Abstract
Despite advances in resuscitation medicine, including target temperature management as part of post-cardiac arrest care, many patients will have a poor neurological outcome, most often resulting in death. It is a commonly held belief that the ability to prognosticate outcome at an early stage after cardiac arrest would allow subsequent health care delivery to be tailored to individual patients. However, currently available predictive methods and biomarkers lack sufficient accuracy and therefore cannot be generally recommended in clinical practice. MicroRNAs have recently emerged as potential biomarkers of cardiovascular diseases. While the biomarker value of microRNAs for myocardial infarction or heart failure has been extensively studied, less attention has been devoted to their prognostic value after cardiac arrest. This review highlights the recent discoveries suggesting that microRNAs may be useful both to predict outcome and to treat patients after cardiac arrest.
Introduction
Although half of the patients resuscitated from cardiac arrest survive without major neurological sequelae, the other half die and some other survivors have severe neurological impairment. This is despite the widespread use of therapeutic hypothermia and its potential neuroprotective effects. Early outcome prognostication of patients resuscitated from cardiac arrest is challenging, mostly due to the paucity of accurate tools. The implementation of mild induced hypothermia has complicated matters further because the metabolism of sedatives is unpredictable and the use of muscle paralysis may confound prognostication [1]. Until recently, clinical neurological examination and neurophysiological tests performed several days after the arrest were the best indicators of outcome [2,3]. The use of circulating biomarkers such as neuron-specific enolase (NSE) improves outcome prediction on a group basis and has been recommended in clinical practice [4,5]. However, the discriminative ability of these tests is sub-optimal in individual patients and management of individuals could potentially benefit from new biomarkers.
Discovered in 2001 in Caenorhabditis elegans [6-8], microRNAs (miRNAs) have attracted great interest in the scientific community. The discovery of their presence and stability in the bloodstream [9,10] revealed their potential as novel disease biomarkers. Multiple groups have addressed the utility of circulating miRNAs as biomarkers of cardiovascular diseases. The vast majority of studies focused on myocardial infarction and heart failure. However, the potential of miRNAs to be used as biomarkers of cardiac arrest has received less attention and, until recently, was totally neglected.
In this article, we first review the current knowledge on available biomarkers used to predict outcome after cardiac arrest. Then, we discuss why it is critical to identify new biomarkers, and how these new tools may enable improvements in health care and outcome of patients after cardiac arrest. Finally, we present recent data suggesting that miRNAs might be useful biomarkers and therapeutic targets in this setting.
Current biomarkers: limitations
In order to predict outcome of patients with post-anoxic coma after circulatory arrest, biomarkers of neuronal damage have been extensively studied. Creatine phosphokinase brain-brain (CK-BB), NSE and the astroglial protein S100 calcium binding protein B (S100B) [11-14] have been evaluated in cerebrospinal fluid and blood of cardiac arrest patients. We have recently shown that combining serum levels of S100B and bispectral index monitoring accurately predicts outcome after cardiac arrest [15]. For a systematic review of the existing literature on biomarkers of cardiac arrest, see [16,17].
The cutoff values of CK-BB and S100B required to obtain sufficient specificity (and therefore sufficiently low false positive rates) are substantially elevated. Consequently, these biomarkers have low sensitivity, and are of limited prognostic value.
Although NSE was identified in the late 1980s as a potential marker of neurological outcome after cardiac arrest [18], its clinical utility is still a subject of debate. NSE is an isoenzyme of the glycolytic enzyme enolase (2-phospho-D-glycerate hydrolase) and is mostly of neuronal and neuroendocrine origin. Its levels are elevated after ischemic stroke, intracerebral hemorrhage, and traumatic and ischemic brain injury, rising just hours after neuronal damage. These properties make NSE a potential useful biomarker for neurologic outcome after cerebral injury. The 2006 American Academy of Neurology guidelines on prediction of outcome in comatose survivors after cardiopulmonary resuscitation advocated the use of NSE as a biomarker to estimate neurologic outcome with a cutoff value of >33 μg/L, which provided a false positive rate of 0% (95% confidence interval 0 to 3%) [3,19]. However, more recent studies do not support this guideline. Grubb and colleagues [20] found a NSE cutoff value of >71.0 μg/L 24 to 48 hours after cardiopulmonary resuscitation resulted in a false positive rate of 0% (95% confidence interval 0 to 43%) and a sensitivity of 14%. Other studies showed cutoff values of 30 to 80 μg/L for poor neurologic outcome and death [21,22]. Krumnikl and colleagues [23] published a case report of a patient with good neurologic outcome after a period of extended in-hospital cardiopulmonary resuscitation with a highest NSE value of 116.8 μg/L. Interestingly, hypothermia may affect serum levels of NSE. Tiainen and colleagues [24] found that the cutoff value of NSE, 48 hours after cardiopulmonary resuscitation and target temperature management, needed to be two to three times higher compared with patients not undergoing mild induced hypothermia (>25 versus 8.8 μg/L). Steffen and colleagues [25] also found higher cutoff values after hypothermia (NSE 78.9 versus 26.9 μg/L). In contrast, Wolff and colleagues [26] reported lower cutoff values after hypothermia. In addition, there is presently a lack of standardization of the measurements of NSE. Several available laboratory tests show variability of up to 40% between NSE values on the same samples [27].
