Abstract
Background
The microscopic residual tumor at the bronchial margin after radical surgery (R1 resection) affects prognosis negatively in non-small-cell lung cancer (NSCLC) patients. For patients with good performance status, a potential cure still exists. Here, we report the outcomes of concurrent paclitaxel-based chemo-radiotherapy (CRT) for NSCLC patients with microscopically positive bronchial margins or peribronchial infiltration.
Methods
A retrospective search in the clinical database was conducted in three hospitals. Patients were identified and evaluated if treated with radiotherapy combined with paclitaxel-based chemotherapy. The objects analyzed were local control time, progression-free survival (PFS), overall survival (OS), and treatment-related toxicity.
Results
Sixty-one patients with microscopic residual tumor at the bronchial stump following pulmonary lobectomy were identified. Forty-six patients who had received concurrent paclitaxel-based CRT were analyzed. The median follow-up was 40 months (range: 15.0–77.5 months). The 1-, 2- and 3-year survival rates were 97.8%, 60.9% and 36.9%, respectively. The local recurrences were recorded in 19.6% (9/46) patients. Median PFS and OS for the evaluated cohort were 23.0 [95% confidence interval (CI): 21.3–24.7] and 32.0 (95% CI: 23.7–40.3) months, respectively. The most common side effects were hematological toxicity (neutropenia, 93.5%; anemia, 89.1%; and thrombocytopenia, 89.1%) and no treatment-related deaths. Grade ≥2 acute radiation-induced pneumonitis and esophagitis were recorded in 43.5% (20/46) and 26.1% (12/46) patients, respectively. By univariate analysis, non-squamous cell lung cancer was associated with a significantly longer survival time (45.1 vs 26.4 months, p = 0.013).
Conclusions
For NSCLC patients with post-surgical microscopic residual tumor at the bronchial stump, concurrent paclitaxel-based chemo-radiotherapy achieved promising outcomes with accepted treatment-related toxicity.
Background
Anatomic pulmonary lobectomy with radical lymph node dissection is the primary treatment for operable non-small-cell lung cancer (NSCLC) [1]. Complete resection of NSCLC should be confirmed pathologically when all resection margins are free from tumor (R0 resection). The incidence of microscopic residual tumor at the bronchial margin (R1 resection) is 4–5% (range: 1.2–17%) of all lung operations [2]. Although the classification of an R1 resection at the bronchial margin is not uniform in the literature, Wind et al. concluded that it could be divided into submucosal residual disease, peribronchial residual disease, and extrabronchial residual disease [2]. Microscopic residual tumor might negatively affect prognosis, with 1- and 5-year survival rates among these patients between 20-50% and 0–20%, respectively [2]. So far, there have been no randomized trials comparing different treatment strategies in such patients. Nevertheless, the panel of the National Comprehensive Cancer Network (NCCN) still recommended that repeat resection or chemo-radiotherapy should be considered if the patients have positive bronchial margins [1]. In such patients, a potential for cure still exists.
Liewald et al. reported that in patients after R1 resection, reoperation might improve survival in Stage I (64 vs 21 months) and Stage II (38 vs 12 months) disease [3]. Snijder et al. reported 28 patients with Stage I NSCLC and microscopic residual tumor at the bronchial margin [4]. The 5-year survival rate of the patients who underwent reoperation was 40% as compared with 27% in patients that did not. Therefore, reoperation in patients with Stage I and II NSCLC and R1 resection of the bronchial resection margin is recommended [1,3-5]. Similarly, postoperative radiotherapy (PORT) is often given in clinical practice if microscopic residual tumor is present at the resection margin, based on the results of several retrospective studies showing a reduction in the local recurrence rates [6-8]. However, the value of PORT is controversial and some studies have reported high local recurrence rates following PORT in this specific population [4,9]. Thus, the NCCN panel indicated that CRT is an alternative strategy for Stage II or III disease with bronchial positive margins [1].
In clinical practice, patients with NSCLC after a R1 resection at the bronchial margin may be considered as potentially curable if their performance status is good. Concurrent CRT consisting of cisplatin and etoposide, paclitaxel and cisplatin (TP), and paclitaxel and carboplatin (TC) regimens has been used for salvage and definitive treatment, according to the NCCN guidelines [1].
In this study, we retrospectively evaluated the clinical outcomes of patients treated with curative-intent CRT, giving detailed information of the survival and related side effects, with the intention of proving suitable treatment for patients after R1 resection at the bronchial margin.
