Highlights
-
•
Rates of development differ across brain regions linked to reward and inhibition.
-
•
Adolescent risk-taking has been attributed in part to normative neurodevelopment.
-
•
Significant risky behavior in mid-adolescence is not characteristic of typical youth.
-
•
Youth with behavior disorders show increased behavioral and brain responses to reward.
-
•
Maturational theories of adolescent risk-taking can consider individual differences.
Keywords: Reward, Punishment, Self-control, Adolescence, Risk-taking, fmri
Abstract
Functional magnetic resonance imaging (fMRI) has illuminated the development of human brain function. Some of this work in typically-developing youth has ostensibly captured neural underpinnings of adolescent behavior which is characterized by risk-seeking propensity, according to psychometric questionnaires and a wealth of anecdote. Notably, cross-sectional comparisons have revealed age-dependent differences between adolescents and other age groups in regional brain responsiveness to prospective or experienced rewards (usually greater in adolescents) or penalties (usually diminished in adolescents). These differences have been interpreted as reflecting an imbalance between motivational drive and behavioral control mechanisms, especially in mid-adolescence, thus promoting greater risk-taking. While intriguing, we caution here that researchers should be more circumspect in attributing clinically significant adolescent risky behavior to age-group differences in task-elicited fMRI responses from neurotypical subjects. This is because actual mortality and morbidity from behavioral causes (e.g. substance abuse, violence) by mid-adolescence is heavily concentrated in individuals who are not neurotypical, who rather have shown a lifelong history of behavioral disinhibition that frequently meets criteria for a disruptive behavior disorder, such as conduct disorder, oppositional-defiant disorder, or attention-deficit hyperactivity disorder. These young people are at extreme risk of poor psychosocial outcomes, and should be a focus of future neurodevelopmental research.
“Everybody's youth is a dream, a form of chemical madness.”- F. Scott Fitzgerald
1. Introduction
Understanding the neural underpinnings of adolescent behavior is of increasing interest, and is enabled by functional magnetic resonance imaging (fMRI) technology for non-invasive probes of human brain function. This research has led to an influential theory that attributes behavior-related mortality and morbidity in adolescents to overactive incentive-motivational circuitry relative to underactive frontocortical behavior control neurocircuitry. In this review, we present a case that in light of epidemiological and longitudinal data, this brain functioning imbalance is likely specific to a subset of youth with disruptive behavior disorders (DBD), and is not especially pronounced or significant in neurotypical youth. We first briefly describe the neuroanatomy of reward-related decision-making, and the fMRI studies of these brain regions that give rise to these opponent-process theories. We then discuss how longitudinal studies, laboratory behavioral studies, and fMRI studies of youth with DBD indicate that these individuals, who are at extreme risk of substance use disorder (SUD) are likely the youth who would show an aberrant opponent-process. We conclude with some directions for future research.
2. Neurodevelopmental models of adolescent risk-taking
Adolescents are renowned for risky behavior, from skateboard stunts to binge drinking and unprotected sex. Reports of adolescents committing violent crime grab headlines. Empirical assessments with psychometric questionnaires and laboratory tasks have also supported a peak in venturesomeness or risk-seeking in mid-adolescence (reviewed in (Steinberg, 2004)). While age-comparison findings with laboratory decision tasks are somewhat inconsistent, the results generally support either a linear decline in risky-choice from adolescence to adulthood (Deakin et al., 2004, Overman et al., 2004), or a developmental peak in pursuit of risky choices in mid-adolescence relative to younger children and adults (Steinberg, 2005, Figner et al., 2009, Burnett et al., 2010). The advent of fMRI has sparked intense interest in whether trajectories of brain maturation contribute to adolescent risk-taking, where developmental differences in structure and function of brain regions involved in incentive processing and behavioral control are touted (and funded) as having a potential public health impact.
Where in the brain do we look? Portions of ventral striatum (VS); including nucleus accumbens (NAcc) have been extensively linked with motivational processing (reviewed in (Knutson et al., 2009)). Notably, adolescents show greater ambiguity tolerance (willingness to take risks when odds are not known) compared to adults, but not greater explicit risk tolerance (Tymula et al., 2012). When the probability of reward in a goal-directed task is uncertain, a wide variety of rewarding stimuli activate cortico-basal ganglia system that includes the oribitofrontal cortex (OFC), anterior cingulate cortex (ACC), insula, thalamus, and dorsal and ventral striatum (Delgado, 2007, Dolan, 2007, Seymour et al., 2007). In these tasks, punishment (i.e., the loss of money) often recruits a similar set of neural circuits, albeit areas in the VS often show less pronounced or even negative activation relative to baseline (e.g. (Delgado et al., 2000, Tom et al., 2007)). Inhibiting approach to potential rewards that may also result in a penalty involves frontal cortex structures, which have been extensively linked to cognitive control in both lesion studies (Bechara et al., 2001, Bechara and Van Der Linden, 2005) and in functional imaging studies (Durston et al., 2002, Ridderinkhof et al., 2004b). For example, in healthy adolescents and adults, cognitive control tasks activate a neural network that includes the dorsolateral and inferior prefrontal regions, ACC, and inferior parietal cortex (Rubia et al., 2001, Aron et al., 2004, Luna and Sweeney, 2004, Buchsbaum et al., 2005).
Initial developmental surveys using magnetic resonance imaging (MRI) documented morphological brain differences from childhood to adulthood in several brain regions. For example, frontocortical gray matter volume follows an inverted-U pattern, peaking around age 12, while temporal lobe gray matter volume increases nearly linearly throughout adolescence (Giedd et al., 1999, Sowell et al., 1999, Sowell et al., 2001). Meanwhile, frontocortical white matter volume as a proportion of total frontocortical volume increases from childhood to adulthood (reviewed in (Marsh et al., 2008)). Finally, developmental diffusion tensor imaging (DTI) studies indicate that organization of this increased frontocortical white matter is composed of increasingly orderly fiber tracts, in that fractional anisotropy of white matter water flow increases from childhood to adulthood (Barnea-Goraly et al., 2005, Imperati et al., 2011, Jernigan et al., 2011).
