS-induced IL-1β secretion was not increased in Ripk3K51A-KD BMDCs (Fig. 4G). In fact, Ripk3K51A-KD BMDCs secreted reduced level of IL-1β (Fig. 4F). However, the reduced IL-1β secretion was likely caused by reduced RIPK3 expression in Ripk3K51A-KD BMDCs (Fig. 4F). Importantly, GSK’872 still enhanced IL-1β secretion by LPS-treated Ripk3K51A-KD BMDCs (Fig. 4G). These results strongly suggest that RIPK3 kinase activity is not required for LPS-induced caspase 8 activation. Rather, the RIPK3 kinase inhibitors promote IL-1β secretion by inducing a conformational change that is amenable for assembly of the alternative caspase 8 complex.
Although the low doses of the RIPK3 kinase inhibitor we used in BMDCs did not cause any toxicity (Supplemental Fig. 3A), higher concentrations of GSK’872 enhanced caspase 8 activation and apoptosis in RIPK3-positive cells (25). Strikingly, the apoptosis induced by high doses of the RIPK3 kinase inhibitors requires a similar RIPK1-RIPK3-FADD-caspase 8 complex (25). Therefore, we used this system to further examine the requirement of the RIP homotypic interaction motif (RHIM), which is essential for necroptosis (13), for RIPK3-dependent caspase 8 activation. We expressed wild type or mutant RIPK3 in Ripk3−/− 3T3 cells under the control of doxycycline. Expression of wild type RIPK3 and RIPK3-D161N, but not RIPK3-K51A, led to caspase 8-dependent apoptosis (25) (Supplemental Fig. 3B). The cell death induced by RIPK3-D161N is apoptotic, since it was reversed by the pan-caspase inhibitor z-VAD-fmk (Supplemental Fig. 3C). However, z-VAD-fmk did not rescue cell death in wild type RIPK3-expressing cells due to a switch from apoptosis to necroptosis (Supplemental Fig. 3C). Caspase 8-dependent apoptosis was enhanced by GSK’872 in cells expressing wild type RIPK3, RIPK3-K51A and RIPK3-D161N (Supplemental Fig. 3B), indicating that the RIPK3 kinase inhibitor enhanced caspase 8 activation independent of the inhibition of RIPK3 kinase activity. In contrast, tetra-alanine substitution in the RHIM abolished caspase 8 activation and apoptosis in wild type RIPK3 and RIPK3-D161N expressing cells (Supplemental Fig. 3B) (25). In addition, the effect of GSK’872 was no longer observed in the RHIM mutant (Supplemental Fig. 3B). These results indicate that RHIM-mediated interaction is crucial for necroptosis as well as RIPK3-dependent caspase 8 activation and pro-IL-1β processing.
Discussion
RIPK3 is touted as a major driver of inflammation due to necroptosis-associated release of intracellular immunogenic contents. However, recent evidence shows that RIPK3 can promote inflammation independent of necroptosis (33). Specifically, RIPK3 positively promotes IL-1β secretion in macrophages or DCs when caspase 8 or IAP proteins are depleted (19–21). In contrast, several recent studies show that RIPK3 is dispensable for IL-1β secretion in macrophages when caspase 8 and IAP functions are preserved (5–9). In this study, we show that RIPK3 functions as an unexpected positive promoter of caspase 8 activation and IL-1β secretion in BMDCs. This is a surprising result because caspase 8 cleaves RIPK1 and RIPK3 and is widely recognized as a negative regulator of necroptosis (16–18). The RIPK3-dependent effect on IL-1β secretion indicates that under certain conditions, RIPK3 is a positive activator of caspase 8. Our results therefore revealed a complex interplay between the RIP kinases and FADD/caspase 8 in cell death and inflammatory signaling.
Mechanistically, we show that RIPK3 promotes assembly of an alternative caspase 8 activating complex that comprises of RIPK1, RIPK3, FADD and caspase 8. The TLR3/4 adaptor TRIF is also essential for caspase 8 activation. However, because antibodies that recognize endogenous mouse TRIF are not available (data not shown), we cannot confirm if TRIF is also a component of this complex. RIPK3 interacts with RIPK1 or TRIF via the RHIM (14, 15, 34, 35). Interestingly, the RHIM of TRIF was reported to be important for caspase 8-dependent IL-1β maturation (2). Therefore, we speculate that similar RHIM-mediated interaction among TRIF, RIPK1 and RIPK3 may be involved in the assembly of the caspase 8 activating complex downstream of TLR4. This notion is bolstered by the observation that RIPK3 is dispensable in situations where caspase 8 activation occurs independent of TRIF, such as that in β-glucan or Fas-induced IL-1β secretion (4, 9). Hence, it appears that there are multiple distinct mechanisms by which caspase 8 facilitates pro-IL-1β processing.