Methods
Patient data
R1 resection was defined as invasive microscopic residual tumor at the bronchial margin, or peribronchial infiltration without any tumor lesion at the bronchial stump area at baseline computed tomography (CT) 4 weeks after surgery. Between March 2007 and August 2012, 61 NSCLC patients received CRT for bronchial positive margin at West China Hospital, Second People’s Hospital of Sichuan, and Second Affiliated Hospital of Anhui Medical University. Forty-six patients received paclitaxel-based CRT. All of the patients had histologically proven NSCLC. This retrospective study was carried out with the approval of the Ethics Committee of West China Hospital, the Second People’s Hospital of Sichuan and the Second Affiliated Hospital of Anhui Medical University.
The basic and clinical characteristics of the study population are summarized in Table 1. The median age of the patients was 57 years; most of them were male and had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–1. Twenty-eight and fourteen patients had squamous cell carcinoma and adenocarcinoma, respectively. The initial tumor stage (Staging system, American Joint Committee on Cancer, 6th edition) [10] after surgery was Stage II (11; 23.9%), Stage IIIa (29; 63.0%), and Stage IIIb (6; 13.1%). The median follow-up was 40.0 months (range: 15.0–77.5 months).
Table 1.
Basic and clinical characteristics of the patients in present study (n = 46)
| Characteristics | Number of patients (%) |
|---|---|
| Age (years) | |
| Median (range) | 57 (39–75) |
| Gender | |
| Male/Female | 35 (76.1)/11 (23.9) |
| ECOG a performance status | |
| 0-1 | 42 (91.3) |
| 2 | 4 (8.7) |
| Pathology | |
| Squamous-cell carcinoma | 28 (60.9) |
| Adenocarcinoma | 14 (30.4) |
| Other types | 4 (8.7) |
| T staging after surgery b | |
| T2/T3/T4 | 11 (23.9)/21 (45.7)/14 (30.4) |
| N staging after surgery b | |
| N0/N1/N2 | 10 (21.7)/22 (47.8)/14 (30.4) |
| Tumor stage after surgery b | |
| II | 11 (23.9) |
| IIIa | 29 (63.0) |
| IIIb | 6 (13.1) |
| Follow-up time (months) | |
| Median (range) | 40 (15.0-77.5) |
a: Eastern Cooperative Oncology Group; b: Staging system, 6th edition, American Joint Committee on Cancer, 2002.
Concurrent CRT
Paclitaxel-based chemotherapy
The regimens consisted of paclitaxel 175 mg/m2 plus cisplatin 75 mg/m2 (TP regimen), paclitaxel 175 mg/m2 plus or carboplatin (AUC = 5) (TC regimen), or paclitaxel 175 mg/m2 plus oxaliplatin 135 mg/m2 (TO regimen) on Day 1 given every 3 weeks. The only adverse effects were Grade ≥3 acute treatment-induced pneumonitis or esophagitis, and if prolonged, chemotherapy was discontinued. Otherwise, the chemotherapy was suspended until recovery and the drug dose was reduced by 25% in the subsequent cycle.
According to the NCCN guidelines [1], adjuvant chemotherapy (including concurrent cycles with radiotherapy) was delivered at a maximum of four cycles.
Radiotherapy
All patients underwent CT simulation. Gross tumor volume (GTV) was defined as the site of the bronchial positive margin (2 cm around the bronchial stump). As described in Table 2, the clinical tumor volume (CTV) enclosed the GTV with an 8-mm margin and the high-risk draining lymph node stations followed the classification by Mountain et al. [11]. For the planning target volume (PTV), a 10-mm margin was added isotropically to the CTV (PTV1) and GTV (PTV2). The dose-volume constraints for the lungs were set as follows: V20 < 22% and mean lung dose <12 Gy. A maximum dose of 45 Gy was allowed to the spinal cord (planning risk volume).
Table 2.
Lymph node stations a irradiated as the CTV b
| N0–1after surgery | N2after surgery | |
|---|---|---|
| Right | ||
| Upper/Middle lobectomy | 10,7 and 4R | 10, 7, 4R [irradiate 2R if 4R (+)] |
| Lower lobectomy | 10 and 7 | 10, 7 [irradiate 8/9 if 8/9 (+)] |
| Left | ||
| Upper lobectomy | 10, 7, 4 L and 5 | 10, 7, 4 L and 5 [irradiate 2 L if 4 L (+)] |
| Lower lobectomy | 10 and 7 | 10, 7 [irradiate 8/9 if 8/9 (+)] |
a: Followed the lymph node classification [11]; b: clinical target volume.
