Abstract
Women diagnosed with breast cancer within 5 years postpartum have poor survival rates. The process of postpartum mammary gland involution, whereby the lactating gland remodels to its pre-pregnant state, promotes breast cancer progression in xenograft models. Macrophage influx occurs during mammary gland involution, implicating immune modulation in the promotion of postpartum breast cancer. Herein, we characterize the postpartum murine mammary gland and find an orchestrated influx of immune cells similar to that which occurs during wound healing. Further, the normal involuting gland may be in an immunosuppressed state as discerned by the transient presence of Foxp3+ regulatory T cells and IL-10+ macrophages with T cell suppressive function. To determine the influence of the postpartum immune microenvironment on mammary tumor promotion, we developed an immune-competent model. In this model, mammary tumors in the involution group are six-fold larger than nulliparous group tumors, have decreased CD4+ and CD8+ T cell infiltrates and contain a greater number of macrophages with the ability to inhibit T cell activation. Targeting involution with a neutralizing antibody against the immunosuppressive cytokine IL-10 reduces tumor growth in involution group mice but not in nulliparous mice, implicating the involution microenvironment as the primary target of αIL-10 treatment. Relevance to women is implicated, as we find post-lactational human breast tissue has transient high IL-10+ and Foxp3+ immune cell infiltrate. These data show an immune modulated microenvironment within the normal involuting mammary gland suggestive of immunosuppression, that when targeted reduces tumor promotion, revealing possible immune-based strategies for postpartum breast cancer.
Keywords: Macrophages, myeloid derived suppressor cells, regulatory T cells, postpartum breast cancer, immune modulation
Introduction
Postpartum mammary gland involution is a multifaceted tissue remodeling process that returns the lactation-competent gland to a quiescent state ready to respond to new gestational signals1, 2. Postpartum involution is characterized by extensive death of the mammary secretory epithelium followed by repopulation of the gland with a fibrous and adipocyte rich stroma. While involution is a physiologically normal, developmentally orchestrated tissue-remodeling process, it shares striking similarities with pathologically induced wound-healing and tumor-promotional microenvironments3–5. Consistent with tumor promotion, in a SCID xenograft model we reported that breast cancer cells exposed to the postpartum involution microenvironment have increased growth, invasion and metastasis compared to nulliparous hosts5. Of potential relevance, young women diagnosed with breast cancer in the postpartum window have a near three-fold increased risk of recurrence and death compared to nulliparous women, even after adjusting for patient age, cancer stage and breast cancer subtype, identifying a postpartum diagnosis as an independent risk factor for metastasis6–8. Based on these observations, we predict that further understanding of the immune infiltrate during postpartum mammary gland involution will elucidate novel mechanisms of tumor progression and identify potential therapeutic targets for the prevention and treatment of postpartum breast cancer.
Weaning-induced involution in the murine mammary gland occurs in two distinct phases. The early, reversible stage (days 1–3) is characterized by accumulation of milk, distended lumen and extensive death of the secretory mammary epithelium and the second, irreversible stage (days 3–8), with adipocyte repopulation and stromal restructuring1, 2. Molecular profiling of the murine mammary gland involution has provided gene signatures consistent with acute phase, innate and adaptive immune responses; however, the cellular basis for these signatures remains largely unknown4, 9. During involution, mammary epithelial cells themselves express transcripts traditionally associated with immune cells10, 11, and acquire phagocytic capability12, making it difficult to assign immune-like gene signatures to specific cell types.
With regard to the role of the immune system during postpartum involution, little data exist for the adaptive arm; however, innate immune cells have been partially characterized. Granulocytes infiltrate the mouse gland on the first day of involution, implicating their role in the early cell death phase13. Recently, resident macrophages in the mammary gland were found to be essential in early involution, as deletion of colony stimulating factor-1 receptor positive cells during involution inhibited mammary epithelial cell death and restructuring of the gland back to its pre-pregnant state14. While the mechanism by which macrophages facilitate postpartum involution is currently unknown, the absence of early-phase mammary epithelial cell death is consistent with a role for resident tissue macrophages, rather than the macrophages that infiltrate in the second, remodeling phase. Resident mammary macrophages have yet to be phenotypically characterized, however, the second-phase infiltrating macrophages express low iNOS, high arginase-1 and the mannose receptor4, consistent with alternative activation or M2 polarization15. Importantly, the alternatively-activated macrophage phenotype correlates with tumor promotion in breast cancer patients16, and with metastasis in rodent models17.
To better understand the role of the immune system during postpartum mammary gland involution, as well as its potential influence on tumor cells exposed to this microenvironment, we characterized immune cells across involution and developed an immunocompetent mouse model of postpartum breast cancer. During gland involution, we find immune cell infiltrates consistent with acute, proliferative, and resolution phases of wound healing providing further evidence for similarities between the physiologic process of postpartum involution and wound healing. Moreover, we demonstrate for the first time that postpartum mammary tumors have a significant decrease in CD4+ and CD8+ T cell infiltrate and an increase in macrophages with T cell suppressive activity. Further, targeting involution with a neutralizing antibody against the immunosuppressive cytokine IL-10 reduces the tumor promotional microenvironment of involution. Our findings are consistent with the postpartum mammary gland involution having a microenvironment that favors tumor growth, in part through wound-healing like programs, which may account, in part, for the poor prognosis of women diagnosed with postpartum breast cancer.
Materials and Methods
Preclinical mouse models and tissue collection
University of Colorado IACUC approved all mouse procedures. For specific reproductive stages, Balb/c female mice (Jackson Laboratories) were bred with male C57Bl/6 male mice (Jackson Laboratories) in ventilated micro-isolator cages, with 12 hour light/dark cycles. Two days post-parturition litter sizes were standardized to 6–7 pups. Weaning was initiated at lactation days 10–14 (denoted L10). Mice were anesthetized with Isofurane (VetOne) and injected with 20,000 D2A1 cells in 20µl PBS into the right and left 4th mammary gland fat pads of involution day 1 or age-matched nulliparous group mice. The D2A1 cell line was a generous gift from Dr. Ann Chambers (Ontario, Canada) and was maintained as previously described18. For our treatment model, we treated involution mice by intraperitoneal injection with 0.2mg rat anti-IL-10 (JES5-2A5, BioXCell) or isotype control rat IgG (HRPN, BioXCell) every two days beginning on day 8 of lactation, L10, INV2, INV4, INV6 and ending on day 8 of involution, with same injection schedule in age-matched nulliparous controls. Tumor burden was evaluated by caliper measurement twice weekly following tumor cell injection, and calculated as width × length × length × 0.5. Mice were sacrificed at 3-weeks post-injection for tissue and blood isolation. Lymph node-free mammary gland 4–5 (tumor free mice) or tumor tissue was collected for flow cytometry, immunohistochemistry and western blot analysis. Primary blood leukocytes were collected by density gradient cell separation of whole blood using Ficoll then analyzed by flow cytometry. Blood granulocytes were uniformly removed across reproductive groups due to Ficoll separation.
