Abstract
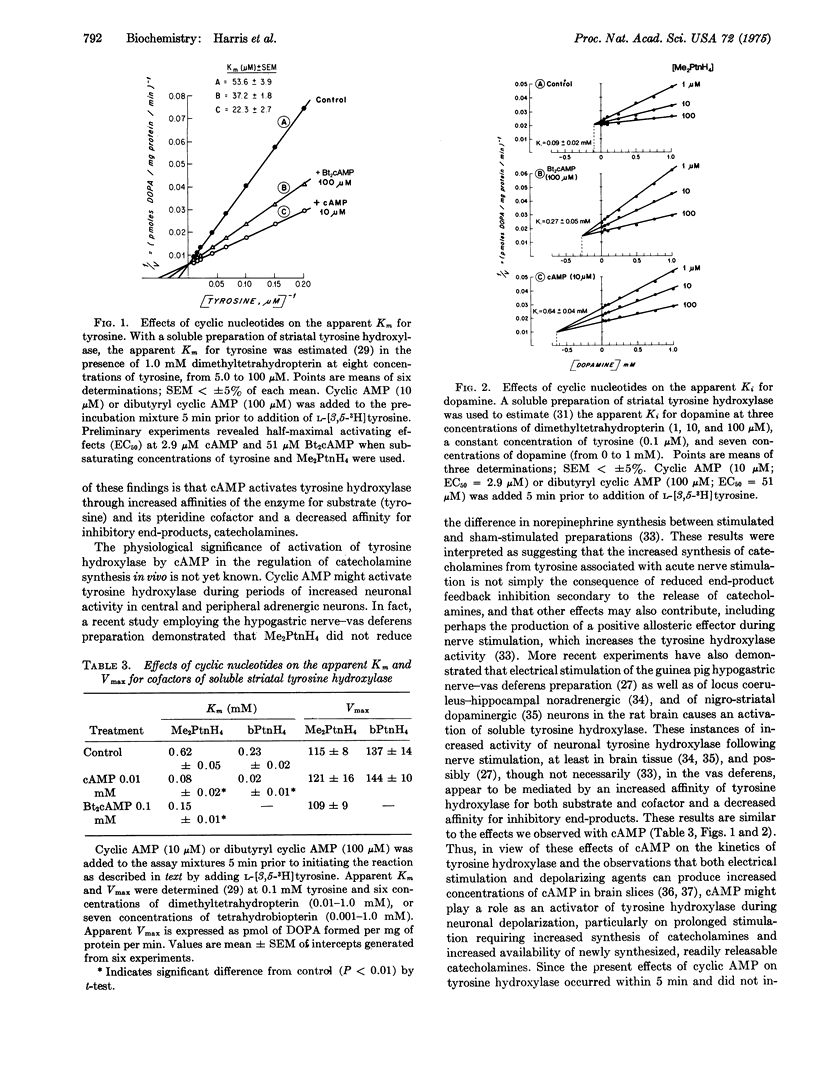
Membrane-permeable derivatives of cyclic AMP (cAMP) produced concentration-dependent increases in activity of tyrosine hydroxylase (L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2) in membrane-limited nerve endings (synaptosomes) prepared from three regions of rat brain. Increased hydroxylation occurred even after preincubation and removal of dibutyryl cyclic AMP. In all brain regions, the hydroxylation of phenylalanine and tyrosine was increased, but dibutyryl cAMP had little effect on activity of tryptophan hydroxylase, no effect on aromatic amino-acid decarboxylase, on uptake of tyrosine or phenylalanine, uptake or efflux of dopamine, or distribution of hydroxylase between cytoplasmic and particulate components of the synaptosomes. Dibutyryl cAMP decreased inhibition of catecholamine synthesis in synaptosomes by dopamine and apomorphine. In a soluble preparation of striatal tyrosine hydroxylase, activity was increased by addition of lower concentrations of cAMP or dibutyryl cAMP than with unbroken nerve endings, when subsaturating concentrations of tyrosine and cofactor were employed, while butyrate, chloride, 5'-AMP, ADP, ATP, and cyclic GMP had no activating effect. Increased activity of soluble tyrosine hydroxylase was reflected in increased affinity (Km) for substrate and cofactor and decreased affinity (Ki) for inhibitory end-product (dopamine), suggesting a change in the physical-chemical state of the enzyme or an activator molecule. Cyclic AMP may activate tyrosine hydroxylase during periods of increased neuronal activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. E., Siggins G. R., Hoffer B. J. Interpreting the failures to confirm the depression of cerebellar Purkinje cells by cyclic AMP. Science. 1974 Aug 16;185(4151):627–629. doi: 10.1126/science.185.4151.627. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Makman M. H. Stimulation by dopamine of adenylate cyclase in retinal homogenates and of adenosine-3':5'-cyclic monophosphate formation in intact retina. Proc Natl Acad Sci U S A. 1972 Mar;69(3):539–543. doi: 10.1073/pnas.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier G., Weiner N. Further studies on the increased synthesis of norepinephrine during nerve stimulation of guinea-pig vas deferens preparation: effect of tyrosine and 6, 7-dimethyltetrahydropterin. J Pharmacol Exp Ther. 1973 Jul;186(1):75–85. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M., Anagnoste B., Shirron C. The effect of trivastal, haloperidol and dibutyryl cyclic AMP on (14C)dopamine synthesis in rat striatum. J Pharm Pharmacol. 1973 Apr;25(4):348–351. doi: 10.1111/j.2042-7158.1973.tb10026.x. [DOI] [PubMed] [Google Scholar]
- Goodman R., Oesch F., Thoenen H. Changes in enzyme patterns produced by high potassium concentration and dibutyryl cyclic AMP in organ cultures of sympathetic ganglia. J Neurochem. 1974 Aug;23(2):369–378. doi: 10.1111/j.1471-4159.1974.tb04368.x. [DOI] [PubMed] [Google Scholar]
- Harris J. E., Baldessarini R. J. Letter: Amphetamine-induced inhibition of tyrosine hydroxylation in homogenates of rat corpus striatum. J Pharm Pharmacol. 1973 Sep;25(9):755–757. doi: 10.1111/j.2042-7158.1973.tb10063.x. [DOI] [PubMed] [Google Scholar]
- Harris J. E., Baldessarini R. J. Uptake of (3H)-catecholamines by homogenates of rat corpus striatum and cerebral cortex: effects of amphetamine analogues. Neuropharmacology. 1973 Jul;12(7):669–679. doi: 10.1016/0028-3908(73)90120-2. [DOI] [PubMed] [Google Scholar]
- Harris J. E., Morgenroth V. H., 3rd, Roth R. H., Baldessarini R. J. Regulation of catecholamine synthesis in the rat brain in vitro by cyclic AMP. Nature. 1974 Nov 8;252(5479):156–158. doi: 10.1038/252156a0. [DOI] [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Oliver A. P., Bloom F. E. Cyclic AMP mediation of norepinephrine inhibition in rat cerebellar cortex: a unique class of synaptic responses. Ann N Y Acad Sci. 1971 Dec 30;185:531–549. doi: 10.1111/j.1749-6632.1971.tb45279.x. [DOI] [PubMed] [Google Scholar]