The patients received a conventional-fraction schedule. The dose prescribed for PTV1 was 50 Gy and that for PTV2 was at ≥60 Gy. Radiotherapy started at the latest on the first day of the second chemotherapy cycle.
The details of the concurrent CRT are shown in Table 3.
Table 3.
Treatment in present study (n = 46)
| Time-interval between resection and start of treatment | |
|---|---|
| Median/range (weeks) | 5/4-6 |
| Radiotherapy | |
| PTV volume a (cm 3 , median/range) | 182.6/162.2-278.4 |
| Irradiation dose for PTV2 b (Gy, median/range) | 60/50-70 |
| Number of fractions (median/range) | 30/25-35 |
| Total lung V 20 (%,median/range) | 21/17-24 |
| Mean lung dose (Gy,median/range) | 11.7/10.3-12.8 |
| Chemotherapy | |
| Chemotherapy regimens c | |
| Paclitaxel and Cisplatin | 31 (67.4%) |
| Paclitaxel and Carboplatin | 11 (23.9%) |
| Paclitaxel and Oxaliplatin | 4 (8.7%) |
| Number of chemotherapy cycles (median/range) | 3 (1–4) |
| Number of concurrent cycles (median/range) | 2 (1–3) |
a: Planning target volume; b: generated according to the GTV; c: all chemotherapy regimens were delivered per three weeks.
Treatment assessment
Local failure was defined as recurrence at the bronchial stump and within the irradiated field. The regional failure was defined as lymph node recurrence outside the irradiated field. Local control was defined as no recurrence in the local and regional fields. Progression was defined as local recurrence or appearance of new lesions. Follow-up evaluations were performed 4 weeks after treatment, every 2–3 months for the first 2 years, and every 6 months thereafter.
Toxicity was evaluated and graded according to the National Cancer Institute Common Toxicity Criteria version 3.0 [12]. A diagnosis of radiation-induced pneumonitis was made on the clinical symptoms (including cough, shortness of breath and fever), with radiological findings in the absence of any other likely cause.
Statistical methods
Statistical analyses were performed using SPSS version 17.0. Progression-free survival (PFS) was measured from the date the treatment began to the date of disease progression, and overall survival (OS) was considered from the start of treatment to the date of data analysis, or date of loss from follow-up for patients alive, or date of death. Patients without local recurrence or progression who discontinued follow-up for any reason were censored on the last day of tumor assessment. The rates of PFS and OS were calculated using the Kaplan-Meier method. Log-rank test and Cox’s proportional hazards regression model were used for univariate survival analysis. Patient age, sex, ECOG performance status, pathological type, disease stage after surgery, chemotherapy regimen, and radiation dose were included in univariate analysis.
Results
All patients received a radiation dose of ≥50 Gy (for PTV1), and 78.3% (36/46) patients completed the planned radiotherapy (for PTV2). The median radiation dose delivered was 60 Gy, with a range of 50–70 Gy (Table 2). Thirty-one, 11 and 4 patients received the TP, TC and TO regimens, respectively, and 6.5% (3/46), 89.1% (41/46) and 4.3% (2/46) of patients received one, two and three cycles of chemotherapy with concurrent radiotherapy, respectively.
Follow-up studies continued until December 2013, with no one lost. Only one patient was diagnosed with brain metastasis at the first follow-up. Local and regional failure was observed in four and two patients, respectively. One patient was diagnosed with local and regional failure. The local control rate was 84.8%. The 1-, 2- and 3-year survival rates were 97.8%, 60.9% and 36.9%, respectively. Median PFS and OS for the evaluated cohort were 23.0 months [95% confidence interval (CI): 21.3-24.7 months) and 32.0 months (95% CI: 23.7-40.3 months), respectively (Figure 1).
Figure 1.
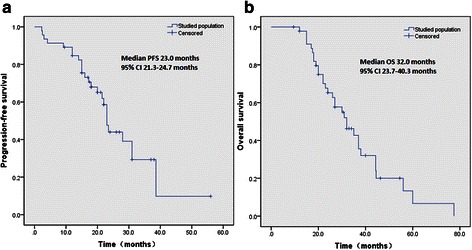
Kaplan-Meier analysis of progression-free survival (a) and overall survival (b) in the present study.