Tissue digestion and immune cell enrichment
Single cell suspensions were prepared from mammary gland or tumor dissection by dicing tissue into 1mm fragments, followed by digestion for 2hours at 37°C in 1mg/mL collagenase (Sigma) and 10µg/mL hyaluronidase (Sigma) in HBSS while rotating. Spleens were diced into 1mm fragments and digested for 20mins at 37°C in 1mg/mL collagenase. Digestion mixtures were quenched using RPMI containing 10% FBS and filtered through a 70µm nylon strainer (BD Biosciences). Total cell digest of mammary gland and spleen were stained with trypan blue and total viable cell count determined by hemocytometer analysis. T cells were negatively selected from spleens using the T cell enrichment set (BD Biosciences), and CD11b+ cells were positively selected from mammary glands or tumors, using CD11b magnetic particles (BD Biosciences) per manufacturer’s instructions. The purity of isolation for all cell types was >90%, as evaluated by flow cytometry.
Leukocyte co-culture assay
Spleens were harvested from mice and T cells enriched as described above. CD11b+ cells were isolated from mammary glands or tumors as described above. Autologous splenic CD3+ T cells (8×105 cells) were co-cultured 1:1 with CD11b+ cells (8×105 cells) in RPMI 1640 (Invitrogen) containing 10% FBS and 1% penicillin-steptomycin (Hyclone) in a tissue culture grade 96-well plate. Mouse T-activator CD3/CD28 Dynabeads (Invitrogen) were used to activate T cells with and without CD11b+ cells and after 96 hours T cells were analyzed by flow cytometry for CD3, CD4, CD8, CD25, and CD69. Percentage T cell activation co-cultured with CD11b+ cells was normalized against % activation by T cells stimulated with CD3/CD28 Dynabeads (Supplementary Fig. S3B and S6B) and was calculated as fold change.
Human tissues
Breast specimens from pre-menopausal women age 20–45 who underwent clinically indicated biopsies or surgical procedures were obtained from a Colorado Multiple Institution Review Board approved protocol. Adjacent normal mammary tissues, confirmed free of pathology by a clinical pathologist, were grouped by reproductive categories of nulliparous (n=7), ≤1 month (n=6), >1–≤6 months (n=4), >6–≤12 months (n=5), >12–≤18 months (n=3), >18–≤24 months (n=7), >2–≤3 years (n=6), >3–≤6 years (n=8), >6–≤10 years (n=6), and >10 years postpartum (n=5).
Statistical analysis
All data were analyzed using the Student’s t test and are expressed as mean +/− Standard error of the mean (SEM) unless otherwise noted. Differences were considered significant when the P values were <0.05. Welch correction was used when variances between the groups were significantly different.
Results
Dynamic influx of immune cells during postpartum mammary gland involution
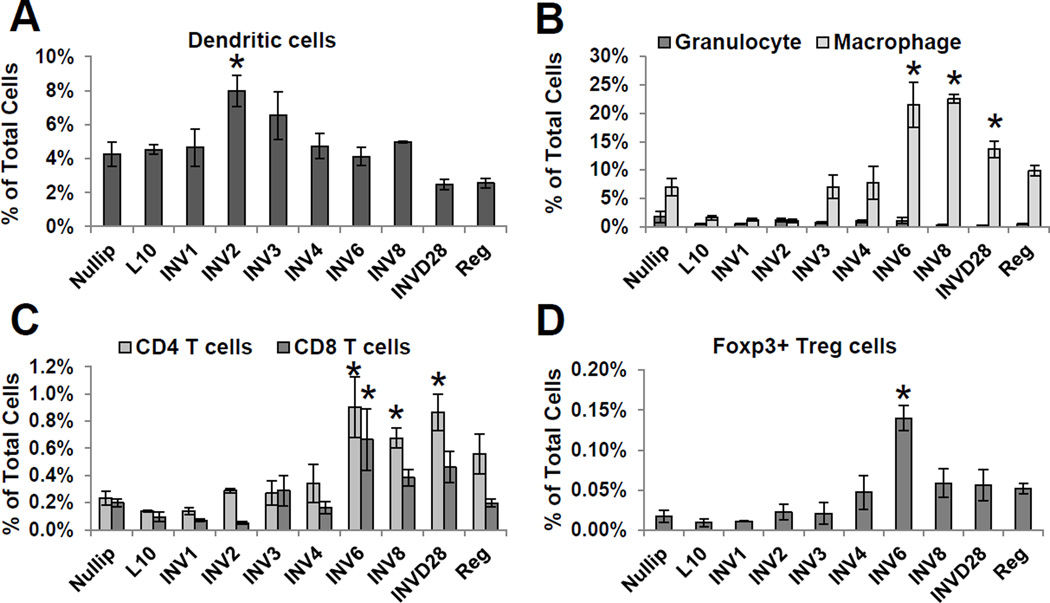
To characterize the immune cell composition of the murine mammary gland during postpartum involution, we utilized flow cytometry on whole mammary gland digests. Dendritic cells, granulocytes, macrophages, CD4+ T cells, CD8+ T cells, and regulatory T cells were investigated in nulliparous (Nullip), lactation day 10 (L10), weaning-induced involution (INV) days 1–4, 6, 8, and 28, and 12 weeks post-weaning regressed (Reg) mice (Supplementary Fig. S1). Dendritic cells were recruited early to the mammary gland, peaking on day 2 of involution (Fig. 1A). Granulocytes, positive for early myeloid lineage markers CD11b and GR1high, remained constant throughout the reproductive cycle (Fig. 1B). Macrophages, dual positive for the immature myeloid lineage marker GR1 and the differentiation macrophage marker F4/80, increased during mid-involution days 6–8 (Fig. 1B), in agreement with published data4, 13. T helper and cytotoxic T cells increased during mid-involution (Fig. 1C). Further, Foxp3+ regulatory T cells (Tregs) increased eight-fold on involution day 6 (Fig. 1D). These patterns of immune cell influx were mostly resolved by involution day 28, with continued elevation in macrophages and CD4 T cells, and fully resolved by 12 weeks postpartum. Taken together, these data demonstrate that the postpartum mammary gland utilizes immune programs to involute. Importantly, the early influx of dendritic cells followed by macrophages and T cells is a temporal pattern of immune cell infiltration consistent with classical wound healing patterns19.
Figure 1. Evidence for wound-like immune cell influx during mammary gland involution.
Flow cytometry analysis of immune cells in mammary gland digests from mice at distinct reproductive stages: nulliparous (Nullip), lactation day 10 (L10), involution (INV) days, 1, 2, 3, 4, 6, 8, and 28, and 12 weeks postpartum regressed (Reg). (A) Dendritic cells (CD11c+MHC-II+), (B) granulocytes (CD45+CD11b+GR1highF4/80−), macrophages (CD45+CD11b+ GR1int/loF4/80+), (C) CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), and (D) regulatory T cells (Treg) (CD3+CD4+CD25+FOXP3+). Mean values ±SEM (n=3–4mice/group), *P<0.05 vs nullip.