Two cross-sectional surveys of resting-state functional connectivity (RSFC) (Fox and Raichle, 2007) during fMRI indicated that from childhood to mid-adulthood, the strength of long-range connections between brain regions tends to increase with age while the strength of short-range connections tends to get weaker with age (Supekar et al., 2009, Dosenbach et al., 2010), and these relative connection strengths can predict an omnibus developmental “age” of the brain (Dosenbach et al., 2010). Whether inter-regional brain connectivity is directly assessed (structurally) by DTI measures of white matter or whether connectivity is inferred from synchronized brain activity between regions during a resting-state, indices of frontocortical network maturation may have clinical or behavioral significance in that decision-making requires extensive cortical integration for the representations of incentive values, potential penalties, future self with the respective outcomes, as well as for formulation of action-plans.
Particularly compelling, however, are findings of developmental (age-group) differences in brain responsiveness to risk and rewards when children, adolescents, and adults perform incentive-laden tasks during functional fMRI. Most experiments indicate that adolescents show greater responsiveness of the VS to rewards than younger children or adults. First, adolescents showed greater left VS activation by notification of money won in a “Wheel of Fortune” gambling task compared to adults (Ernst et al., 2005). Later Galvan et al. (2006) reported that once associations between cartoon cues and rewarding outcomes had become learned, adolescents showed greater VS activation during delivery of unspecified monetary reward compared to activation in adults or younger children. Similarly, Van Leijenhorst et al. (2010a) found that mid-adolescents showed greater VS activation by risky gains than younger children or young adults. A decision-making task also indicated that the adolescent striatum is more sensitive to the delivery of unexpected rewards during cue-reward association learning (Cohen et al., 2010). Moreover, in a slot machine simulation, mid-adolescents also showed more VS activation by reward-predictive slot results than younger children and young adults (Van Leijenhorst et al., 2010b). If one assumes that visual stimuli of happy faces is rewarding, Somerville et al. (2011) reported that compared to younger children and young adults, adolescents emitted more commission errors to (and had greater VS recruitment by) photographs of happy faces assigned as non-target stimuli in a go–nogo task. Finally, in a seminal investigation on the effects of social context on reward processing, Chein et al. (2011) demonstrated that running virtual yellow lights in a simulated driving game resulted in increased VS recruitment in adolescents compared to adults, but only when adolescent peer observers were present in the scanning control room.
Ironically, the first fMRI comparison between adolescents and adults in reward processing, which used a monetary incentive delay (MID) reaction-time task with simple cues (Knutson et al., 2001), indicated that adolescents showed reduced right VS recruitment by reward cues compared to adults, with no age group differences in VS or anterior mesofrontal cortex (mFC) recruitment by reward notifications (Bjork et al., 2004b). This right-lateralized striatal activation decrement in adolescents was replicated in a larger sample, using a jittered variant of the MID task that temporally disentangled reward anticipation activation from reward notification activation (Bjork et al., 2010b). An adolescent decrement in reward-anticipatory striatal activation by the MID task (Cho et al., 2012) and a MID-like task (Hoogendam et al., 2013) was also replicated in other labs. Finally, a longitudinal study showed that reward-anticipatory striatal recruitment by MID task reward cues increases across adolescence (Lamm et al., 2014). Developmental differences may also depend on the component of instrumental behavior. Adolescents showed relatively lower reward-anticipatory activation compared to young adults, but greater consummatory/notification activation by rewards in both an antisaccade task (Geier et al., 2010) and a MID-like reaction-time task (Hoogendam et al., 2013).
These opposing findings may actually provide a useful conceptual foil, and suggest that provided the incentive task for fMRI features either: (a) very engaging visual stimuli (such as casino iconography, social cartoons or driving simulations), (b) decisions between rewarding options, or (c) peers present in the lab to potentially stoke the social reward of taking a risk, normative functional development of human mesolimbic incentive neurocircuitry features a non-linear trajectory, with peak in striatal responsiveness to rewards occurring around age 14–15. Conversely, blunted reward anticipation activation in adolescents tends to be found in more “work-like” reaction-time tasks that feature minimal visual stimuli, require intense vigilance and rapid responses, and/or have no decision-making component. It could be argued that the former class of tasks may be more naturalistically-relevant to real-world risk-taking scenarios for adolescents. Irrespective of developmental directionality, it is important to consider, however, that age-group typically accounts for only a modest portion of variance in VS responses to rewards due to substantial inter-subject variability (c.f. Fig. 2B of Somerville et al., 2010). For example, in the Bjork et al. (2010b) study, age group accounted for only 12% of the variance in right VS responses to high reward cues, despite a significant group-wise difference.
This mid-adolescent peak in reward processing may be occurring against a backdrop of relatively immature or underactive frontocortical behavior control circuitry. A well-established literature indicates that with aging from adolescence to adulthood, cognitive control in rapid stimulus evaluation tasks improves (Bunge and Wright, 2007), in tandem with more focal (potentially more efficient) frontocortical activation during inhibition (Durston et al., 2006). Developmental differences in frontocortical recruitment by potential penalties have also been detected. First, using a wheel-of-fortune (WoF) task, where probabilities for winning and magnitudes of potential wins were explicitly indicated by pie-chart displays, adolescents (compared to adults) showed reduced activation of posterior mFC when choosing lower-probability but more potentially rewarding (i.e. riskier) pie-slices (Eshel et al., 2007). This adolescent activation decrement occurred in a region of mFC consistently recruited by pre-decision conflict (Ridderinkhof et al., 2004a). Second, while performing a monetary game of “chicken” akin to the Balloon Analogue Risk-Taking Task (BART; Lejuez et al., 2002), accrual of risky reward (as a contrast with accruing guaranteed reward) activated posterior mFC in adults, but not in adolescents (Bjork et al., 2007). In both experiments, greater engagement in risk-taking behavior was associated with decrements in posterior mFC recruitment.