Interpreting data from biomarker studies is confounded by differences in study design, inclusion/exclusion criteria of patients, duration of treatment, time of sampling and laboratory evaluation, as well as differences in evaluated endpoints of treatment, making it difficult to compare studies in a systematic review or a meta-analysis in a meaningful way. Other more recently described biomarkers such as procalcitonin [28-30], glial fibrillary acidic protein [31], heparin binding protein [32] and brain-derived natriuretic factor [33] face the same methodological issues and further large scale studies with an accurate methodology are warranted.
Therefore, there is an urgent need for identifying novel biomarkers that can guide patient management at an early stage after cardiac arrest.
How will new biomarkers allow for patient-oriented treatment and improvement of outcome?
Patients who remain unconscious following an out-of-hospital cardiac arrest despite the return of spontaneous circulation utilize considerable health care resources during the first days of admission to the hospital. Patients often require immediate coronary intervention to establish revascularization followed by mechanical ventilation in intensive care where they are treated with mild induced hypothermia, frequently requiring sedation and muscle paralysis. Cardiogenic shock and multiple organ failure may further complicate the clinical course of some survivors, necessitating high dose inotropic drugs, vasopressors, renal replacement therapy, intra-aortic balloon pumps, mechanical assist devices or extra-corporeal membrane oxygenation [34,35]. Despite early aggressive therapy, many of these patients will not survive due to irreversible cerebral damage caused by the initial insult.
New biomarkers may be very valuable if they have sufficient prognostic power when measured early after cardiac arrest. Health care resources may then be applied to patients who are most likely to benefit and futile care for those patients with irreversible severe cerebral damage can be avoided. Furthermore, patients’ relatives, who often experience long delays before receiving reliable information about prognosis, may be informed and guided early.
Emerging biomarkers: microRNAs
miRNAs are short (around 21 nucleotides) non-protein-coding RNA molecules that are evolutionarily conserved and ubiquitously expressed, albeit with a degree of tissue specificity. Since the first version of The miRBase Sequence Database [36] in December 2002, the number of known miRNAs has continued to grow. To date, as many as 30,424 mature miRNAs have been characterized in 206 species, with 2,578 in humans (release 20 June 2013).
Although miRNAs do not encode proteins, their role in gene regulation, and thereby in protein expression, is significant (Figure 1). Synthesized in the nucleus as primary miRNAs by the RNA polymerase III enzyme complex, they are cleaved by a second enzyme complex called Drosha to generate precursor miRNAs, which are exported to the cytoplasm to be finally cleaved by Dicer to form mature miRNAs. Mature miRNAs bind to target mRNA species and prevent their translation into proteins, either by induction of mRNA degradation by RNA-induced silencing complex when there is a perfect match between miRNA sequence and target mRNA, or by translational blockade when the two sequences are mis-matched. In mammals, miRNAs predominantly regulate gene expression by induction of mRNA degradation [37]. This property allows miRNAs to regulate developmental, physiological, as well as pathophysiological processes [38]. In the heart, miRNAs have been shown to regulate many functions, such as apoptosis, angiogenesis, contractility, and hypertrophy [39]. Similarly, miRNAs are abundantly expressed in the brain [40], where they play key roles in development, plasticity and disease evolution [41].
Figure 1.
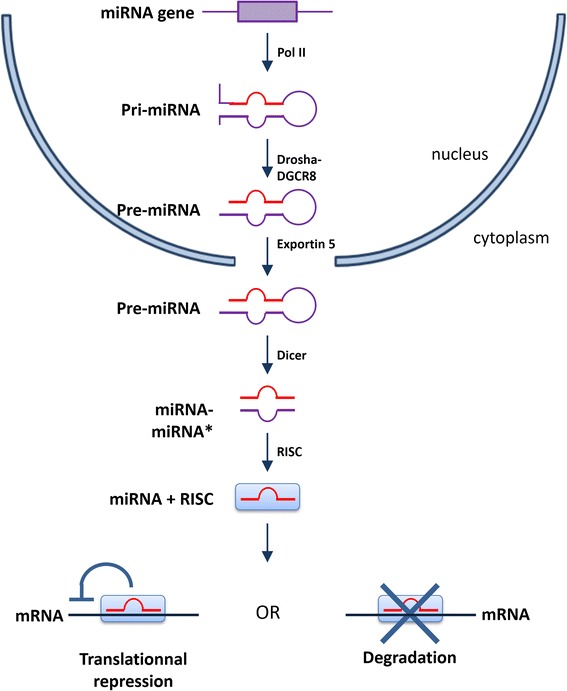
MicroRNA biogenesis. miRNA, microRNA; Pol II, polymerase II; RISC, RNA-induced silencing complex. Adapted from Goretti and colleagues [81].