The ripoptosome is a macromolecular complex that activates apoptosis and is made up of RIPK1, FADD, and caspase 8 (36, 37). Recruitment of RIPK3 switches the ripoptosome towards necroptosis. Thus, the ripoptosome resembles the LPS-induced caspase 8 activating complex in composition. Although its primary function is to promote IL-1β maturation, the LPS-induced caspase 8 complex has the capacity to induce apoptosis when de novo protein synthesis is blocked by CHX. Moreover, RIPK1, RIPK3, FADD and caspase 8 are also components of the necrosome (31). That means that the same adaptors can initiate at least three distinct signaling outcomes: apoptosis, necroptosis and IL-1β processing. How is this remarkable diversity in signaling outcome achieved? Although the details are yet to be determined, additional adaptors may be involved in this decision. For example, formation of the ripoptosome and the necrosome requires depletion of the cellular IAPs. Although depletion of cellular IAPs is not a prerequisite for the caspase 8 complex that cleaves pro-IL-1β, other adaptors may yet determine the signaling outcome of this alternative caspase 8 activating complex. It is also noteworthy that the kinase activities of RIPK1 and RIPK3, which are essential for necrosome and ripoptosome assembly, are dispensable for the LPS-induced caspase 8 activation. Hence, post-translational modifications such as ubiquitination, protein phosphorylation or binding by pathway-specific adaptors may determine the signaling outcome of the RIPK1-RIPK3-FADD-caspase 8 complex.
Supplementary Material
Acknowledgement
We thank D. Porter (Novartis) for LBW242 and V Dixit, E Mocarski, W. Kaiser, K. Rock, M. Kelliher, E. Lien and A. Winoto for providing different knock-out mice used in this study.
This work is supported by NIH grant AI083497 (F.K.M. Chan). K.M. was supported by postdoctoral fellowships from the Uehara Memorial Foundation and the Japan Society for the Promotion of Science.
Abbreviations
- BMDC
bone marrow-derived dendritic cell
- IAP
inhibitor of apoptosis
- SMAC
second mitochondria-derived activator of caspase
- ER
endoplasmic reticulum
Footnotes
J.B. and P.J.G. are employees of GlaxoSmithKline.
References
- 1.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 4.Ganesan S, Rathinam VA, Bossaller L, Army K, Kaiser WJ, Mocarski ES, Dillon CP, Green DR, Mayadas TN, Levitz SM, Hise AG, Silverman N, Fitzgerald KA. Caspase-8 Modulates Dectin-1 and Complement Receptor 3-Driven IL-1beta Production in Response to beta-Glucans and the Fungal Pathogen, Candida albicans. J Immunol. 2014;193:2519–2530. doi: 10.4049/jimmunol.1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, Bryant CE. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1beta production. J Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK, Kelliher MA, Harris PA, Bertin J, Gough PJ, Shayakhmetov DM, Goguen JD, Fitzgerald KA, Silverman N, Lien E. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1beta via caspase-8 in dendritic cells. J Immunol. 2013;191:4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossaller L, Chiang PI, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VA, Mocarski ES, Subramanian D, Green DR, Silverman N, Fitzgerald KA, Marshak-Rothstein A, Latz E. Cutting edge: FAS (CD95) mediates noncanonical IL-1beta and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D. Release of Interleukin-1alpha or Interleukin-1beta Depends on Mechanism of Cell Death. J Biol Chem. 2014;289:15942–15950. doi: 10.1074/jbc.M114.557561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, Fitzgerald P, Oberst A, Dillon CP, Green DR, Cerundolo V, Sher A. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 2014;192:2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK, Wu H. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 19.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Hacker G, Silke J, Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Yabal M, Muller N, Adler H, Knies N, Gross CJ, Damgaard RB, Kanegane H, Ringelhan M, Kaufmann T, Heikenwalder M, Strasser A, Gross O, Ruland J, Peschel C, Gyrd-Hansen M, Jost PJ. XIAP Restricts TNF- and RIP3-Dependent Cell Death and Inflammasome Activation. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Dondelinger Y, Aguileta MA, Goossens V, Dubuisson C, Grootjans S, Dejardin E, Vandenabeele P, Bertrand MJ. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013;20:1381–1392. doi: 10.1038/cdd.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 24.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, Harris PA, Kaiser WJ, Mocarski ES, Bertin J, Gough PJ. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton JW, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, M. Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014 doi: 10.1016/j.molcel.2014.10.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young JA, He TH, Reizis B, Winoto A. Commensal microbiota are required for systemic inflammation triggered by necrotic dendritic cells. Cell Rep. 2013;3:1932–1944. doi: 10.1016/j.celrep.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Franchi L, Nunez G. TLR agonists stimulate Nlrp3-dependent IL-1beta production independently of the purinergic P2×7 receptor in dendritic cells and in vivo. J Immunol. 2013;190:334–339. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK. The Necroptosis Adaptor RIPK3 Promotes Injury-Induced Cytokine Expression and Tissue Repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes MA, Harper N, Butterworth M, Cain K, Cohen GM, MacFarlane M. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell. 2009;35:265–279. doi: 10.1016/j.molcel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PloS one. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McComb S, Cheung HH, Korneluk RG, Wang S, Krishnan L, Sad S. cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ. 2012;19:1791–1801. doi: 10.1038/cdd.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriwaki K, Chan FK. Necrosis-dependent and independent signaling of the RIP kinases in inflammation. Cytokine Growth Factor Rev. 2014;25:167–174. doi: 10.1016/j.cytogfr.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 35.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 36.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.