This combined developmental pattern of a mid-adolescent peak in brain responsiveness to rewards, coupled with immature behavior control neurocircuitry has given rise to an influential dual-process model that ostensibly accounts in part for a proneness for engaging in illegal and risky behaviors beginning in adolescence (Somerville et al., 2010), such as experimentation with drugs and alcohol (Casey and Jones, 2010). Specifically, this opponent-process model posits a functional imbalance resulting from relatively rapid development of motivational circuits of the VS relative to more protracted development of behavior control circuits of frontal cortex, where this imbalance is most pronounced in mid-adolescence, and essentially normalizes or remits by young adulthood. This model seems very plausible in light of differing maturational trajectories among human brain structures (Brown et al., 2012) if morphometric differences with development (such as cortical thickness) reflect underlying functional differences. Moreover, preclinical research indicates greater DA responsiveness in younger relative to older animals (Luciana et al., 2012). Increased fMRI-measured activity in the ventral striatum is generally interpreted as reflecting greater phasic dopamine (DA) activity (Knutson and Gibbs, 2007), based on single unit primate studies of instrumental behavior (Schultz, 2007). A related “triadic” model of neurodevelopment (Ernst, 2014) features a more prominent role of developmental differences in fear/aversion neurocircuitry, and subdivides motivated behavior as the net output of approach and avoidance circuits, where each system of this opponent process is in turn governed by elements of a third, regulatory circuit.
3. Attributing public health burden of youth behavior to neurodevelopmental patterns
Previous reviews have addressed the possibility that the maturation of cognitive control neurocircuitry in adolescence is already sufficient for rational decision-making (Epstein, 2007), at least in explicit, deliberative contexts (Steinberg et al., 2009), where adolescent behaviors that adults consider ill-advised result from rational benefit-cost trade-off calculations (Furby and Beyth-Marom, 1992, Reyna and Farley, 2006). We raise a different concern. Recently, Willoughby and colleagues noted that the mid-adolescent peak in reward processing (and by extension the mid-adolescent functional imbalance between motivation and control neurocircuitry) occurs several years before the real-world peak in mortality and morbidity in youth from behavioral causes such as violence or substance intoxication (Willoughby et al., 2013). The functional imbalance in the prevailing dual-process model occurs at around age 14–16. The actual peak ages of unintentional injury (and especially death) from behavioral causes, however, occurs between ages 19 and 23 (U.S. Centers for Disease Control; c.f. http://www.cdc.gov/injury/wisqars/index.html), as does the age-peak in binge drinking (National Survey on Drug Use and Health; c.f. www.samhsa.gov/data/NSDUH.aspx) as well as delinquency and risky sex (Piquero et al., 2012). Notably, this is the same age range as “adult” subject groups in which striatal responsiveness to rewards reverts to pre-adolescent levels in some fMRI reports (e.g. Van Leijenhorst et al., 2010b).
We note that some degree of drug and alcohol experimentation is arguably normative in adolescence (Spear, 2000), and it could also be argued that the relatively low rates of binge drinking and behavior-related death and injury among younger adolescents are artificially compressed by societal structures and laws that limit their access to alcohol, guns, and motor vehicles. A subset of youth are relatively undaunted by those strictures, however, and are characterized by persistent, clinically-significant levels of antisocial or risky behavior. Decades ago, the “problem behavior theory” (Jessor and Jessor, 1977) first explained how deviant behaviors tend to co-occur within the same subject (e.g. drug use and theft) as a function of environmental factors, social learning, and personality. Subsequent longitudinal studies of youth consistently indicate that subjects who already abuse substances or who have engaged in other dangerous risky behaviors by mid-adolescence (who commonly meet criteria for a disruptive behavior disorder (DBD)) frequently show a longstanding history of behavioral disinhibition since early childhood (reviewed below), which endures for decades in a subset of individuals as a neurobehavioral trait (Casey et al., 2011).
We argue that these youth may in fact be the adolescents who truly show an aberrant opponent-process in mid-adolescence envisioned by these neuromaturational models. We contend that despite how neuromaturational models of adolescent risky behavior are derived almost exclusively from “neurotypical” research subjects (selected for scanning by virtue of absence of psychiatric symptomatology), these models actually describe youth with DBD or other constructs of antisocial behavior, whose excessive risky reward-seeking might result from delayed and/or permanently stunted maturation of frontocortical control circuitry (Shaw et al., 2007), and where atypical maturation likely results in significant individual differences between adolescents in the opponent processing.
4. Behavioral disinhibition in childhood correlates with reward-sensitivity and predicts risky behavior in adolescence
DBD are comprised largely of Attention Deficit Hyperactivity Disorder (ADHD), Oppositional Defiant Disorder (ODD) and the more severe Conduct disorder (CD), which is characterized by a persistent pattern of behaviors that involve violating the rights of others and major societal norms (e.g., fighting, stealing, and truancy), but the disorder is not explicitly defined by early substance use (Pardini et al., 2010). Longitudinal studies indicate that behaviors consistent with CD in childhood and adolescence are one of the robust predictors of the development of substance use disorders, even after controlling for early-onset substance use and other forms of psychopathology (Fergusson et al., 1996, Fergusson et al., 2007, Grant et al., 2001, Elkins et al., 2007, Pardini et al., 2007). For example, children with CD are twice as likely to initiate alcohol use at age 15 than children without the disorder and several times more likely to initiate illicit drug use (Hopfer et al., 2013). CD that precedes the onset of regular substance use is also a predictor of poor substance use disorder (SUD) treatment outcomes in adolescents (Myers et al., 1995, Brown et al., 1996b). One study reported that 95% of adolescents (predominantly age 15–16) in substance use treatment met criteria for CD, and this proportion remained at 47% when CD symptoms related to obtaining alcohol or drugs (e.g., robbing drug dealers) were not counted (Brown et al., 1996a). CD and ODD are extensively comorbid with ADHD (Connor et al., 2010), such that a case could be made for a more dimensional approach to poor behavioral inhibition and robust reward processing as cross-cutting brain circuit abnormalities underlying multiple syndromes (Cuthbert and Insel, 2013).