A PubMed literature search revealed a plethora of research articles related to miRNAs during the past decade. More than 2,600 articles have been published to date in the field of brain and neurological research (Figure 2A). In the cardiovascular system, 2,300 articles have been published on miRNAs since the first identification of cardiac-enriched miRNAs by Lagos-Quintana and colleagues in 2002 [42] (Figure 2B). Significantly for biomarker research, circulating miRNAs are stable (that is, protected from RNase degradation), and can be easily and accurately quantified using conventional PCR techniques. A PubMed search (query (microrna OR mirna OR “micro-rna”) AND (brain OR neuron OR neurological OR cerebral) AND (biomarker OR diagnostic OR prognostic) showed that, to date, 624 articles were related to miRNAs as potential biomarkers of neurological diseases, the vast majority of which address the value of miRNAs as diagnostic biomarkers of brain tumors. With respect to miRNAs as biomarkers of cardiovascular diseases, we found 724 articles (query (microrna OR mirna OR “micro-rna”) AND (heart OR cardiac OR myocardial OR cardiovascular) AND (biomarker OR diagnostic OR prognostic)), the larger part focusing on myocardial infarction (125 articles) and heart failure (106 articles). Interestingly, only two reports focused on cardiac arrest (Figure 3). Furthermore, the large part of these studies addressed the diagnostic value of miRNAs, not their prognostic value.
Figure 2.
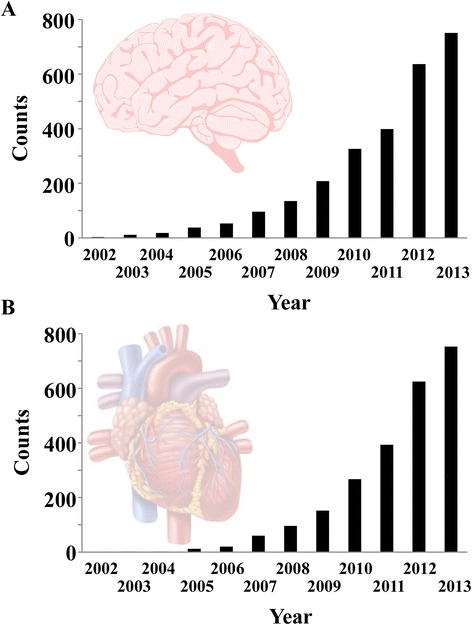
PubMed literature searches of research articles related to microRNAs. (A) Evolution of the number of articles related to microRNAs (miRNAs) in the neurological system. Literature search was performed in PubMed using the query: (microrna OR mirna OR “micro-rna”) AND (brain OR neuron OR neurological OR cerebral). (B) Evolution of the number of articles related to miRNAs in the cardiovascular system. Literature search was performed in PubMed using the query: (microrna OR mirna OR “micro-rna”) AND (heart OR cardiac OR myocardial OR cardiovascular).
Figure 3.
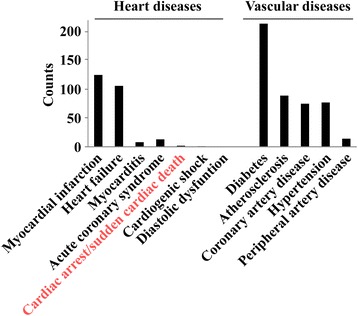
Numbers of articles related to microRNAs and their biomarker value, according to cardiovascular disease type. Literature search was performed in PubMed using the query: (microrna OR mirna OR “micro-rna”) AND (biomarker OR diagnostic OR prognostic) AND (name of the specific disease).
Consequently, miRNAs have emerged as candidate biomarkers of brain and heart diseases.
MicroRNAs and cardiac diseases
Several studies suggested that miRNAs may be used as diagnostic biomarkers of cardiovascular diseases, notably acute myocardial infarction (for recent reviews, see [43-45]). Other reports have addressed the prognostic value of circulating miRNAs after acute myocardial infarction [46-50]. We observed a significant inverse correlation between miR-208b and miR-499 and the left ventricular ejection fraction of patients 4 months after acute myocardial infarction [51]. However, these two miRNAs failed to accurately predict outcome in these patients. In another study involving two independent cohorts of patients with acute myocardial infarction, we found that plasma levels of miR-150, a non-prototypical cardiac miRNA, measured in the first few days following infarction significantly predicts left ventricular remodeling at 4 months [52]. In addition, a combination of several miRNAs, including miR-150, improved the predictive value of brain natriuretic peptide after acute myocardial infarction [53]. While several studies characterized the prognostic value of miRNAs in other cardiovascular conditions such as heart failure, this has not been rigorously investigated in cardiac arrest patients.