Involuting mammary gland immune profile is not apparent in the circulation
To determine whether the dynamic influx of immune cells into the involuting mammary gland coincides with changes in systemic immune cell populations, we analyzed blood samples corresponding to the reproductive stages described above. The dynamic changes in immune cell populations observed in the involuting mammary gland were not mirrored in the blood (Supplemental Fig. 2). Dendritic cells increased two-fold over nulliparous levels by day 4 of involution, and this increase was delayed with respect to the peak levels observed in the mammary gland (Supplemental Fig. 2A). Circulating monocytes, positive for both GR1 and F4/80, increased modestly over nulliparous levels, and preceded the increase in macrophage levels seen in the mammary gland (Supplemental Fig. 2B). Surprisingly, given the significant T cell subset changes within the mammary gland itself, systemic CD4+, CD8+, and Treg levels during involution were not significantly altered (Supplemental Fig. 2C–D). These data indicate that the immune cell influx observed in the mammary gland is largely independent of circulating immune cell levels, and likely driven by local, mammary specific events.
Involution macrophages exhibit markers of immune suppression
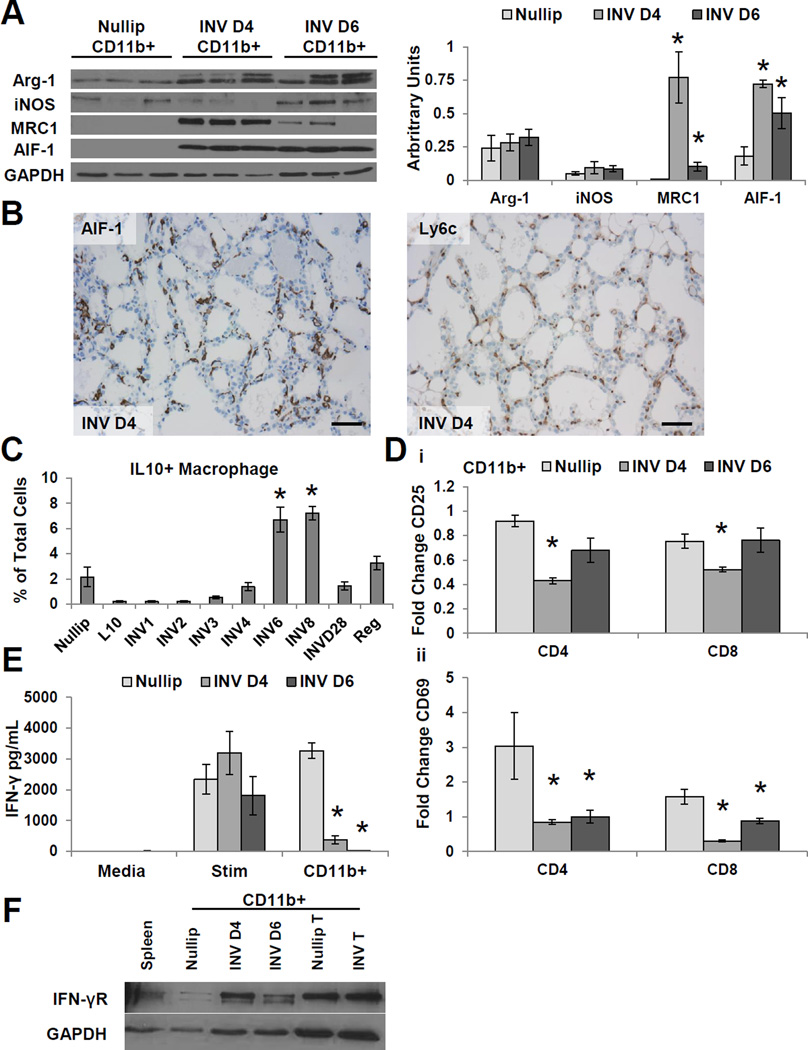
Tumor microenvironments enriched in macrophages predict poor outcomes in breast cancer20, 21. Macrophages present in the involuting mammary gland have been previously described by immunohistochemistry as alternatively activated/M2 macrophages, as they express arginase-1 (Arg-1) and the mannose receptor (MRC1)4. To further characterize the macrophage programing state and their function during normal involution, we isolated CD11b+ cells from nulliparous and day 4 and 6 involuting mouse mammary glands and evaluated them for classical M1 and M2 markers. The isolated CD11b+ cells were found to be approximately 90% positive for GR1 and F4/80, data consistent with a macrophage phenotype (Supplemental Fig. S3A). Both nulliparous and involution CD11b+ cells yielded similar levels of the M2 marker Arg-1 and the M1 marker iNOS (Fig. 2A). However, the involuting CD11b+ cells compared to nulliparous mammary gland derived CD11b+ cells expressed significantly higher levels of the M2 marker MRC1 and the inflammatory cytokine Allograft Inflammatory Factor-1 (AIF-1) (Fig. 2A). During tissue injury, resolution-macrophages have been shown to express both M1 and M2 markers23; suggesting involution macrophages may have a similar programing state to macrophages present during wound healing. As an independent assessment of tissue macrophages within the involuting gland, tissue sections from day 4 involuting mammary glands were stained for AIF-1 and Ly6c (Fig. 2B), a marker of GR1+ monocytes that have been shown to influx into inflamed tissues24. The presence of Ly6C+ cells within the involuting mammary gland is consistent with an immature macrophage phenotype25, 26.
Figure 2. Macrophages from the involuting mammary gland display markers of immune suppression.
(A) Representative immunoblot of CD11b+ cell lysates isolated from Nullip, INV D4, and INV D6 mammary glands for macrophage markers: Arg-1, iNOS, MRC1, AIF-1, and GAPDH. Protein expression normalized to GAPDH by densitometry (right panel). Mean arbitrary units ±SEM (n=5–6mice/group), *P<0.02 vs nullip. (B) Immunohistochemistry of INV D4 mammary gland tissue for macrophage markers AIF-1 and Ly6c. Scale bar, 120µm. (C) IL-10+ macrophages (CD45+CD11b+GR1int/loF4/80+) in the mammary gland. Mean values ±SEM (n=3–4mice/group), *P<0.03 vs nullip. (D) CD25+ (Di) and CD69+ (Dii) T cells following stimulation and co-culture with CD11b+ cells from nullip, INV D4 or INV D6 mammary glands, data normalized to stimulated T cells. Fold change ±SEM (n=5mice/group analyzed in duplicate), *P<0.0001 vs nullip (E) CD11b+ cells from INV D4 and INV D6 mammary glands suppress IFN-γ production in mixed leukocyte assays. Left, media only; middle, T cells stimulated with CD3/CD28 beads; and right stimulated T cells co-cultured with CD11b+ cells. Fold change ±SEM (n=5/group analyzed in duplicate), *P<0.0001 vs Nullip CD11b+. (F) Representative immunoblot of CD11b+ cell lysates isolated from Nullip, INV D4, INV D6 mammary glands, Nullip and INV tumors for IFN-γ receptor (IFN-γR) and GAPDH.