The preponderance of laboratory behavioral evidence suggests that hypersensitivity to rewards may serve as a characteristic or liability factor for DBD (reviewed in Byrd et al., 2014), especially in DBD youth with low levels of anxiety and those exhibiting callous-unemotional (e.g., lack of empathy/guilt) traits (O’Brien et al., 1994). For example, hospitalized adolescents with DBD emit more frequent free-operant responses for reward in a task that rewards each press as a function of time since last press (Dougherty et al., 2003). Moreover, when their lower IQ was controlled for, these patients also showed increased choices of smaller immediate rewards over larger-but delayed rewards in an “experiential” variant of the discrete-choice delay-discounting task (where subjects had to sit and acutely endure the delays). In adulthood, individuals with SUD tend to value smaller immediate rewards over larger delayed rewards on discounting tasks (Kirby et al., 1999), and this response style is particularly pronounced in individuals with SUD and features of CD (Petry, 2002, Bjork et al., 2004a, Bobova et al., 2009). These findings parallel animal literature, in that rodents with a reward-dominant style prior to drug exposure more rapidly self-administer cocaine (Dalley et al., 2007, Belin et al., 2008) and exhibit punishment resistant drug-seeking behavior (Belin et al., 2008, Economidou et al., 2009a, Economidou et al., 2009b) relative to control rodents.
Although reversal-learning decision tasks do not directly probe reward-sensitivity per se, the deficits in reversal learning performance characteristic of drug abusers or subjects at risk for drug use (Izquierdo and Jentsch, 2012), may exaggerate the effects of reward sensitivity. For example, children and adolescents with externalizing disorders (CD in particular) and those exhibiting callous-unemotional traits continue responding to previously rewarded cues even when contingencies change and the response results in escalating punishments (Fonseca and Yule, 1995, Ernst et al., 2003, Matthys et al., 2004, Byrd et al., 2014), similar to substance abusing adolescents and adults (Damasio et al., 2000, Lane et al., 2007). Decrements in reversal learning are thought to contribute to addiction if the subject is insensitive to increasing negative consequences of substance use
An important counterpoint to this narrative, however, is the reward deficiency hypothesis (RDH) of addiction (Blum et al., 2000). The RDH attributes substance use initiation, and especially chronic use of substances, to a hypofunctioning reward system. Drugs of abuse, by virtue of their ability to trigger a dopamine surge, are uniquely able to stimulate deficient mesolimbic incentive neurocircuitry compared to natural rewards, leading to a bias toward drugs. This bias may be especially critical when substances of abuse themselves degrade mesolimbic function with repeated exposure (Koob and Le Moal, 2008). Evidence for the RDS in fMRI findings is mixed, however, with studies showing both greater as well as lesser mesolimbic responses to reward prospects or deliveries in abusers of alcohol and cannabis (Hommer et al., 2011). Studies of tobacco smokers, however, consistently show reduced VS activation by rewards in accord with the RDH including in teens (Peters et al., 2011). This may be a specific effect of nicotinic acetylcholine desensitization with chronic exposure (or perhaps acute nicotine withdrawal) to reduce phasic stimulus-elicited dopamine responses (Faure et al., 2014).
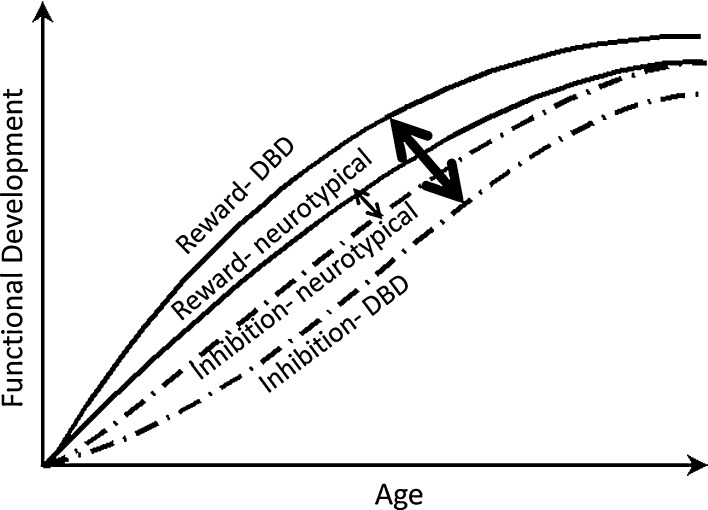
In addition to evidence for greater reward-sensitivity in at-risk youth, problems with cognitive control have also been implicated in the development of both DBD and SUD. Cognitive control refers to a set of abilities necessary to engage in adaptive decision-making in response to changing environmental demands, including sustained attention, response inhibition, and performance monitoring. Children and adolescents with CD exhibit performance deficits on cognitive control tasks compared to normally developing youth (Oosterlaan et al., 1998, Toupin et al., 2000, Loeber et al., 2007), as do adolescents with a history of chronic and problematic substance use (Tapert and Brown, 2000, Medina et al., 2007). In addition, problems with cognitive control appear to be particularly pronounced in individuals with SUD and a history of early CD (Finn et al., 2002). While chronic substance use may cause cognitive control deficits over time (Tapert et al., 2002b), poor performance on cognitive control tasks has been shown to prospectively predict the development of heavy and problematic substance use (Aytaclar et al., 1999, Tapert et al., 2002a). In a longitudinal study, Castellanos-Ryan et al. (2011) administered neurocognition measures to mid-adolescents and found that the link between sensation-seeking personality at 14 and binge drinking at 16 was mediated by reward sensitivity measured in an incentivized go–nogo task, and the link between self-reported impulsivity at 14 and actual CD symptoms at 16 was mediated by inhibitory task performance. To reiterate, whereas an aberrant opponent-process between reward-motivation and inhibition neurocircuity has been postulated to underlie normative developmental differences between adolescents and other age groups in risky behavior, an equally compelling case could be made that an aberrant opponent process is what underlies individual differences between adolescents in neurocircuit function- to promote greater risk of addiction, criminal offending, and violence in some individuals. This is illustrated in Fig. 1.
Fig. 1.
Conceptual trajectories of neurocircuit function from birth to young adulthood in neurotypical youth and in youth with disruptive behavior disorders (DBD). Solid lines represent incentive-motivational (“reward”) functioning, and dashed lines represent frontocortical inhibitory/behavior control functioning. The thin double-headed arrow denotes a modest imbalance between incentive-motivational and inhibitory control neurocircuitry in typical adolescents. The thick double-headed arrow denotes a clinically-significant imbalance between incentive-motivational and inhibitory control neurocircuitry in adolescents with DBD.