Treatment-related toxicity
All the patients were evaluated for treatment-related toxicity (Table 4). The combination of chemotherapy (either TP, TC or TO regimens) and radiotherapy proved to be tolerable. The most common toxicity was neutropenia (93.5%, 43/46). Grade 3 and 4 neutropenia was observed in 16 (34.8%) and one (2.2%) patients, respectively. Other major toxicities G1/2 included anemia, thrombocytopenia and acute esophagitis. Grade 3 treatment-related acute pneumonitis was observed in three (6.5%), nausea and vomiting in 8 (17.3%) patients. No grade 5 toxicity was recorded among any patients.
Table 4.
The treatment-related toxicities in present study (n = 46)
| Toxicitiesa | Toxicity grades, n (%) | ||||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hematological | |||||
| Neutropenia | 3 (6.5) | 12 (26.1) | 14 (30.4) | 16 (34.8) | 1 (2.2) |
| Anemia | 5 (10.9) | 25 (54.3) | 16 (34.8) | 0 | 0 |
| Thrombocytopenia | 5 (10.9) | 21 (45.7) | 20 (43.4) | 0 | 0 |
| Non-hematological | |||||
| Nausea and vomiting | 9 (19.6) | 17 (37.0) | 12 (26.1) | 8 (17.3) | 0 |
| Acute esophagitis | 6 (13.0) | 28 (60.9) | 12 (26.1) | 0 | 0 |
| Acute pneumonitis | 8 (17.3) | 18 (39.2) | 17 (37.0) | 3 (6.5) | 0 |
a: According to the Common Toxicity Criteria for Adverse Events, version 3.0.
Systemic treatment after disease progression
Twenty-seven patients (58.7%) were recorded with disease progression during follow-up. Local recurrence and tumor metastasis were observed in nine (19.6%) and 18 (39.1%) patients, respectively. Most of them (92.6%) received systemic treatment after disease progression, and only two patients received palliative radiotherapy. Among the patients with squamous-cell lung cancer, eight patients had received the gemcitabine/platinum regimen. Among the patients with non-squamous cell lung cancer, five patients had received the pemetrexed/platinum regimen and 5 patients had received tyrosine kinase inhibitors (TKIs: erlotinib or gefitinib).
Univariate survival analysis
Because of the small number of patients, only univariate analysis was performed according to the basic and clinical characteristics of the patients. The details are shown in Table 5. Age, sex, ECOG performance status, disease stage after surgery, chemotherapy regimen, and radiation dose did not significantly affect survival time. Pathological type (non-squamous cell lung cancer) was significantly associated with improved OS (median: 45.1 months), compared with patients with squamous-cell lung cancer (median OS: 26.4 months, p = 0.013) (Figure 2).
Table 5.
Prognostic factors by log-rank test and univariate survival analysis a in present study
| Factors | Group | Number | Median OSb(months) | Log-rank testpvalue | Univariate analysispvalue |
|---|---|---|---|---|---|
| Age | <57 years | 24 | 33.2 | 0.876 | 0.853 |
| ≧57 years | 22 | 30.8 | |||
| Gender | Male | 35 | 31.3 | 0.949 | 0.932 |
| Female | 11 | 32.8 | |||
| ECOG c performance status | 0-1 | 41 | 32.4 | 0.906 | 0.887 |
| 2 | 4 | 29.6 | |||
| Pathology | Squamous-cell lung cancer | 28 | 26.4 | 0.013 | 0.017 |
| Non-squamous cell lung cancer | 18 | 45.1 | |||
| Staging after surgery d | II | 11 | 35.2 | 0.654 | 0.613 |
| III | 35 | 30.0 | |||
| Chemotherapy regimen | TP | 31 | 33.7 | 0.393 | 0.308 |
| TC/TO | 15 | 30.5 | |||
| Irradiation dose | ≧60 Gy | 40 | 33.6 | 0.505 | 0.496 |
| <60 Gy | 16 | 29.3 |
a: Cox’s proportional hazards regression model; b: overall survival; c: Eastern Corporative Oncology Group; d: According to the AJCC 6th staging system.
Figure 2.