MicroRNAs and brain injury
MicroRNAs in the ischemic brain
The role of miRNAs in regulating brain development, plasticity and nervous system diseases, including cancer, has been reviewed previously [40,41,54,55]. After cardiac arrest, the brain, as well as other peripheral organs, is subjected to oxygen and nutrient deprivation as a consequence of cessation of blood flow. Expression of miRNAs in the brain is altered following cerebral ischemia [56], and several candidate miRNAs have been identified. In rodent models, miR-233 is up-regulated in ischemic brain and controls the response to neuronal injury by down-regulating the expression of glutamate receptors. This protects neurons from calcium influx mediated by extracellular glutamate accumulation that characterizes the excitotoxicity phase of brain ischemia. A lack of miR-223 leads to memory deficits and neuronal cell death after stroke [57]. The miR-200 and miR-182 families are down-regulated in the brain of hibernating squirrels and inhibition of their activities protects neuronal cells from oxygen and glucose deprivation-induced death [58]. In a rat model of global cerebral ischemia, miR-181c regulates microglial-mediated neuronal apoptosis following ischemia/reperfusion injury [59]. miR-181c directly targets the 3’ untranslated region of TNF-α mRNA, inhibiting apoptosis mediated by TNF-α from activated microglial cells [59]. These data suggest that miRNAs are functionally important mediators of neurological impairment in ischemic brain. Thus, miRNAs may represent not only new biomarkers for neurological prognosis but also novel candidates for neuroprotective therapy targets that may be investigated following cardiac arrest.
Neuroprotection and microRNAs
A few miRNAs have been identified in therapeutic strategies aiming at protecting the ischemic brain. Valproic acid, a histone deacetylase inhibitor that reduces neurological sequelae and improves motor activity following stroke in rodents [60], regulates miR-331 expression in ischemic neuronal cells [56]. Combined therapy with bortezomib, a proteasome inhibitor approved for treatment of patients with multiple myeloma [61], and tissue plasminogen activator, which is neuroprotective after stroke in aged rats, is associated with an increase of miR-146a expression in cerebral endothelial cells [56,62]. However, whether these miRNAs are neuroprotective per se remains to be demonstrated.
Mild induced hypothermia and microRNAs
Numerous experimental models and two randomized clinical trials have suggested that mild induced hypothermia improves neurological outcome in patients who remain unconscious following out-of-hospital cardiac arrest. This treatment is now standard care in many intensive care units. However, the use of therapeutic hypothermia has been recently challenged by the results of our TTM trial (Target Temperature Management After Cardiac Arrest), which showed that lowering body temperature to 33°C in unconscious survivors of out-of-hospital cardiac arrest did not confer protection compared with 36°C [35]. The putative mechanisms of neuroprotection have been extensively explored in experimental models but there has been little focus on the expression and function of miRNAs. Recent studies have reported that hypothermia regulates miRNAs expression. Truettner and colleagues [63] showed that miRNAs are dysregulated in the brain of hypothermic rats. Pilotte and colleagues [64] showed that hypothermia regulates miRNA expression through enhanced processing of pre-miRNAs by Dicer. The cold-responsive protein Rbm3, a glycine-rich RNA-binding protein, is implicated in disinhibition of Dicer in this process [65]. In pigs subjected to cardiogenic shock, mild induced hypothermia down-regulated plasma levels of miR-122 [66]. Further research is needed to determine whether miRNAs are key players in the neuroprotective effects of cooling. If this could be demonstrated, miRNAs would represent a novel class of neuroprotective agents that would deserve further testing.
MicroRNAs as therapeutic target
Several lines of evidence support the concept that miRNAs functionally involved in the response of the brain to ischemic injury and miRNAs participating in the neuroprotective effects of hypothermia may be interesting therapeutic targets, either to protect the brain from neurological damage or to stimulate neurological repair after cardiac arrest. This assumption is supported by recent reports, including that of Selvamani and colleagues [67] showing that antagomirs to Let7f or miR-1 are able to extend the neuroprotection afforded by insulin-like growth factor-1 in a rat model of cerebral ischemia. In addition, locked nucleic acid anti-miR130a reduced infarct volume and promoted recovery after transient focal cerebral ischemia in rats [68].
The finding that exosomes conveying miRNAs are able to cross the blood-brain barrier [69] suggests that simple intravenous injection of artificial exosomes may represent an effective way of delivering miRNAs to the ischemic brain. Therefore, miRNAs are promising therapeutic targets that may be further tested, alone or in adjunction with hypothermia, to improve neurological recovery of patients with cardiac arrest.