5. Brain signatures of increased reward sensitivity and reduced cognitive control in youth with behavior problems
As mentioned previously, developmental differences in VS recruitment by rewards during fMRI have been interpreted as reflecting underlying development of DA activity in the mesolimbic system (Luciana et al., 2012). Might individual differences in DA functioning also apply to individual differences in risk traits? In Positron Emission Tomography (PET) studies of adults, displacement of radiolabeled DA ligands in VS by rewards have correlated within-subject with fMRI-measured VS recruitment by rewards (Schott et al., 2008, Buckholtz et al., 2010), and dynamic DA synthesis in VS correlated with sensation-seeking personality (Lawrence and Brooks, 2014). Third, antisocial and psychopathic personality features in adults have been associated with increased amphetamine-induced dopamine release in nucleus accumbens (Buckholtz et al., 2010). Therefore, it stands to reason that individual differences between adolescents in VS responsiveness to fMRI rewards could also be DA-mediated.
By extension, greater VS activation by rewards in fMRI might be expected in youth with greater impulsivity or sensation-seeking personality. For example, in adolescents with no psychiatric disorder, individual differences in sensation-seeking (Bjork et al., 2008), and individual differences in a tally of self-reported risky behaviors and substance use (Bjork et al., 2011) correlated positively with mesolimbic recruitment by cues for operant rewards. Moreover, striatal sensitivity to rewards correlated positively with self-reported likelihood to engage in future risky behavior in both adolescents and adults (Galvan et al., 2007). Researchers have also begun directly examining brain function abnormalities in youth with significant antisocial behavior (AB) using cognitive control tasks as well as various monetary reward tasks (recently reviewed in Hyde et al., 2013). These studies collectively implicate (correlate) altered brain activity in limbic circuits (such as insula and VS) in syndromes of antisocial behavior, with differences between findings likely resulting from differing definitions of antisocial behavior such as the presence versus absence psychopathic features, including callous-unemotional traits. For example, adolescents with DBD also showed significantly greater VS recruitment by reward notifications in the MID task (Bjork et al., 2010a). Gatzke-Kopp et al. (2009) reported that when an operant response was no longer rewarded, AB subjects persisted in striatal activation by reward-linked cues, while controls instead recruited error-monitoring circuitry of the ACC when response contingencies changed.
In large-scale twin studies of psychiatric diagnoses, a latent neurobiological trait has been suggested that underlies both addiction and DBD (Kendler et al., 2003). Recent functional neuroimaging studies suggest that abnormalities in the neural network subserving cognitive control may represent a mechanism for this latent trait or common risk factor for the development of both CD and SUD. In comparison to controls, adolescents with CD have been shown to exhibit reduced dorsomedial prefrontal activity during attention allocation (Rubia et al., 2009) and reduced posterior ACC and inferior parietal reactivity when committing response inhibition errors (Rubia et al., 2008). Reduced dorsal pre-frontal activation during response inhibition has also been found in adolescents with high levels of neurobehavioral disinhibition, which includes features of CD (McNamee et al., 2008). Individuals with SUD exhibit similar abnormalities, including reduced brain activation in the dorsolateral/dorsomedial prefrontal cortex (Tapert et al., 2001, Eldreth et al., 2004, Chang et al., 2006, Schweinsburg et al., 2008), ACC (Kaufman et al., 2003, Bolla et al., 2004, Eldreth et al., 2004, Forman et al., 2004, Lee et al., 2005, Li et al., 2008), and posterior parietal cortex (Tapert et al., 2001) during cognitive control tasks. However, it remains unclear whether these deficits are a consequence of chronic use, a risk factor for the development SUD, or a combination of the two.
6. Conclusions and future directions
Functional neuroimaging has opened a window into the development of human motivation and behavior control, to reveal divergent trajectories across different brain systems critical for risk-taking decision-making (e.g. Somerville et al., 2011). Innovative naturalistic tasks during fMRI also hold promise to reveal mechanisms of many behavioral proclivities of adolescents, such as elements of social behavior (Jones et al., 2014). We caution, however, that due to the preponderance of youth with childhood histories of behavior problems among adolescents with significant risky behavior by mid-adolescence, coupled with how the peak in real-world severe risky behavior actually occurs later in young adulthood, it seems doubtful that severe risk-taking behavior in adolescence is significantly accounted for by individual differences in normative neurodevelopment. We suggest instead that severe risky behavior in adolescence can result from problematic individual differences in opponent process function between motivational versus inhibitory brain circuits that are present prior to adolescence, where these individual differences may interact with or be derived from altered neurodevelopment.
Future research on brain mechanisms of significant adolescent risk-taking would therefore benefit from a greater emphasis on individual differences between adolescents, such as histories of behavior disorders. Divergent findings in this emerging literature could in turn be resolved by careful phenotyping of emotionality components, such as callous-unemotional traits, or reactive versus instrumental aggression (Hyde et al., 2013). Moreover, it remains largely unexplored how changes in either persistence, escalation or remittance of drug use, delinquency, or risky behaviors track with brain changes from childhood to adulthood. Large-scale longitudinal neuroimaging projects that follow subjects past their peak crime- and risk-prone young adult ages will be critical in uncovering the functional and structural brain underpinnings of behavior change with development. In order to more convincingly demonstrate that changes in brain structure and function are causally associated with the initial escalation and subsequent decline in risky behaviors, linkages between these dynamic processes must be examined within-individuals from adolescence into early adulthood.
Finally, we caution that attributing real-world behavior to brain activation differences remains speculative, particularly since there is considerable heterogeneity in the factors underlying substance misuse and antisocial behavior in youth. Longitudinal studies are needed to address the functional significance of individual differences (whether developmental differences or differences between adolescents) in brain activation with respect to a causal explanation of real-world risky behaviors, akin to the decades of rigorous longitudinal research that has characterized the influence of socio-contextual factors on adolescent behavioral outcomes.
Conflict of interest
The authors declare no conflict of interest involving the preparation and submission of this manuscript.
Acknowledgments
This work was supported by a grant provided to Dustin Pardini by the National Institute on Drug Abuse (DA034608-02).