Given the role for alternatively activated/M2 macrophages in tumor induced immune suppression4, 21, we evaluated for markers of immune suppression in macrophages isolated from the normal involuting gland. Approximately 30% of the involution CD11b+, F4/80+, GR1int/low macrophages identified by flow cytometry were positive for the cytokine IL-10 (Fig. 2C); with intracellular IL-10 levels peaking during the remodeling phase at involution days 6–8. Macrophage expression of IL-10 is consistent with a reparative programing state of wound healing27, where it contributes to local immune suppression28. To test the ability of these CD11b+ cells to suppress T cell activation, we utilized an ex vivo assay where autologous splenic T cells in the presence of stimulatory CD3/CD28 beads were co-cultured with mammary gland CD11b+ cells. In this assay, CD11b+ cells isolated from involution day 4 and 6 mice significantly inhibited the upregulation of T cell activation markers CD69 and CD25, and reduced T cell IFN-γ levels in the media compared to CD11b+ cells isolated from nulliparous mammary glands (Fig. 2D–E and Supplemental Fig. S3B). We did not observe an increase in apoptotic T cells when T cells were co-cultured with CD11b+ cells (Supplemental Fig. S4). CD11b+ cells isolated from involuting mammary glands express high levels of the IFN-γ receptor compared to nulliparous CD11b+ cells (Fig. 2F); suggesting involution CD11b+ cells may deplete IFN-γ from the co-cultures. The IFN-γ receptor has previously been shown to be expressed by suppressive monocytic-myeloid derided suppressor cells (MO-MDSCs)29 and in our study CD11b+ cells enriched from mammary gland tumors also express the IFN-γ receptor (Fig. 2F). In summary, the phenotype and function of involution CD11b+ cells are similar to immature myeloid cells (iMCs) or MO-MDSC, previously described in the literature as CD11b+GR1int/lowLy6c+Ly6glow cells that express low levels of F4/8025, 26, 30.
Postpartum, immune competent model elucidates a role for macrophages in tumor promotion
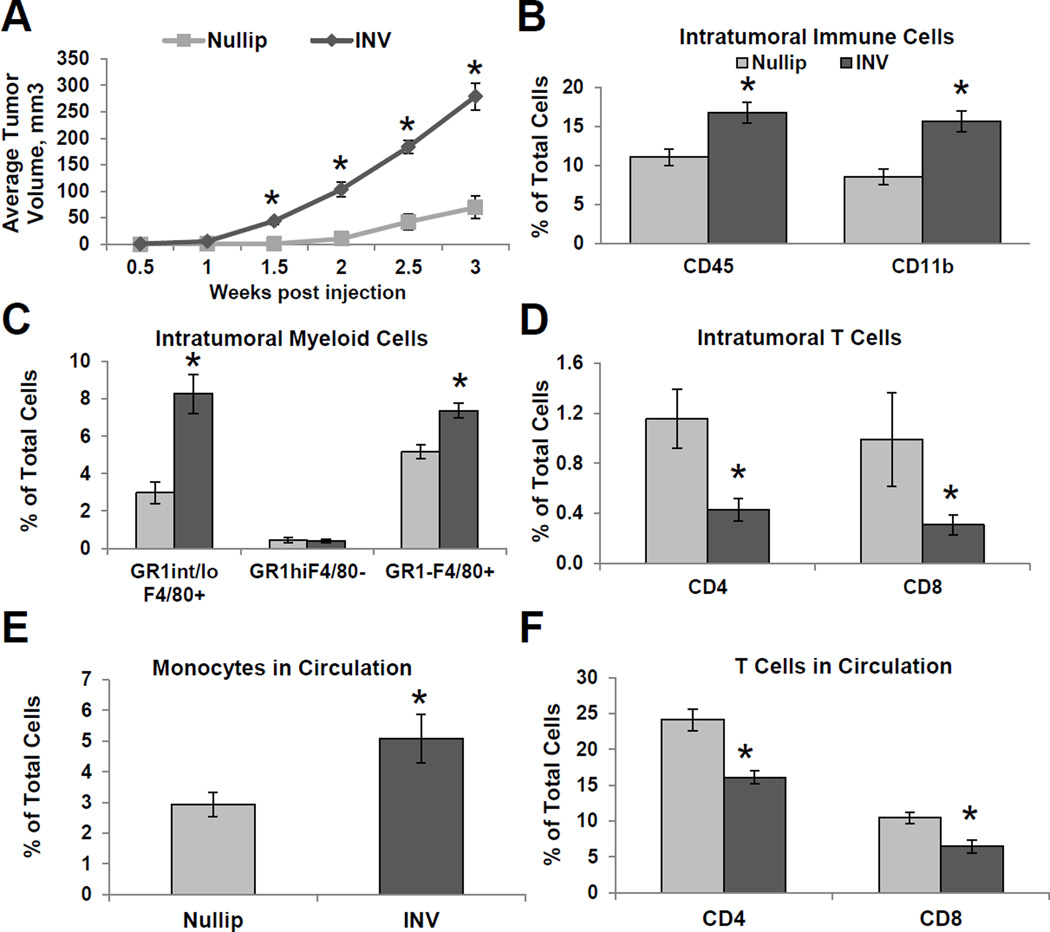
The influx of immature macrophages during normal mammary gland involution led us to hypothesize that the immune microenvironment of the involuting gland favors the growth of breast cancer. To begin to address this question, we injected murine mammary D2A1 carcinoma cells into the mammary fat pads of Balb/cJ mice that were nulliparous or at day 1 of weaning-induced involution. In this immune competent model, D2A1 cells formed invasive carcinomas that were negative for the estrogen receptor, progesterone receptor and Her 2 neu (Supplemental Fig. S5A). Beginning at 1.5 weeks post-tumor cell injection, the involution group mice had significantly larger tumors compared to the nulliparous group (Fig. 3A). By three weeks post-injection, the involution group mice had an average tumor size that was six-fold greater than the nulliparous group. Additional tumor studies taken out to 5 weeks post-injection show the involution group have a continued tumor growth advantage over nulliparous (Supplemental Fig. S5B).
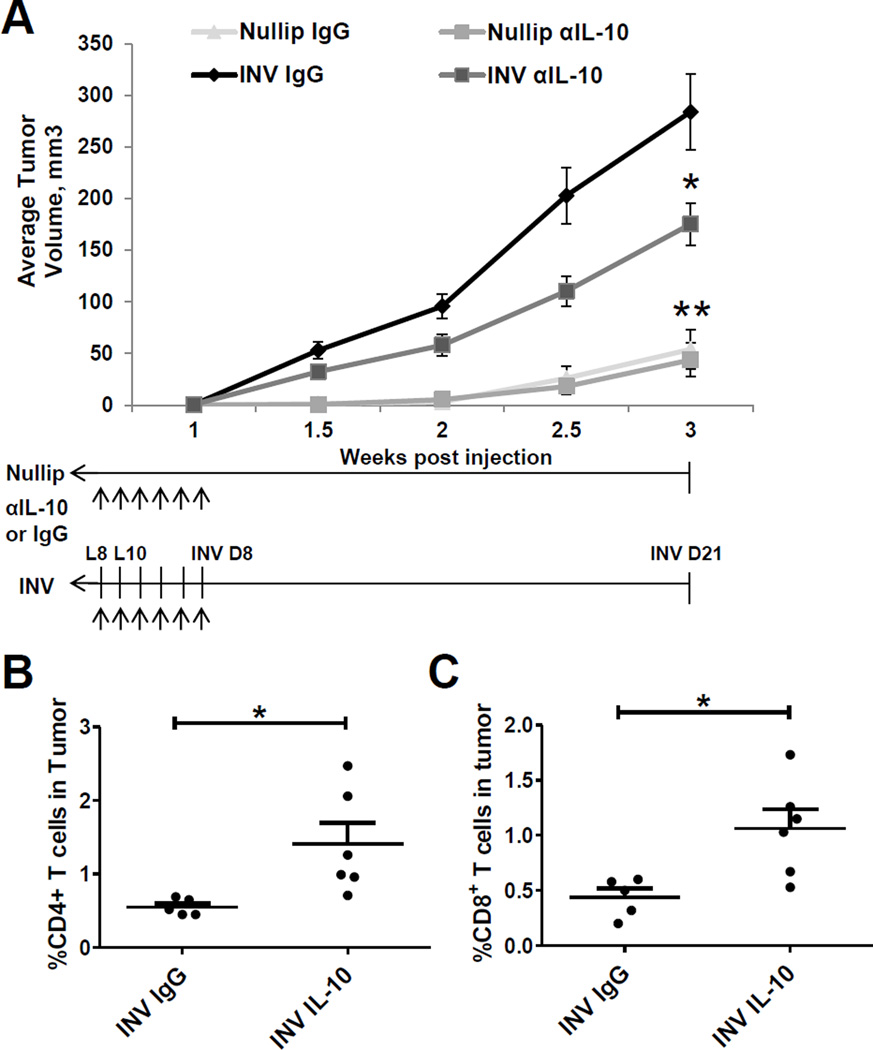
Figure 3. Postpartum involution increases tumor growth and immune cell infiltration.