MicroRNAs as prognostic biomarkers after cardiac arrest
The biomarker value of miRNAs after cerebral ischemia has been suggested by the observation that specific miRNAs have been detected in the blood after ischemic stroke in both animals [70] and humans [71]. In addition, some of these miRNAs might be potential biomarkers of ischemic stroke [71-73]. As a first attempt to identify miRNAs with prognostic value after cardiac arrest, we performed a proof-of-concept study in which we compared the plasma miRnome of 14 patients with favorable outcome and 14 patients with poor outcome after cardiac arrest [74]. Using microarrays covering almost 700 miRNAs (miRBase release 12.0), we observed a miRNA biosignature linked to outcome. Among miRNAs differentially expressed between patients with favorable outcome and patients with poor outcome, miR-122 and miR-21 were significant predictors of neurological outcome (areas under the receiver-operating characteristic curve of 0.73 and 0.77, respectively) and mortality (P < 0.05) at 6 months. We could verify that miR-122 and miR-21 were reliably expressed by neuronal cells, as also shown elsewhere [75,76], comforting our working hypothesis that miRNAs originating from dying neurons after cardiac arrest can be measured in the bloodstream. Consistent with this hypothesis was the demonstration that exosomes, which carry miRNAs outside cells, are able to cross the blood-brain barrier [69]. In addition, disruption of the blood-brain barrier has been shown after cerebral ischemia, which may facilitate the release of neuron-derived miRNAs into the bloodstream [77]. Sheinerman and colleagues [78] identified brain-enriched miRNAs in the blood of patients with mild cognitive impairment, an early stage of multiple neurodegenerative diseases. Thus, brain-derived miRNAs present in the bloodstream after cardiac arrest may indicate neurological damage. Since the extent of neurological damage is a critical determinant of post-cardiac arrest recovery, it is expected that circulating miRNAs may have an interesting prognostic value in this setting. A recent study from our group showing that brain-enriched miR-124 is associated with neurological outcome after cardiac arrest confirmed this assumption [79]. In future studies, the added value of this novel category of biomarkers over existing tools will have to be determined. Also, the sensitivity and specificity of miRNAs, as well as their usefulness for early prediction, will have to outperform current electrophysiological and neuroimaging tools. Finally, the techniques used to quantify miRNAs, which are still time-consuming, will have to be improved, both in terms of reproducibility, rapidity, cost, and standardization.
Conclusion and future perspectives
The discovery of miRNAs as regulators of gene expression has generated considerable excitement amongst researchers. A number of studies have been conducted addressing their potential as diagnostic, prognostic or therapeutic targets in cerebral and cardiovascular diseases. The value of miRNAs as biomarkers in patients resuscitated following cardiac arrest has, however, received little attention to date. Pilot studies suggest that miRNAs may be useful predictors of neurological outcome and survival after cardiac arrest and adequately powered studies should be undertaken to validate these preliminary findings. Ideally, these studies will determine the optimal time for blood sampling, evaluate the added value of miRNAs over existing prognostic tools, and consider multimarker strategies. Importantly, while it has been shown that cardiac-enriched miRNAs are released very early after cardiac injury [51], the kinetics of release of brain-derived miRNAs after cardiac arrest will have to be accurately characterized. The following main technical issues regarding the measurement of circulating levels of miRNAs will have to be considered: advantages and drawbacks of assessing miRNAs in whole blood versus plasma, and appropriate normalization procedure. Interestingly, miRNAs may function not only as novel biomarkers but also as potential therapeutic targets following cardiac arrest [80].
Acknowledgements
The authors thank Dr. Daniel Wagner, Department of Cardiology, Centre Hospitalier Luxembourg, for continuous support. We also thank Emeline Goretti for providing Figure 1. Supported by grants from the National Research Fund of Luxembourg to YD (# C10/BM/785036).
Abbreviations
- CK-BB
Creatine phosphokinase brain-brain
- miRNA
microRNA
- NSE
Neuron-specific enolase
- TNF
Tumor necrosis factor
Footnotes
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Yvan Devaux, Email: yvan.devaux@crp-sante.lu.
Pascal Stammet, Email: Stammet.Pascal@chl.lu.
Hans Friberg, Email: hans.friberg@skane.se.
Christian Hassager, Email: Christian.hassager@rh.regionh.dk.
Michael A Kuiper, Email: m.kuiper@hccg-online.eu.
Matt P Wise, Email: mattwise@doctors.org.uk.
Niklas Nielsen, Email: niklas.nielsen@telia.com.