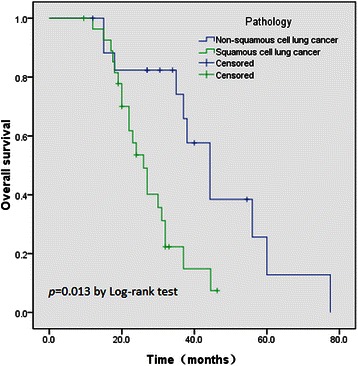
Kaplan-Meier analysis of overall survival in the present study, according to the pathology type of the patients.
Discussion
To our knowledge, this is the first report of concurrent CRT for post-surgical microscopic residual tumor at the bronchial margin in patients with NSCLC. Paclitaxel-based chemotherapy and concurrent radiotherapy achieved a median OS of 32.0 months among those NSCLC patients after R1 resection, with tolerable treatment-related toxicity. Although this was a retrospective evaluation with a small sample, our data suggest that selected NSCLC patients after R1 resection may benefit from aggressive and curative-intent concurrent CRT.
Reports from Liewald et al. and Snijder et al. show that for Stage I and II NSCLC patients with positive margins, repeat resection could improve OS [3,4] and is recommended by NCCN [1] for Stage II patients. In the present study, 11 patients (23.9%) with Stage II NSCLC did not undergo repeat resection, but received CRT. As recorded in our database, four patients refused reoperation because of limited cardiopulmonary function, and the others did not want a second operation. Among these patients, the median survival time was 35.2 months, which was similar to the reported data of repeat resection in Stage II cases by Liewald et al. (median OS: 38 months) [3].
The NCCN panel recommends the concurrent CRT for R2 resection or mediastinal recurrence, and the sequential CRT for R1 resection [1]. However, there is no direct evidence of any disadvantage of concurrent settings in NSCLC patients with R1 resection. Concurrent chemoradiation improves the clinical outcomes of Stage IIIA or IIIB disease [13-15]. In the present study, salvage CRT was well-tolerated and toxicity was as expected from thoracic CRT. The median OS was 32.0 months, which is comparable to those data collected from patients with bronchial stump recurrence [16]. Recently, Bar et al. reported the outcomes of CRT for loco-regional recurrence of NSCLC after surgery, and the median survival after recurrence was 26.9 months [17]. Thus, for patients with good ECOG performance status after R1 resection, concurrent CRT is still a treatment of choice.
It should be mentioned that three patients (6.5%) developed acute grade 3 radiation pneumonitis after treatment. Two patients had received right lower lobectomy and one left lower lobectomy. The delivered dose was 60, 62 and 60 Gy respectively, and the chemotherapy regimen was paclitaxel and cisplatin. They were diagnosed with Grade 3 radiation pneumonitis between 2 and 4 weeks after radiotherapy and finally recovered after steroid therapy. The incidence of RP is somewhat lower than the concurrent CRT for locally advanced NSCLC. Among several parameters based on dose-volume histograms, Vdose and mean lung dose (MLD) are important predictive factors of acute radiation pneumonitis [18,19]. In definitive chemo-radiotherapy for NSCLC, the NCCN panel suggests that a V20 value of 30-35% and MLD <20 Gy are thresholds for symptomatic radiation pneumonitis [1]. However, there is little information of such a threshold in the post-lobectomy situation. Uno et al. reported that in a 21-patient population, three patients developed grade ≥2 radiation pneumonitis after concurrent CRT [20]. They found that the V20 < 20%/MLD <10 Gy might be predictive factors for grade ≥2 radiation pneumonitis in post-lobectomy patients receiving definitive radiotherapy. In our practice, the lung constraints were set as V20 < 22% and MLD <12 Gy. All these constraints need more studies for validation.
In this study, the drug doses were modified in 19 (41.3%) patients during treatment. In addition, the granulocyte colony stimulating factor (G-CSF) is routinely applied for secondary prophylaxis in our practice when patients have grade 1 or 2 neutropenia in the preceding cycle. In some situations, we use G-CSF prophylactically among patients after chemotherapy to avoid any break in radiotherapy. So, the rate of grade 4 neutropenia (1 patient, 2.2%) was lower than expected. No grade ≥3 acute esophagitis was observed in this study. To avoid any break in radiotherapy as a consequence of grade 3 acute esophagitis, we usually prescribe a liquid combination (500 ml 0.9% physiological saline injection, 10 ml 1% lidocaine injection, and 10 mg dexamethasone mixed with each other, 15 ml P.O three times per day) among the patients with acute grade 2 esophagitis.