(A) Average tumor volume from Nullip and INV group mice ±SEM (n=10mice/group), *P<0.001 vs nullip. (B) Percent leukocyte (CD45+) and myeloid cells (CD45+CD11b+) is increased in INV group tumors. Mean % of total cells ±SEM (n=5mice/group), *P<0.01 vs nullip. (C) Mean frequency of GR1int/loF4/80+, GR1highF4/80−, and GR1−F4/80+ shows elevated macrophages in INV tumors ±SEM (n=5mice/group), *P<0.003 vs nullip. (D) Mean frequency of CD4+ T cells (CD3+CD4+) and CD8+ T cells (CD3+CD8+) shows decreased infiltration in INV tumors ±SEM (n=5mice/group), *P<0.005 vs nullip. Mean frequency of circulating (E) monocytes and (F) CD4+ and CD8+ T cells ±SEM (n=5mice/group), *P<0.04 vs nullip.
Macrophage infiltration in human breast cancer correlates with recurrence in lymph-node positive patients20 and overall poor clinical outcomes31, 32. To investigate if involution group tumors have increased macrophage content or changes in other immune cell profiles, we characterized the mammary mouse tumors for immune cell infiltrates. Tumors arising in the involution microenvironment had higher levels of total leukocytes (CD45+), with the vast majority (93%) of the leukocytes being CD11b+ cells (Fig. 3B). The CD11b+ were further characterized as GR1−F4/80+ and GR1int/loF4/80+, and both populations were present in significantly higher levels within tumors from the involution group compared to the nulliparous group (Fig. 3C and Supplemental Fig. S5C). Granulocytes (CD11b+GR1high) made up a small percentage of the total CD11b+ cells within the tumor, and were not significantly different between groups (Fig. 3C). Since effector T cells are instrumental in orchestrating immune rejection of a tumor, we next characterized infiltrating T cells in the primary tumors as well as in the peripheral blood. Analysis of the primary tumors and peripheral blood showed that involution group had significantly lower numbers of CD4+ and CD8+ T cells both within the tumor and systemically (Fig. 3D–F and Supplemental Fig. S5C). When comparing the ratio of macrophages to CD8+ T cells present within the tumors to that observed in the normal gland, we observed a nineteen-fold increase in macrophages over CD8+ T cells within nulliparous tumors and a seventy-fold increase in the involution tumors (p=0.009). The large increase in macrophages and decrease in infiltrating CD8+ T cells in the involution group is indicative of involution tumor macrophages suppressing effector T cell infiltration, and possibly function.
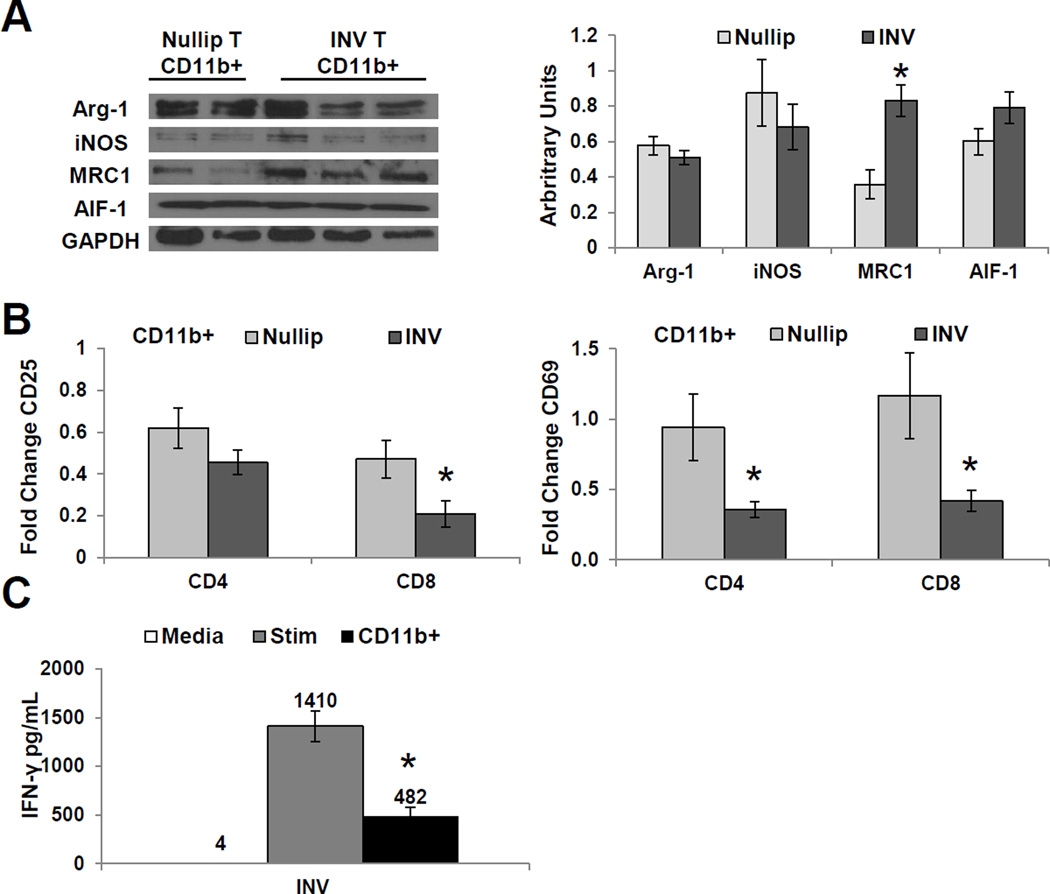
Further characterization of tumor infiltrating macrophages show that CD11b+ cells isolated from involution tumors had similar expression of Arg-1, AIF-1, and iNOS compared to nulliparous group CD11b+ cells; however, involution CD11b+ cells had significantly higher levels of the M2 marker, MRC1 (Fig. 4A). Enriched CD11b+ cells from mammary tumors were approximately 75% GR1int/loF480+ and 15% GR1+F4/80− by flow cytometry (Supplemental Fig. S6A). Functionally, we found these involution tumor infiltrating CD11b+ cells suppressed effector T cell responses more than CD11b+ cells isolated from nulliparous tumors, as analyzed by CD25, CD69 and IFN-γ levels (Fig. 4B–C and Supplementary Fig. S6B). In summary, these murine data show that postpartum mammary tumors are characterized by immune cell infiltrates that display markers previously associated with immune suppression; observations consistent with the transient presence of macrophages in the involuting mammary gland durably influencing the tumors that arise during this unique developmental window.