References
- 1.Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35:2196–2204. doi: 10.1097/01.CCM.0000281517.97507.6E. [DOI] [PubMed] [Google Scholar]
- 2.Arrich J, European Resuscitation Council Hypothermia After Cardiac Arrest Registry Study Group Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35:1041–1047. doi: 10.1097/01.CCM.0000259383.48324.35. [DOI] [PubMed] [Google Scholar]
- 3.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S, Quality Standards Subcommittee of the American Academy of Neurology Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 4.Oksanen T, Tiainen M, Skrifvars MB, Varpula T, Kuitunen A, Castren M, Pettila V. Predictive power of serum NSE and OHCA score regarding 6-month neurologic outcome after out-of-hospital ventricular fibrillation and therapeutic hypothermia. Resuscitation. 2009;80:165–170. doi: 10.1016/j.resuscitation.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Rundgren M, Karlsson T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009;80:784–789. doi: 10.1016/j.resuscitation.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 7.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 8.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 9.Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, Goren Y, Chajut A. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karkela J, Bock E, Kaukinen S. CSF and serum brain-specific creatine kinase isoenzyme (CK-BB), neuron-specific enolase (NSE) and neural cell adhesion molecule (NCAM) as prognostic markers for hypoxic brain injury after cardiac arrest in man. J Neurol Sci. 1993;116:100–109. doi: 10.1016/0022-510X(93)90095-G. [DOI] [PubMed] [Google Scholar]
- 12.Bottiger BW, Mobes S, Glatzer R, Bauer H, Gries A, Bartsch P, Motsch J, Martin E. Astroglial protein S-100 is an early and sensitive marker of hypoxic brain damage and outcome after cardiac arrest in humans. Circulation. 2001;103:2694–2698. doi: 10.1161/01.CIR.103.22.2694. [DOI] [PubMed] [Google Scholar]
- 13.Tirschwell DL, Longstreth WT, Jr, Rauch-Matthews ME, Chandler WL, Rothstein T, Wray L, Eng LJ, Fine J, Copass MK. Cerebrospinal fluid creatine kinase BB isoenzyme activity and neurologic prognosis after cardiac arrest. Neurology. 1997;48:352–357. doi: 10.1212/WNL.48.2.352. [DOI] [PubMed] [Google Scholar]
- 14.Longstreth WT, Jr, Clayson KJ, Sumi SM. Cerebrospinal fluid and serum creatine kinase BB activity after out-of-hospital cardiac arrest. Neurology. 1981;31:455–458. doi: 10.1212/WNL.31.4_Part_2.455. [DOI] [PubMed] [Google Scholar]
- 15.Stammet P, Wagner DR, Gilson G, Devaux Y. Modeling serum level of S100B and bispectral index to predict outcome after cardiac arrest. J Am Coll Cardiol. 2013;62:851–858. doi: 10.1016/j.jacc.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 16.Scolletta S, Donadello K, Santonocito C, Franchi F, Taccone FS. Biomarkers as predictors of outcome after cardiac arrest. Expert Rev Clin Pharmacol. 2012;5:687–699. doi: 10.1586/ecp.12.64. [DOI] [PubMed] [Google Scholar]
- 17.Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Abe R, Tateishi Y, Hattori N, Shimada T, Hirasawa H. S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care. 2009;13:R121. doi: 10.1186/cc7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roine RO, Somer H, Kaste M, Viinikka L, Karonen SL. Neurological outcome after out-of-hospital cardiac arrest. Prediction by cerebrospinal fluid enzyme analysis. Arch Neurol. 1989;46:753–756. doi: 10.1001/archneur.1989.00520430047015. [DOI] [PubMed] [Google Scholar]
- 19.Zandbergen EG, Hijdra A, Koelman JH, Hart AA, Vos PE, Verbeek MM, de Haan RJ, PROPAC Study Group Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66:62–68. doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 20.Grubb NR, Simpson C, Sherwood RA, Abraha HD, Cobbe SM, O’Carroll RE, Deary I, Fox KA. Prediction of cognitive dysfunction after resuscitation from out-of-hospital cardiac arrest using serum neuron-specific enolase and protein S-100. Heart. 2007;93:1268–1273. doi: 10.1136/hrt.2006.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prohl J, Rother J, Kluge S, de Heer G, Liepert J, Bodenburg S, Pawlik K, Kreymann G. Prediction of short-term and long-term outcomes after cardiac arrest: a prospective multivariate approach combining biochemical, clinical, electrophysiological, and neuropsychological investigations. Crit Care Med. 2007;35:1230–1237. doi: 10.1097/01.CCM.0000261892.10559.85. [DOI] [PubMed] [Google Scholar]
- 22.Reisinger J, Höllinger K, Lang W, Steiner C, Winter T, Zeindlhofer E, Mori M, Schiller A, Lindorfer A, Wiesinger K, Siostrzonek P. Prediction of neurological outcome after cardiopulmonary resuscitation by serial determination of serum neuron-specific enolase. Eur Heart J. 2007;28:52–58. doi: 10.1093/eurheartj/ehl316. [DOI] [PubMed] [Google Scholar]
- 23.Krumnikl JJ, Bottiger BW, Strittmatter HJ, Motsch J. Complete recovery after 2 h of cardiopulmonary resuscitation following high-dose prostaglandin treatment for atonic uterine haemorrhage. Acta Anaesthesiol Scand. 2002;46:1168–1170. doi: 10.1034/j.1399-6576.2002.460920.x. [DOI] [PubMed] [Google Scholar]