Another issue that should be discussed here is the target delineation. As all tumors are microscopic at the bronchial margin, it was difficult to define the GTV. Griess et al. [21] and Cotton [22] have reported that even after resection, in which there is a macroscopic tumor-free margin >2 cm, the incidence of R1 resection was still around 6% among these resections. From the report by Olszyna-Serementa et al., 80 patients with R1 resection have been analyzed [23]. They concluded that the PORT results in a relatively better survival in these patients. They also suggested that the elective nodal irradiation was useful for local control in pN0–1 patients. At present, the precise definition of GTV and CTV in an R1-resection situation had not been concluded and needs more clinical investigation.
By univariate survival analysis, we found that pathological type (non-squamous cell lung cancer) was significantly longer survival time, compared with patients with squamous-cell lung cancer (45.1 vs 26.4 months, p = 0.013). This result differs from the studies of Ghiribelli et al. [5] and Liewald et al. [3]. This may in part be explained by imbalances in the selection of subsequent treatment regimens with respect to histological subtypes. Currently, it is well known that several new anti-tumor drugs (including pemetrexed and TKIs) could significantly prolong the survival time among patients with metastatic non-squamous cell lung cancer, since a series of landmark trials has been published [24-27]. It might be the reason that these patients survive longer than the others in the present study.
Limitations of the present study should be mentioned. First, the retrospective nature of the study and the small number of the patients must be paid attention when interpreting the results. Second, the patients analyzed in this study had good ECOG performance status (0 or 1). Some patients could not tolerate the adjuvant CRT or chemotherapy alone, if their performance status was 2 or 3.
Conclusions
Concurrent paclitaxel-based chemo-radiotherapy is a feasible treatment strategy for NSCLC patients after R1 resection, with as-expected treatment-related toxicity. However, the most suitable chemotherapy regimen and the optimal radiotherapy planning (target delineation and normal tissue constraints) are not established and require further investigation.
Acknowledgement
We would like to thank Drs. Lin Zhou, Xiaojuan Zhou, Xingxin Zhang, Jianxin Xue, Yan Zhang, Yanying Li, Min Yu and Binwen Zou at the Department of Thoracic Oncology, Cancer Center, West China Hospital for their technical support for this work.
Part of the material has been presented in the poster session of the 56th Annual Meeting of American Society for Radiation Oncology, San Francisco, USA.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MZ and TL contributed in collection and analysis of data and drafting the manuscript; YL, CS and NL contributed in collection and analysis of data; YX, JZ, ZD, YW, MH, FP, JW, LR and YL provided the critical revision of the manuscript and the administrative support; YG provided the conception of this study and the final approval of the version to be published. And all authors read and approved the final manuscript.
Contributor Information
Meixiang Zhou, Email: 395261330@qq.com.
Tao Li, Email: litao1315@sina.com.
Yongmei Liu, Email: lymi75@163.com.
Changjin Sun, Email: cjsun@163.com.
Na Li, Email: lina_anhuimu@163.com.
Yong Xu, Email: xy868996@163.com.
Jiang Zhu, Email: zhujiang1@medmail.com.cn.
Zhenyu Ding, Email: dingzy333@163.com.
Yongsheng Wang, Email: wangys@scu.edu.cn.
Meijuan Huang, Email: hmj107@163.com.
Feng Peng, Email: pfwork@126.com.
Jin Wang, Email: wangjin_hxyy@scu.edu.cn.
Li Ren, Email: renlihx@163.com.
You Lu, Email: radyoulu@hotmail.com.
Youling Gong, Email: gongyouling@hotmail.com.
References
- 1.Non-small cell lung cancer. National Comprehensive Cancer Network, Guidelines Version 2; 2014. www.nccn.org.