Figure 4. Tumor infiltrating macrophages from involution group tumors suppress T cell activation.
(A) Representative immunoblot of CD11b+ cells isolated from Nullip or INV group tumors for: Arg-1, iNOS, MRC1, AIF-1, and GAPDH (left panel). Protein expression normalized to GAPDH by densitometry (right panel) shows INV tumor macrophages enriched for M2 marker, MRC1. Mean arbitrary units ±SEM (n=5–6mice), *P<0.004 vs nullip. (B) CD25+ and CD69+ T cells following stimulation and co-culture with CD11b+ cells from Nullip and INV tumors, data normalized to stimulated T cells. Fold change ±SEM (n=5–6mice/group analyzed in duplicate), *P<0.05 vs nullip. (C) CD11b+ cells from INV group tumors suppress IFN-γ production in mixed leukocyte assays. Fold change ±SEM (n=5/group analyzed in duplicate), *P<0.0001 vs Stim vs nullip.
IL-10 depletion inhibits postpartum mammary gland tumor growth
The cytokine IL-10+ has been implicated in tumor promotion through immunosuppression33–35, and the infiltration of IL-10+ macrophages during involution identifies this cytokine as a potential mediator of postpartum tumor promotion. To determine whether IL-10 influences tumor promotion, we systemically depleted the cytokine using an IL-10 neutralizing monoclonal antibody (mAb) in nulliparous and involution groups with treatment being limited to 10 days. Compared to isotype IgG controls, involution group mice treated with an IL-10 mAb had significantly decreased tumor size (Fig. 5A). In contrast, treatment of nulliparous group mice with αIL-10 did not affect tumor size, consistent with the tumor promotional effect of IL-10 being specific to the postpartum involution microenvironment. Importantly, while αIL-10 treatment ended on day 8 of involution, a delay in tumor growth persisted to study end, at 3 weeks post weaning. Characterization of intratumoral CD4 and CD8 T cells at study end showed that αIL-10 treated involution group mice had significantly higher levels of infiltrating T cells compared to involution IgG treated mice (Fig. 5B and C). There was no significant change in CD4 and CD8 T cells between nulliparous αIL-10 and IgG-group tumors (data not shown), suggesting that αIL-10 is specifically targeting the involution microenvironment. The demonstrated efficacy of this involution-limited treatment further supports postpartum involution as a window of opportunity for targeted therapies.
Figure 5. Postpartum mammary gland tumor growth is delayed by IL-10 depletion.
(A) Primary tumor growth of nullip and INV group mice treated with αIL-10 neutralizing antibody or rat IgG1 isotype control with treatment schedules depicted with arrows. Average tumor volume ±SEM (n=5–12mice/group), *P<0.04 vs INV IgG, ** P<0.0001 vs INV IgG. Mean frequency of CD4+ (B) and CD8+ (C) T cells are elevated in αIL-10 INV group tumors ±SEM (n=5–6mice/group), *P<0.05 vs INV IgG.
Il-10+ and Foxp3+ immune cells infiltrate the involuting human postpartum breast
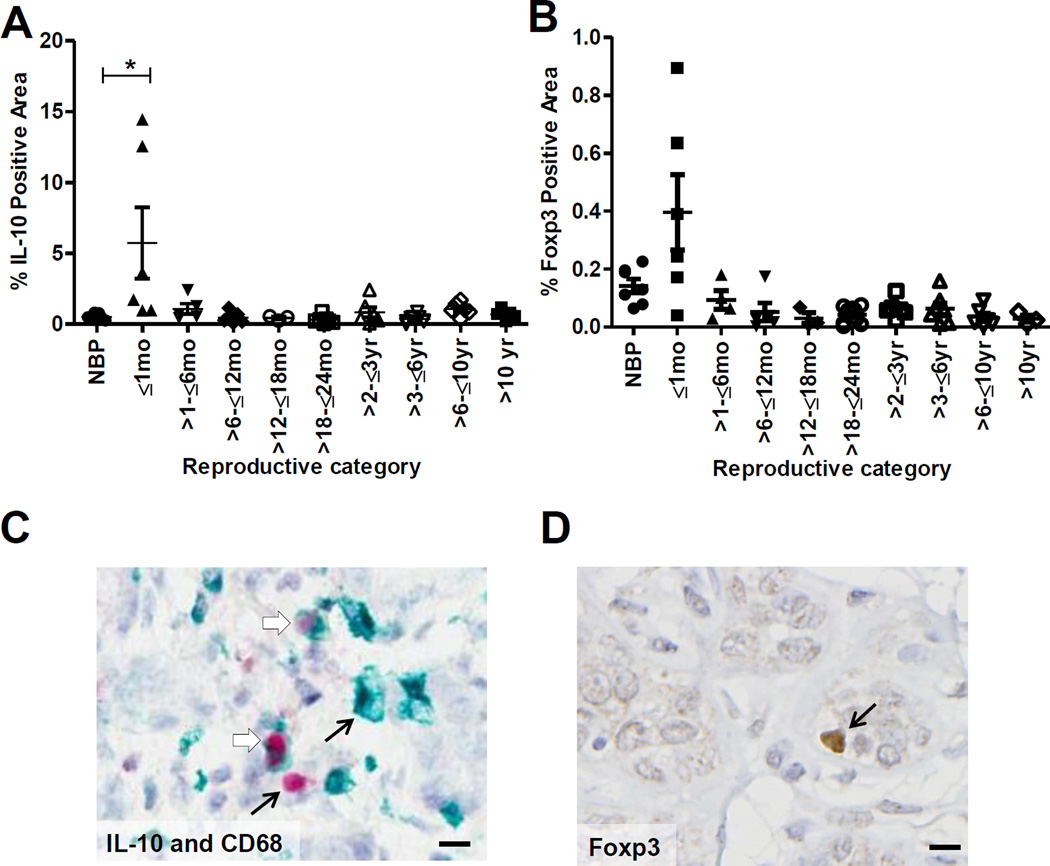
To investigate potential relevance of immune modulation in human postpartum breast involution, we evaluated young women’s adjacent normal breast tissue for IL-10+ and Foxp3+ cells by immunohistochemistry. We observed significant increase in IL-10+ cells during active breast involution, ≤1 month post-lactation (Fig. 6A). In a single involuting case, we found ~ 50% of the IL-10+ cells were CD68+, 25% were CD4+, 15% were CD19+ and <2% were CD8+ (Fig. 6B and Supplemental Fig. S7A–C). These data indicate infiltration of IL-10+ subsets of macrophages as well as T and B cells. Involution breast tissue also contained an elevated number of Foxp3+ immune cells that co-stained for CD4, suggesting the presence of regulatory T cells (Fig. 6C and Supplemental Fig. S7D). The increase in IL10+ and Foxp3+immune cells in the human breast specifically during postpartum involution is suggestive of an immune suppressed microenvironment.