Footnotes
Available online 12 August 2014
References
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends Cognit. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aytaclar S., Tarter R.E., Kirisci L., Lu S. Association between hyperactivity and executive cognitive functioning in childhood and substance use in early adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:172–178. doi: 10.1097/00004583-199902000-00016. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Menon V., Eckert M., Tamm L., Bammer R., Karchemskiy A., Dant C.C., Reiss A.L. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb. Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bechara A., Dolan S., Denburg N., Hindes A., Anderson S.W., Nathan P.E. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A., Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr. Opin. Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Belin D., Mar A.C., Dalley J.W., Robbins T.W., Everitt B.J. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Chen G., Smith A.R., Hommer D.W. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J. Child Psychol. Psychiatry. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Hommer D.W., Grant S.J., Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Hommer D.W. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Psychosocial problems and recruitment of incentive neurocircuitry: exploring individual differences in healthy adolescents. Dev. Cognit. Neurosci. 2011;1:570–577. doi: 10.1016/j.dcn.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Danube C.L., Hommer D.W. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J. Neurosci. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Braverman E.R., Holder J.M., Lubar J.F., Monastra V.J., Miller D., Lubar J.O., Chen T.J., Comings D.E. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoact. Drugs. 2000;32(Suppli–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Bobova L., Finn P.R., Rickert M.E., Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp. Clin. Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K., Ernst M., Kiehl K., Mouratidis M., Eldreth D., Contoreggi C., Matochik J., Kurian V., Cadet J., Kimes A., Funderburk F., London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J. Neuropsychiatry Clin. Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.A., Gleghorn A., Schuckit M.A., Myers M.G., Mott M.A. Conduct disorder among adolescent alcohol and drug abusers. J. Stud. Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Brown S.A., Gleghorn A., Schuckit M.A., Myers M.G., Mott M.A. Conduct disorders among adolescent alcohol and drug abusers. J. Stud. Alcohol. 1996;57:314–324. doi: 10.15288/jsa.1996.57.314. [DOI] [PubMed] [Google Scholar]
- Brown T.T., Kuperman J.M., Chung Y., Erhart M., McCabe C., Hagler D.J., Jr., Venkatraman V.K., Akshoomoff N., Amaral D.G., Bloss C.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kaufmann W.E., Kenet T., Kennedy D.N., Murray S.S., Sowell E.R., Jernigan T.L., Dale A.M. Neuroanatomical assessment of biological maturity. Curr. Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum B.R., Greer S., Chang W.L., Berman K.F. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum. Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz J.W., Treadway M.T., Cowan R.L., Woodward N.D., Benning S.D., Li R., Ansari M.S., Baldwin R.M., Schwartzman A.N., Shelby E.S., Smith C.E., Cole D., Kessler R.M., Zald D.H. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Burnett S., Bault N., Coricelli G., Blakemore S.J. Adolescents’ heightened risk-seeking in a probabilistic gambling task. Cognit. Dev. 2010;25:183–196. doi: 10.1016/j.cogdev.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd A.L., Loeber R., Pardini D.A. Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clin. Child Fam. Psychol. Rev. 2014;17:125–156. doi: 10.1007/s10567-013-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Somerville L.H., Gotlib I.H., Ayduk O., Franklin N.T., Askren M.K., Jonides J., Berman M.G., Wilson N.L., Teslovich T., Glover G., Zayas V., Mischel W., Shoda Y. Behavioral and neural correlates of delay of gratification 40 years later. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N., Rubia K., Conrod P.J. Response inhibition and reward response bias mediate the predictive relationships between impulsivity and sensation seeking and common and unique variance in conduct disorder and substance misuse. Alcohol Clin. Exp. Res. 2011;35:140–155. doi: 10.1111/j.1530-0277.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- Chang L., Yakupov R., Cloak C., Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev. Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.T., Fromm S., Guyer A.E., Detloff A., Pine D.S., Fudge J.L., Ernst M. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 2012;66C:508–521. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.R., Asarnow R.F., Sabb F.W., Bilder R.M., Bookheimer S.Y., Knowlton B.J., Poldrack R.A. A unique adolescent response to reward prediction errors. Nat. Neurosci. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor D.F., Steeber J., McBurnett K. A review of attention-deficit/hyperactivity disorder complicated by symptoms of oppositional defiant disorder or conduct disorder. J. Dev. Behav. Pediatr. 2010;31:427–440. doi: 10.1097/DBP.0b013e3181e121bd. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Insel T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J.W., Fryer T.D., Brichard L., Robinson E.S.J., Theobald D.E.H., Laane K., Pena Y., Murphy E.R., Shah Y., Probst K., Abakumova I., Aigbirhio F.I., Richards H.K., Hong Y., Baron J.C., Everitt B.J., Robbins T.W. Nucleus Accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L., Parvizi J., Hichwa R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Deakin J., Aitken M., Robbins T., Sahakian B.J. Risk taking during decision-making in normal volunteers changes with age. J. Int. Neuropsychol. Soc. 2004;10:590–598. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- Delgado M.R. Reward-related responses in the human striatum. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D.C., Fiez J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dolan R.J. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos. Trans. R. Soc. Lond.: Ser. B: Biol. Sci. 2007;362:787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Nelson S.M., Wig G.S., Vogel A.C., Lessov-Schlaggar C.N., Barnes K.A., Dubis J.W., Feczko E., Coalson R.S., Pruett J.R., Jr., Barch D.M., Petersen S.E., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D.M., Bjork J.M., Harper R.A., Marsh D.M., Moeller F.G., Mathias C.W., Swann A.C. Behavioral impulsivity paradigms: a comparison in hospitalized adolescents with disruptive behavior disorders. J. Child Psychol. Psychiatry. 2003;44:1145–1157. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S., Thomas K.M., Worden M.S., Yang Y., Casey B.J. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Economidou D., Pelloux Y., Robbins T.W., Dalley J.W., Everitt B.J. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol. Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Economidou D., Pelloux Y., Robbins T.W., Dalley J.W., Everitt B.J. High impulsivity predicts relapse to cocaine seeking after extended access: effect of treatment with atomoxetine. Eur. Neuropsychopharm. 2009;19:S82–S83. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Eldreth D.A., Matochik J.A., Cadet J.L., Bolla K.I. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Elkins I.J., Mcgue M., Iacono W.G. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch. Gen. Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Epstein R., 2007. The myth of the teen brain. In: Scientific American Mind, pp 57–63.