- Huang M., Daly J. W. Interrelationships among the levels of ATP, adenosine and cyclic AMP in incubated slices of guinea-pig cerebral cortex: effects of depolarizing agents, psychotropic drugs and metabolic inhibitors. J Neurochem. 1974 Aug;23(2):393–404. doi: 10.1111/j.1471-4159.1974.tb04371.x. [DOI] [PubMed] [Google Scholar]
- KLAINER L. M., CHI Y. M., FREIDBERG S. L., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. IV. The effects of neurohormones on the formation of adenosine 3',5'-phosphate by preparations from brain and other tissues. J Biol Chem. 1962 Apr;237:1239–1243. [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W., McIlwain H. The effect of electrical stimulation upon the accumulation of adenosine 3',5'-phosphate in isolated cerebral tissue. J Neurochem. 1969 Apr;16(4):485–491. doi: 10.1111/j.1471-4159.1969.tb06847.x. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. The influence of chemical agents on the accumulation of adenosine 3',5'-Phosphate in slices of rabbit cerebellum. Mol Pharmacol. 1968 Jul;4(4):367–378. [PubMed] [Google Scholar]
- Karobath M., Baldessarini R. J. Formation of catechol compounds from phenylalanine and tyrosine with isolated nerve endings. Nat New Biol. 1972 Apr 19;236(68):206–208. doi: 10.1038/newbio236206a0. [DOI] [PubMed] [Google Scholar]
- Karobath M. Serotonin synthesis with rat brain synaptosomes. Effects of serotonin and monoamineoxidase inhibitors. Biochem Pharmacol. 1972 May 1;21(9):1253–1263. doi: 10.1016/0006-2952(72)90287-0. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971 Dec 24;174(4016):1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R. T., Mandell A. J. Regulatory properties of soluble and particulate rat brain tyrosine hydroxylase. J Biol Chem. 1972 May 25;247(10):3114–3122. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetñanský R., Gewirtz G. P., Weise V. K., Kopin I. J. Effect of dibutyryl cyclic-AMP on adrenal catecholamine-synthesizing enzymes in repeatedly immobilized hypophysectomized rats. Endocrinology. 1971 Jul;89(1):50–55. doi: 10.1210/endo-89-1-50. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgenroth V. H., 3rd, Boadle-Biber M., Roth R. H. Tyrosine hydroxylase: activation by nerve stimulation. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4283–4287. doi: 10.1073/pnas.71.11.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach M. J. Stimulation of release of adrenal catecholamine by adenosine 3':5'-cyclic monophosphate and theophylline in the absence of extracellular Ca 2+ . Proc Natl Acad Sci U S A. 1972 Apr;69(4):834–836. doi: 10.1073/pnas.69.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. H., Salzman P. M., Morgenroth V. H., 3rd Noradrenergic neurons: allosteric activation of hippocampal tyrosine hydroxylase by stimulation of the locus coeruleus. Biochem Pharmacol. 1974 Oct 1;23(19):2779–2784. doi: 10.1016/0006-2952(74)90051-3. [DOI] [PubMed] [Google Scholar]
- Shiman R., Akino M., Kaufman S. Solubilization and partial purification of tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1971 Mar 10;246(5):1330–1340. [PubMed] [Google Scholar]
- Shimizu H., Daly J. W., Creveling C. R. A radioisotopic method for measuring the formation of adenosine 3',5'-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969 Dec;16(12):1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten G. F., Thoa N. B., Kopin I. J., Axelrod J. Enhanced release of dopamine -hydroxylase and norepinephrine from sympathetic nerves by dibutyryl cyclic adenosine 3', 5'-monophosphate and theophylline. Mol Pharmacol. 1973 Mar;9(2):178–183. [PubMed] [Google Scholar]