- 24.Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34:2881–2886. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 25.Steffen IG, Hasper D, Ploner CJ, Schefold JC, Dietz E, Martens F, Nee J, Krueger A, Jorres A, Storm C. Mild therapeutic hypothermia alters neuron specific enolase as an outcome predictor after resuscitation: 97 prospective hypothermia patients compared to 133 historical non-hypothermia patients. Crit Care. 2010;14:R69. doi: 10.1186/cc8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009;133:223–228. doi: 10.1016/j.ijcard.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Stern P, Bartos V, Uhrova J, Bezdickova D, Vanickova Z, Tichy V, Pelinkova K, Prusa R, Zima T. Performance characteristics of seven neuron-specific enolase assays. Tumour Biol. 2007;28:84–92. doi: 10.1159/000098441. [DOI] [PubMed] [Google Scholar]
- 28.Annborn M, Dankiewicz J, Erlinge D, Hertel S, Rundgren M, Smith JG, Struck J, Friberg H. Procalcitonin after cardiac arrest - an indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation. 2013;84:782–787. doi: 10.1016/j.resuscitation.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Fries M, Kunz D, Gressner AM, Rossaint R, Kuhlen R. Procalcitonin serum levels after out-of-hospital cardiac arrest. Resuscitation. 2003;59:105–109. doi: 10.1016/S0300-9572(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 30.Stammet P, Devaux Y, Azuaje F, Werer C, Lorang C, Gilson G, Max M. Assessment of procalcitonin to predict outcome in hypothermia-treated patients after cardiac arrest. Crit Care Res Pract. 2011;2011:631062. doi: 10.1155/2011/631062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashida H, Kaneko T, Kasaoka S, Oshima C, Miyauchi T, Fujita M, Oda Y, Tsuruta R, Maekawa T. Comparison of the predictability of neurological outcome by serum procalcitonin and glial fibrillary acidic protein in postcardiac-arrest patients. Neurocrit Care. 2010;12:252–257. doi: 10.1007/s12028-009-9318-5. [DOI] [PubMed] [Google Scholar]
- 32.Dankiewicz J, Linder A, Annborn M, Rundgren M, Friberg H. Heparin-binding protein: an early indicator of critical illness and predictor of outcome in cardiac arrest. Resuscitation. 2013;84:935–939. doi: 10.1016/j.resuscitation.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Mörtberg E, Zetterberg H, Nordmark J, Blennow K, Rosengren L, Rubertsson S. S-100B is superior to NSE, BDNF and GFAP in predicting outcome of resuscitation from cardiac arrest with hypothermia treatment. Resuscitation. 2011;82:26–31. doi: 10.1016/j.resuscitation.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen N, Hovdenes J, Nilsson F, Rubertsson S, Stammet P, Sunde K, Valsson F, Wanscher M, Friberg H, Hypothermia N. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53:926–934. doi: 10.1111/j.1399-6576.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Åneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 39.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 40.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat Rev Neurosci. 2012;13:528–541. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/S0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 43.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 44.Fiedler J, Thum T. MicroRNAs in myocardial infarction. Arterioscler Thromb Vasc Biol. 2013;33:201–205. doi: 10.1161/ATVBAHA.112.300137. [DOI] [PubMed] [Google Scholar]
- 45.Salic K, De Windt LJ. MicroRNAs as biomarkers for myocardial infarction. Curr Atheroscler Rep. 2012;14:193–200. doi: 10.1007/s11883-012-0238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Widera C, Gupta SK, Lorenzen JM, Bang C, Bauersachs J, Bethmann K, Kempf T, Wollert KC, Thum T. Diagnostic and prognostic impact of six circulating microRNAs in acute coronary syndrome. J Mol Cell Cardiol. 2011;51:872–875. doi: 10.1016/j.yjmcc.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Eitel I, Adams V, Dieterich P, Fuernau G, de Waha S, Desch S, Schuler G, Thiele H. Relation of circulating microRNA-133a concentrations with myocardial damage and clinical prognosis in ST-elevation myocardial infarction. Am Heart J. 2012;164:706–714. doi: 10.1016/j.ahj.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, Komuro I. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res. 2013;113:322–326. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 49.Devaux Y, McCann GP, Wagner DR, Squire IB. Prognostic microRNAs after AMI. Circ Res. 2013;113:e46–e47. doi: 10.1161/CIRCRESAHA.113.302030. [DOI] [PubMed] [Google Scholar]
- 50.Devaux Y, Mueller M, Haaf P, Goretti E, Twerenbold R, Zangrando J, et al. Diagnostic and prognostic value of circulating microRNAs in patients with acute chest pain. J Intern Med;2013. doi:10.1111/joim.12183. [DOI] [PubMed]
- 51.Devaux Y, Vausort M, Goretti E, Nazarov PV, Azuaje F, Gilson G, Corsten MF, Schroen B, Lair ML, Heymans S, Wagner DR. Use of circulating microRNAs to diagnose acute myocardial infarction. Clin Chem. 2012;58:559–567. doi: 10.1373/clinchem.2011.173823. [DOI] [PubMed] [Google Scholar]