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014 doi: 10.1016/j.bandc.2014.01.006. pii: S0278-2626(14)00008-6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Grant S.J., London E.D., Contoreggi C.S., Kimes A.S., Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am. J. Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E., Blair J., Pine D.S. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Eshel N., Nelson E.E., Blair R.J., Pine D.S., Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure P., Tolu S., Valverde S., Naude J. Role of nicotinic acetylcholine receptors in regulating dopamine neuron activity. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.05.040. [DOI] [PubMed] [Google Scholar]
- Fergusson D.M., Horwood L.J., Ridder E.M. Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence: results of a 25-year longitudinal study. Drug Alcohol Depend. 2007;88(Suppl 1):S14–S26. doi: 10.1016/j.drugalcdep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Fergusson D.M., Lynskey M.T., Horwood L.J. The short-term consequences of early onset cannabis use. J. Abnorm. Child Psychol. 1996;24:499–512. doi: 10.1007/BF01441571. [DOI] [PubMed] [Google Scholar]
- Figner B., Mackinlay R.J., Wilkening F., Weber E.U. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. J. Exp. Psychol. Learn. Mem. Cognit. 2009;35:709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Finn P.R., Mazas C.A., Justus A.N., Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcoholism-Clin. Exp. Res. 2002;26:186–206. [PubMed] [Google Scholar]
- Fonseca A.C., Yule W. Personality and antisocial behavior in children and adolescents: an enquiry into Eysenck's and Gray's theories. J. Abnorm. Child Psychol. 1995;23:767–781. doi: 10.1007/BF01447476. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Dougherty G.G., Casey B.J., Siegle G.J., Braver T.S., Barch D.M., Stenger V.A., Wick-Hull C., Pisarov L.A., Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol. Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Furby L., Beyth-Marom R. Risk taking in adolescence: a decision-making perspective. Dev. Rev. 1992;12:1–44. [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B.J. Risk-taking and the adolescent brain: who is at risk? Dev. Sci. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L.M., Beauchaine T.P., Shannon K.E., Chipman J., Fleming A.P., Crowell S.E., Liang O., Johnson L.C., Aylward E. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. J. Abnorm. Psychol. 2009;118:203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grant B.F., Stinson F.S., Harford T.C. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. J. Subst. Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Hommer D.W., Bjork J.M., Gilman J.M. Imaging brain response to reward in addictive disorders. Ann. N. Y. Acad. Sci. 2011;1216:50–61. doi: 10.1111/j.1749-6632.2010.05898.x. [DOI] [PubMed] [Google Scholar]
- Hoogendam J.M., Kahn R.S., Hillegers M.H., van Buuren M., Vink M. Different developmental trajectories for anticipation and receipt of reward during adolescence. Dev. Cognit. Neurosci. 2013;6:113–124. doi: 10.1016/j.dcn.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer C., Salomonsen-Sautel S., Mikulich-Gilbertson S., Min S.J., McQueen M., Crowley T., Young S., Corley R., Sakai J., Thurstone C., Hoffenberg A., Hartman C., Hewitt J. Conduct disorder and initiation of substance use: a prospective longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52 doi: 10.1016/j.jaac.2013.02.014. 511-518 e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Hariri A.R., 2013. Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: review, integration, and directions for research. Dev. Rev., DR 33. [DOI] [PMC free article] [PubMed]
- Imperati D., Colcombe S., Kelly C., Di Martino A., Zhou J., Castellanos F.X., Milham M.P. Differential development of human brain white matter tracts. PLoS One. 2011;6:e23437. doi: 10.1371/journal.pone.0023437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A., Jentsch J.D. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T.L., Baare W.F., Stiles J., Madsen K.S. Postnatal brain development: structural imaging of dynamic neurodevelopmental processes. Prog. Brain Res. 2011;189:77–92. doi: 10.1016/B978-0-444-53884-0.00019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessor R., Jessor S.L. Academic Press; New York: 1977. Problem Behavior and Psychosocial Development: A Longitundinal Study of Youth. [Google Scholar]
- Jones R.M., Somerville L.H., Li J., Ruberry E.J., Powers A., Mehta N., Dyke J., Casey B.J. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cogn. Affect. Behav. Neurosci. 2014;14:683–697. doi: 10.3758/s13415-014-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J.N., Ross T.J., Stein E.A., Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J. Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Prescott C.A., Myers J., Neale M.C. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch. Gen. Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kirby K.N., Petry N.M., Bickel W.K. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol.-Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Delgado M.R., Phillips P.E.M. Representation of subjective value in the striatum. In: Glimcher P.W., editor. Neuroeconomics: Decision-Making and the Brain. Elsevier; New York: 2009. pp. 389–406. [Google Scholar]
- Knutson B., Gibbs S.E. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Addiction and the brain antireward system. Annu. Rev. Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lamm C., Benson B.E., Guyer A.E., Perez-Edgar K., Fox N.A., Pine D.S., Ernst M. Longitudinal study of striatal activation to reward and loss anticipation from mid-adolescence into late adolescence/early adulthood. Brain Cognit. 2014 doi: 10.1016/j.bandc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S.D., Cherek D.R., Tcheremissine O.V., Steinberg J.L., Sharon J.L. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addict. Behav. 2007;32:977–990. doi: 10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lawrence A.D., Brooks D.J. Ventral striatal dopamine synthesis capacity is associated with individual differences in behavioral disinhibition. Front. Behav. Neurosci. 2014;8:86. doi: 10.3389/fnbeh.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.M.C., Zhou W.H., Luo X.J., Yuen K.S.L., Ruan X.Z., Weng X.C. Neural activity associated with cognitive regulation in heroin users: a fMRI study. Neurosci. Lett. 2005;382:211–216. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Read J.P., Kahler C.W., Richards J.B., Ramsey S.E., Stuart G.L., Strong D.R., Brown R.A. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J. Exp. Psychol. Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Li C.S.R., Huang C., Yan P.S., Bhagwagar Z., Milivojevic V., Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R., Pardini D.A., Stouthamer-Loeber M., Raine A. Do cognitive, physiological, and psychosocial risk and promotive factors predict desistance from delinquency in males? Dev. Psychopathol. 2007;19:867–887. doi: 10.1017/S0954579407000429. [DOI] [PubMed] [Google Scholar]