- 2.Wind J, Smit EJ, Senan S, Eerenberg JP. Residual disease at the bronchial stump after curative resection for lung cancer. Eur J Cardiothorac Surg. 2007;32:29–34. doi: 10.1016/j.ejcts.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Liewald F, Hatz RA, Dienemann H, Sunder-Plassmann L. Importance of microscopic residual disease at the bronchial margin after resection for non-small-cell carcinoma of the lung. J Thorac Cardiovasc Surg. 1992;104:408–12. [PubMed] [Google Scholar]
- 4.Snijder RJ, Brutel de la Riviere A, Elbers HJJ, van der Bosch JMM. Survival in resected stage I lung cancer with residual tumor at the bronchial resection margin. Ann Thorac Surg. 1998;65:212–6. doi: 10.1016/S0003-4975(97)01114-4. [DOI] [PubMed] [Google Scholar]
- 5.Ghiribelli C, Voltolini L, Paladini P, Luzzi L, Di Bisceglie M, Gotti G. Treatment and survival after lung resection for non-small cell lung cancer in patients with microscopic residual disease at the bronchial stump. Eur J Cardiothorac Surg. 1999;16:555–9. doi: 10.1016/S1010-7940(99)00310-3. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H, Yamaguchi Y. Survival of noncuratively resected lung cancer. Lung Cancer. 1994;11:229–42. doi: 10.1016/0169-5002(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 7.Massard G, Doddoli C, Gasser B, Durcrocq X, Kessler R, Schumacher C, et al. Prognostic implications of a positive bronchial resection margin. Eur J Cardiothorac Surg. 2000;17:557–65. doi: 10.1016/S1010-7940(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann HS, Taege C, Lautenschlager C, Neef H, Silber RE. Microscopic (R1) and macroscopic (R2) residual disease in patients with resected non-small cell lung cancer. Eur J Cardiothorac Surg. 2002;21:606–10. doi: 10.1016/S1010-7940(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 9.Lacasse Y, Bucher HC, Wong E, Griffith L, Walter S, Ginsberg RJ, et al. Incomplete resection in non-small cell lung cancer: need for a new definition. Ann Thorac Surg. 1998;65:220–6. doi: 10.1016/S0003-4975(97)01190-9. [DOI] [PubMed] [Google Scholar]
- 10.AJCC Cancer Staging Manual . American Joint Committee on Cancer. 6. New York: Springer; 2002. [Google Scholar]
- 11.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–23. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 12.Common Toxicity Criteria version 3.0. National Cancer Institute. http://ctep.cancer.gov/reporting/ctc.html. [PubMed]
- 13.Albain KS, Crowley JJ, Turrisi AT, III, Gandara DR, Farrar WB, Clark JI, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non–small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20:3454–60. doi: 10.1200/JCO.2002.03.055. [DOI] [PubMed] [Google Scholar]
- 14.Curran WJ, Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–60. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–91. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 16.Jeremic B, Bamberg M. External beam radiation therapy for bronchial stump recurrence of non-small-cell lung cancer after complete resection. Radiother Oncol. 2002;64:251–7. doi: 10.1016/S0167-8140(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 17.Bar J, Ng D, Moretto P, Goss GD, Sun A, MacRae R, et al. Chemoradiotherapy for locoregional recurrence of non-small-cell lung cancer after surgical resection: a retrospective analysis. Clin Lung Cancer. 2013;14:200–4. doi: 10.1016/j.cllc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues G, Lock M, Souza DD, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer-a systematic review. Radiother Oncol. 2004;71:127–38. doi: 10.1016/j.radonc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Senan S, Ruysscher DD, Giraud P, Mirimanoff R, Budach V, on behalf of the Radiotherapy Group of the European Organization for Research and Treatment of Cancer (EORTC) Literature-based recommendations for treatment planning and execution in high-dose radiotherapy for lung cancer. Radiother Oncol. 2004;71:139–46. doi: 10.1016/j.radonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Uno T, Isobe K, Kawakami H, Ueno N, Kawata T, Yamamoto S, et al. Dose-volume factors predicting radiation pneumonitis in patients receiving salvage radiotherapy for postlobectomy locoregional recurrent non-small-cell lung cancer. Int J Clin Oncol. 2006;11:55–9. doi: 10.1007/s10147-005-0542-5. [DOI] [PubMed] [Google Scholar]
- 21.Griess DF, McDonald JR, Clagett OT. The proximal extension of carcinoma of the lung in the bronchial wall. J Thorac Surg. 1945;14:362–8. [Google Scholar]
- 22.Cotton RE. The bronchial spread of lung cancer. Br J Dis Chest. 1959;53:142–50. doi: 10.1016/S0007-0971(59)80004-8. [DOI] [PubMed] [Google Scholar]
- 23.Olszyna-Serementa M, Socha J, Wierzchowski M, Kepka L. Patterns of failure after postoperative radiotherapy for incompletely resected (R1) non-small cell lung cancer: Implications for radiation target volume design. Lung Cancer. 2013;80:179–84. doi: 10.1016/j.lungcan.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Hanna N, Shepherd FA, Fossella FV, Pereira JR, Marinis FD, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 25.Scagliotti G, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 26.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]