- 52.Devaux Y, Vausort M, McCann G, Zangrando J, Kelly D, Razvi N, Zhang L, Ng LL, Wagner DR, Squire IB. MicroRNA-150: a novel marker of left ventricular remodeling after acute myocardial infarction. Circ Cardiovasc Genet. 2013;6:290–298. doi: 10.1161/CIRCGENETICS.113.000077. [DOI] [PubMed] [Google Scholar]
- 53.Devaux Y, Vausort M, McCann GP, Kelly D, Collignon O, Ng LL, Wagner DR, Squire IB. A panel of 4 microRNAs facilitates the prediction of left ventricular contractility after acute myocardial infarction. PLoS One. 2013;8:e70644. doi: 10.1371/journal.pone.0070644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 55.Kagias K, Nehammer C, Pocock R. Neuronal responses to physiological stress. Front Genet. 2012;3:222. doi: 10.3389/fgene.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunsberger JG, Fessler EB, Wang Z, Elkahloun AG, Chuang DM. Post-insult valproic acid-regulated microRNAs: potential targets for cerebral ischemia. Am J Transl Res. 2012;4:316–332. [PMC free article] [PubMed] [Google Scholar]
- 57.Harraz MM, Eacker SM, Wang X, Dawson TM, Dawson VL. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc Natl Acad Sci. 2012;109:18962–18967. doi: 10.1073/pnas.1121288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee YJ, Johnson KR, Hallenbeck JM. Global protein conjugation by ubiquitin-like-modifiers during ischemic stress is regulated by microRNAs and confers robust tolerance to ischemia. PLoS One. 2012;7:e47787. doi: 10.1371/journal.pone.0047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L, Dong LY, Li YJ, Hong Z, Wei WS. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J Neuroinflammation. 2012;9:211. doi: 10.1186/1742-2094-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- 61.Voorhees PM, Dees EC, O’Neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316–6325. [PubMed] [Google Scholar]
- 62.Zhang L, Chopp M, Liu X, Teng H, Tang T, Kassis H, Zhang ZG. Combination therapy with VELCADE and tissue plasminogen activator is neuroprotective in aged rats after stroke and targets microRNA-146a and the toll-like receptor signaling pathway. Arterioscler Thromb Vasc Biol. 2012;32:1856–1864. doi: 10.1161/ATVBAHA.112.252619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Truettner JS, Alonso OF, Bramlett HM, Dietrich WD. Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J Cereb Blood Flow Metab. 2011;31:1897–1907. doi: 10.1038/jcbfm.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pilotte J, Dupont-Versteegden EE, Vanderklish PW. Widespread regulation of miRNA biogenesis at the Dicer step by the cold-inducible RNA-binding protein, RBM3. PLoS One. 2011;6:e28446. doi: 10.1371/journal.pone.0028446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci U S A. 2005;102:1865–1870. doi: 10.1073/pnas.0409764102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersson P, Gidlof O, Braun OO, Gotberg M, van der Pals J, Olde B, Erlinge D. Plasma levels of liver-specific miR-122 is massively increased in a porcine cardiogenic shock model and attenuated by hypothermia. Shock. 2012;37:234–238. doi: 10.1097/SHK.0b013e31823f1811. [DOI] [PubMed] [Google Scholar]
- 67.Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sepramaniam S, Ying LK, Armugam A, Wintour EM, Jeyaseelan K. MicroRNA-130a represses transcriptional activity of Aquaporin 4 M1 promoter. J Biol Chem. 2012;287:12006–12015. doi: 10.1074/jbc.M111.280701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lakhal S, Wood MJA. Exosome nanotechnology: an emerging paradigm shift in drug delivery. Bioessays. 2011;33:737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 70.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 71.Gan CS, Wang CW, Tan KS. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genet Mol Res. 2012;11:147–152. doi: 10.4238/2012.January.27.1. [DOI] [PubMed] [Google Scholar]
- 72.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, Zheng C, Cheng Q, Chen S, Yang GY. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci (Elite Ed) 2011;3:1265–1272. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 74.Stammet P, Goretti E, Vausort M, Zhang L, Wagner DR, Devaux Y. Circulating microRNAs after cardiac arrest. Crit Care Med. 2012;40:3209–3214. doi: 10.1097/CCM.0b013e31825fdd5e. [DOI] [PubMed] [Google Scholar]
- 75.Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. J Neurosci. 2012;32:17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheinerman KS, Tsivinsky VG, Crawford F, Mullan MJ, Abdullah L, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging (Albany NY) 2012;4:590–605. doi: 10.18632/aging.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilje P, Gidlof O, Rundgren M, Cronberg T, Al-Mashat M, Olde B, Friberg H, Erlinge D. The brain-enriched microRNA miR-124 in plasma predicts neurological outcome after cardiac arrest. Crit Care. 2014;18:R40. doi: 10.1186/cc13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tisherman SA, Rittenberger J. Should our crystal ball after cardiac arrest include one of the building blocks of life? Crit Care Med. 2012;40:3321–3323. doi: 10.1097/CCM.0b013e31826536c9. [DOI] [PubMed] [Google Scholar]
- 81.Goretti E, Wagner DR, Devaux Y. Regulation of endothelial progenitor cell function by microRNAs. Minerva Cardioangiol. 2013;61:591–604. [PubMed] [Google Scholar]