- Luciana M., Wahlstrom D., Porter J.N., Collins P.F. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev. Psychol. 2012;48:844–861. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Sweeney J.A. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann. N. Y. Acad. Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Marsh R., Gerber A.J., Peterson B.S. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys W., van Goozen S.H., Snoek H., van Engeland H. Response perseveration and sensitivity to reward and punishment in boys with oppositional defiant disorder. Eur. Child Adolesc. Psychiatry. 2004;13:362–364. doi: 10.1007/s00787-004-0395-x. [DOI] [PubMed] [Google Scholar]
- McNamee R.L., Dunfee K.L., Luna B., Clark D.B., Eddy W.F., Tarter R.E. Brain activation, response inhibition, and increased risk for substance use disorder. Alcoholism-Clin. Exp. Res. 2008;32:405–413. doi: 10.1111/j.1530-0277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- Medina K.L., Hanson K.L., Schweinsburg A.D., Cohen-Zion M., Nagel B.J., Tapert S.F. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J. Int. Neuropsychol. Soc. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M.G., Brown S.A., Mott M.A. Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcoholism: Clin. Exp. Res. 1995;19:1528–1536. doi: 10.1111/j.1530-0277.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- O’Brien B.S., Frick P.J., Lyman R.D. Reward dominance among children with disruptive behavior disorders. J. Psychopathol. Behav. Assess. 1994;16:131–145. [Google Scholar]
- Oosterlaan J., Logan G.D., Sergeant J.A. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. J. Child Psychol. Psyciatr. 1998;39:411–425. [PubMed] [Google Scholar]
- Overman W.H., Frassrand K., Ansel S., Trawalter S., Bies B., Redmond A. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42:1838–1851. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Pardini D., White H.R., Stouthamer-Loeber M. Early adolescent psychopathology as a predictor of alcohol use disorders by young adulthood. Drug Alcohol Depend. 2007;88:S38–S49. doi: 10.1016/j.drugalcdep.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini D.A., Frick P.J., Moffitt T.E. Building an evidence base for DSM-5 conceptualizations of oppositional defiant disorder and conduct disorder: introduction to the special section. J. Abnorm. Psychol. 2010;119:683–688. doi: 10.1037/a0021441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Bromberg U., Schneider S., Brassen S., Menz M., Banaschewski T., Conrod P.J., Flor H., Gallinat J., Garavan H., Heinz A., Itterman B., Lathrop M., Martinot J.L., Paus T., Poline J.B., Robbins T.W., Rietschel M., Smolka M., Strohle A., Struve M., Loth E., Schumann G., Buchel C., Consortium I. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am. J. Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Petry N.M. Discounting of delayed rewards in substance abusers: relationship to antisocial personality disorder. Psychopharmacology. 2002;162:425–432. doi: 10.1007/s00213-002-1115-1. [DOI] [PubMed] [Google Scholar]
- Piquero A., Hawkins D., Kazemian L. From Juvenile Delinquency to Adult Crime: Criminal Careers, Justice Policy, and Prevention. Oxford University Press; Oxford, England: 2012. Criminal career patterns; pp. 14–46. [Google Scholar]
- Reyna V.F., Farley F. Risk and rationality in adolescent decision-making: Implications for theory, practice, and public policy. Psychol. Sci. Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., van den Wildenberg W.P., Segalowitz S.J., Carter C.S. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognit. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Smith A.B., Mohammad M., Scott S., Brammer M.J. Shared and disorder-specific prefrontal abnormalities in boys with pure attention-deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J. Child Psychol. Psyciatr. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Smith A.B., Mohammed M., Scott S., Giampietro V., Taylor E., Brammer M.J. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am. J. Psychiatry. 2008;165:889–897. doi: 10.1176/appi.ajp.2008.07071084. [DOI] [PubMed] [Google Scholar]
- Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T., Simmons A., Williams S.C., Giampietro V., Andrew C.M., Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Schott B.H., Minuzzi L., Krebs R.M., Elmenhorst D., Lang M., Winz O.H., Seidenbecher C.I., Coenen H.H., Heinze H.J., Zilles K., Duzel E., Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schweinsburg A.D., Nagel B.J., Schweinsburg B.C., Park A., Theilmann R.J., Tapert S.F. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res.—Neuroimaging. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B., Daw N., Dayan P., Singer T., Dolan R. Differential encoding of losses and gains in the human striatum. J. Neurosci. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Eckstrand K., Sharp W., Blumenthal J., Lerch J.P., Greenstein D., Clasen L., Evans A., Giedd J., Rapoport J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cognit. Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cognit. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Delis D., Stiles J., Jernigan T.L. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J. Int. Neuropsychol. Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat. Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann. N. Y. Acad. Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cognit. Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Cauffman E., Woolard J., Graham S., Banich M. Are adolescents less mature than adults? Minors’ access to abortion, the juvenile death penalty, and the alleged APA “flip-flop”. Am. Psychol. 2009;64:583–594. doi: 10.1037/a0014763. [DOI] [PubMed] [Google Scholar]
- Supekar K., Musen M., Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert S.F., Baratta M.V., Abrantes A.M., Brown S.A. Attention dysfunction predicts substance involvement in community youths. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Tapert S.F., Brown G.G., Kindermann S.S., Cheung E.H., Frank L.R., Brown S.A. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism-Clin. Exp. Res. 2001;25:236–245. [PubMed] [Google Scholar]
- Tapert S.F., Brown S.A. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1054. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert S.F., Granholm E., Leedy N.G., Brown S.A. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J. Int. Neuropsychol. Soc. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tom S.M., Fox C.R., Trepel C., Poldrack R.A. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Toupin J., Dery M., Pauze R., Mercier H., Fortin L. Cognitive and familial contributions to conduct disorder in children. J. Child Psychol. Psyciatr. 2000;41:333–344. [PubMed] [Google Scholar]
- Tymula A., Rosenberg Belmaker L.A., Roy A.K., Ruderman L., Manson K., Glimcher P.W., Levy I. Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17135–17140. doi: 10.1073/pnas.1207144109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L., Moor B.G., Op de Macks Z.A., Rombouts S.A., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Willoughby T., Good M., Adachi P.J., Hamza C., Tavernier R. Examining the link between adolescent brain development and risk taking from a social-developmental perspective. Brain Cognit. 2013;83:315–323. doi: 10.1016/j.bandc.2013.09.008. [DOI] [PubMed] [Google Scholar]