Figure 6. Human postpartum involution breast tissue shows increased IL-10+ and Foxp3+ cell infiltration.
Quantitative immunohistologic assessment of (A) IL-10 and (C) Foxp3 in histologically normal, adjacent human breast tissue obtained from premenopausal women: nulliparous (NBP), ≤1 month, >1–≤6 months, >6–≤12 months, >12–≤18 months, >18–≤24 months, >2–≤3 years, >3–≤6 years, >6–≤10 years, and >10 years postpartum. Mean % positive area ±SEM, *P<0.02 vs NBP. Immunohistochemistry of (B) dual stain for CD68 (green) and IL-10 (red) and (D) Foxp3. Pictures represent normal adjacent breast tissue obtained from a women ≤1 month post-lactation. Black arrows represent single stained cells and open arrows represent dual stained cells. Scale bar, 10µm.
Discussion
We show for the first time that murine postpartum mammary gland involution is characterized by an orchestrated influx of immune cells that mirror classic wound healing. Moreover, mammary tumors exposed to the involution microenvironment are infiltrated by immature macrophages that inhibit T cell activation ex vivo and involution tumors have a growth advantage that is IL-10 dependent. These data are consistent with the transient wound healing programs of involution creating a pro-tumorigenic immune microenvironment within the tumor that persists beyond the narrow window of postpartum mammary gland remodeling. Further, we show potential relevance to women as increased Il-10+ and Foxp3+ immune cell infiltrate are observed in the human postpartum involuting breast. This demonstrated link between mammary gland involution and wound healing-like immune environment provides compelling evidence to investigate immune based mechanisms for the poor prognosis of postpartum breast cancer.
In previous studies, we and others report similarities between normal mammary gland weaning-induced involution and wound healing microenvironments, including deposition and remodeling of fibrillar collagen, increased matrix metalloproteinase activity, elevated M2-like macrophages, and T helper 2 (Th2)-associated cytokines IL-4, IL-13, TGF-β, as well as gene expression profiles consistent with immune infiltrate3, 4, 9, 13, 36–38. While these markers are consistent with wound healing, evidence for involvement of immune cells classically associated with wound healing and resolution have been lacking. Here we identify the immune cell composition across postpartum mammary gland involution and demonstrate resemblance with the inflammation, proliferation, and remodeling/resolution phases associated with classic wound healing19, 39. Wound healing begins with an initial infiltration of phagocytic neutrophils and antigen presenting dendritic cells, which prevent infection and initiate an adaptive immune response, respectively19, 39. Similarly, we find the first immune cells to enter the gland post weaning are dendritic cells. Next, in both wounds and mammary gland involution, this initial inflammatory response is followed by macrophage and lymphocyte infiltration, which in wounds are known to augment the inflammatory phase and the transition to the proliferative phase19, 39. Finally, during wound healing, transition to wound-resolution occurs with the loss of immune cells, in part through programmed death40. We find complete resolution in the involuting mammary gland occurring between 4 and 12 weeks post weaning. Importantly, when apoptotic cells are phagocytized by macrophages, macrophages are stimulated to an immune-suppressive phenotype, characterized by TGF-β and IL-10 expression23, 27, 41–43. We show a similar immune-suppressed mechanism may occur during mammary gland involution, as the monocytic cells present in involuting tissue display markers of alternative activation, express the classically immunosuppressive cytokine IL-10 and are capable of inhibiting effector T cell activation markers ex vivo. Further, we find an eight-fold increase in regulatory T cells during mid to late involution. Tregs provide a checkpoint against inflammation during wounding by suppressing multiple cell types including CD4+ and CD8+ T cells, B cells, natural killer (NK), NKT cells, and antigen presenting cells44. Thus, the observed increases in macrophages with T cell suppressive function and regulatory T cells during involution are further suggestive of classic wound resolution.
The association between wound healing and tumor promotion has been recognized as a potential therapeutic target for several decades45. Relevance in breast cancer has recently been obtained, as mammary gland biopsies in rodents promotes lung metastasis through recruitment of immune cells46 and a skin-wound adjacent to a mammary tumor stimulates tumor progression47. These studies support a potential for tumor promotional effects of wound healing programs associated with normal mammary gland involution. Hormone regulation of immune cell infiltrate during breast involution remains a provocative but uninvestigated area. Studies have shown biologically-active estrogens and androgens in breast extracellular matrix (ECM); ECM that promotes invasiveness, tumorigenicity, and metastases in rodent models48. Further, in rodent models, circulating estrogens are responsible for recruiting ERα+ bone marrow cells that promote mammary tumor growth49, 50.
In order to investigate the potential role of the involution immune cell milieu in breast cancer progression, we developed an immune-competent murine model of postpartum breast cancer. In this model, we demonstrate that tumor size is increased six-fold in the involution microenvironment compared to nulliparous hosts. Additionally, mammary tumors in the postpartum group have high levels of immature macrophages and reduced cytotoxic T cell levels, an immune cell profile predictive of poor prognosis in human breast cancer patients20, 21. Further, we show potential relevance in women, as normal adjacent breast tissue from postpartum women within 1 month of cessation of lactation have infiltration of IL-10+ and Foxp3+ expressing immune cells compared to all other reproductive stages characterized. Of note, expression of these classic immune suppression markers in the normal involuting gland is confined to a relatively narrow developmental window; however, our data show that postpartum tumors display an immune profile consistent with tumor progression beyond this developmental window. Such persistence could be explained by the establishment of a positive feedback loop within the tumor. Evidence that a transient inflammatory signal can initiate a positive feedback loop resulting in a persistent, inflammatory autocrine-loop within tumor cells has been recently described51.
We further demonstrate that neutralizing the immunosuppressive cytokine IL-10 during involution decreases growth of postpartum tumors, demonstrating a causal relationship between tumor progression and IL-10 as well as the feasibility of targeting this interaction. Cytokine IL-10 depletion during involution resulted in increased T cell infiltration to tumors, data consistent with loss of immune suppression. While the correlation of immune cell infiltrate and poor prognosis in breast cancer patients has been well studied17, 20, 31, 32, evaluation of the immune and cytokine presence specifically in postpartum breast cancers is lacking and deserves further attention. Of note, in our mouse model, αIL-10 treatment did not decrease tumor levels to that observed in nulliparous hosts, indicating additional pro-tumorigenic targets exist during postpartum involution. Supportive of this, fibular collagen has also been identified as a stromal mediator of postpartum tumor progression, and COX-2 inhibition during involution blocked both fibular collagen deposition and tumor progression in this model5.
In summary, our study demonstrates the dynamic composition of immune cells during normal mammary gland involution and suggests the window of mammary gland involution as a localized immune-suppressed state. We find murine postpartum tumors to be characterized by low T-cell infiltrate and the presence of immature macrophages with T cell suppressive activity. These data justify future research investigating the role of immune suppression in postpartum breast cancers and raise the possibility of using immune therapies for the prevention and treatment of these poor prognostic breast cancers.
Supplementary Material
Novelty and Impact Statements.
Young women diagnosed with postpartum breast cancer have poor prognosis for unknown reasons, and in all age groups regardless of reproductive history, breast cancers characterized by high macrophage number and low T cell number have poor outcomes. Here we link a transient immune suppressed microenvironment of postpartum involution with tumor monocyte infiltrate and low T cells, implicating immune suppression in the poor prognosis of young women’s postpartum breast cancer.
Acknowledgements
The authors thank Michelle Borakove, Erica Goddard, Pat Bell, Ethan Cabral, and Oscar Ramirez for technical assistance; and Max Kullberg, Jaime Fornetti and Kim Jordan for conceptual and intellectual discussions; and Peter Henson for critical review of the manuscript. We are very grateful to the patients for their contribution to this research. This work was supported by the Colorado’s NIH/NCI Tissue Biobanking and Processing, and Flow Cytometry Shared Resource Cancer Center Support Grant P30CA046934, by the NIH/NCATS Colorado CTSI Grant Number UL1 TR001082. [Contents are the authors’ sole responsibility and do not necessarily represent official NIH views]; by the Department of Defense Breast Cancer Research Program grant BC101904, University of Colorado Safeway Gift Fund Grant, Grohne Family Foundation Award and National Institute of Health (1R01CA169175) to PS & VB and the Department of Defense Breast Cancer Research Pre-doctoral Training grant BC100910 to HM.
Footnotes
Conflict of interest disclosure: The authors have no conflicting financial interests.
References
- 1.Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Dano K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog. 2004;41:207–220. doi: 10.1002/mc.20058. [DOI] [PubMed] [Google Scholar]
- 3.Schedin P, O'Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia. 2007;12:71–82. doi: 10.1007/s10911-007-9039-3. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, Man YG, Borges V, Schedin P. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176:1241–1255. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons TR, O'Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan AC, Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 7.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 2009;14:87–98. doi: 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, Urquhart A, Schedin P, Borges VF. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2013 doi: 10.1007/s10549-013-2437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6:R92–R109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes K, Wickenden JA, Allen JE, Watson CJ. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J Pathol. 2012;227:106–117. doi: 10.1002/path.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen AV, Pollard JW. Transforming growth factor beta3 induces cell death during the first stage of mammary gland involution. Development. 2000;127:3107–3118. doi: 10.1242/dev.127.14.3107. [DOI] [PubMed] [Google Scholar]
- 12.Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod. 2008;78:586–594. doi: 10.1095/biolreprod.107.065045. [DOI] [PubMed] [Google Scholar]
- 13.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, Bell AK, Ferrier RK, Sandilands GP, Gusterson BA. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–R91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development. 2012;139:269–275. doi: 10.1242/dev.071696. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 17.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris VL, Tuck AB, Wilson SM, Percy D, Chambers AF. Tumor progression and metastasis in murine D2 hyperplastic alveolar nodule mammary tumor cell lines. Clin Exp Metastasis. 1993;11:103–112. doi: 10.1007/BF00880071. [DOI] [PubMed] [Google Scholar]
- 19.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 20.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Deininger MH, Seid K, Engel S, Meyermann R, Schluesener HJ. Allograft inflammatory factor-1 defines a distinct subset of infiltrating macrophages/microglial cells in rat and human gliomas. Acta Neuropathol. 2000;100:673–680. doi: 10.1007/s004010000233. [DOI] [PubMed] [Google Scholar]
- 23.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu W, Roos A, Schlagwein N, Woltman AM, Daha MR, van Kooten C. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 2006;107:4930–4937. doi: 10.1182/blood-2005-10-4144. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Ohshima T, Kondo T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem Biophys Res Commun. 1999;265:194–199. doi: 10.1006/bbrc.1999.1455. [DOI] [PubMed] [Google Scholar]
- 29.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 30.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 31.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 32.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 33.Bolpetti A, Silva JS, Villa LL, Lepique AP. Interleukin-10 production by tumor infiltrating macrophages plays a role in Human Papillomavirus 16 tumor growth. BMC Immunol. 2010;11:27. doi: 10.1186/1471-2172-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 35.Hagenbaugh A, Sharma S, Dubinett SM, Wei SH, Aranda R, Cheroutre H, Fowell DJ, Binder S, Tsao B, Locksley RM, Moore KW, Kronenberg M. Altered immune responses in interleukin 10 transgenic mice. J Exp Med. 1997;185:2101–2110. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schedin P, Strange R, Mitrenga T, Wolfe P, Kaeck M. Fibronectin fragments induce MMP activity in mouse mammary epithelial cells: evidence for a role in mammary tissue remodeling. J Cell Sci. 2000;113(Pt 5):795–806. doi: 10.1242/jcs.113.5.795. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien J, Hansen K, Barkan D, Green J, Schedin P. Non-steroidal anti-inflammatory drugs target the pro-tumorigenic extracellular matrix of the postpartum mammary gland. Int J Dev Biol. 2011;55:745–755. doi: 10.1387/ijdb.113379jo. [DOI] [PubMed] [Google Scholar]
- 38.Maller O, Martinson H, Schedin P. Extracellular matrix composition reveals complex and dynamic stromal-epithelial interactions in the mammary gland. J Mammary Gland Biol Neoplasia. 2010;15:301–318. doi: 10.1007/s10911-010-9189-6. [DOI] [PubMed] [Google Scholar]
- 39.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 40.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 41.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 43.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy TJ, Ni Choileain N, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 45.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 46.Hobson J, Gummadidala P, Silverstrim B, Grier D, Bunn J, James T, Rincon M. Acute inflammation induced by the biopsy of mouse mammary tumors promotes the development of metastasis. Breast Cancer Res Treat. 2013;139:391–401. doi: 10.1007/s10549-013-2575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stuelten CH, Barbul A, Busch JI, Sutton E, Katz R, Sato M, Wakefield LM, Roberts AB, Niederhuber JE. Acute wounds accelerate tumorigenesis by a T cell-dependent mechanism. Cancer Res. 2008;68:7278–7282. doi: 10.1158/0008-5472.CAN-08-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleming JM, Miller TC, Quinones M, Xiao Z, Xu X, Meyer MJ, Ginsburg E, Veenstra TD, Vonderhaar BK. The normal breast microenvironment of premenopausal women differentially influences the behavior of breast cancer cells in vitro and in vivo. BMC Med. 2010;8:27. doi: 10.1186/1741-7015-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, Kuperwasser C. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67:2062–2071. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- 50.Iyer V, Klebba I, McCready J, Arendt LM, Betancur-Boissel M, Wu MF, Zhang X, Lewis MT, Kuperwasser C. Estrogen promotes ER-negative tumor growth and angiogenesis through mobilization of bone marrow-derived monocytes. Cancer Res. 2012;72:2705–2713. doi: 10.1158/0008-5472.CAN-11-3287. [DOI] [PubMed] [Google Scholar]
- 51.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.