Abstract
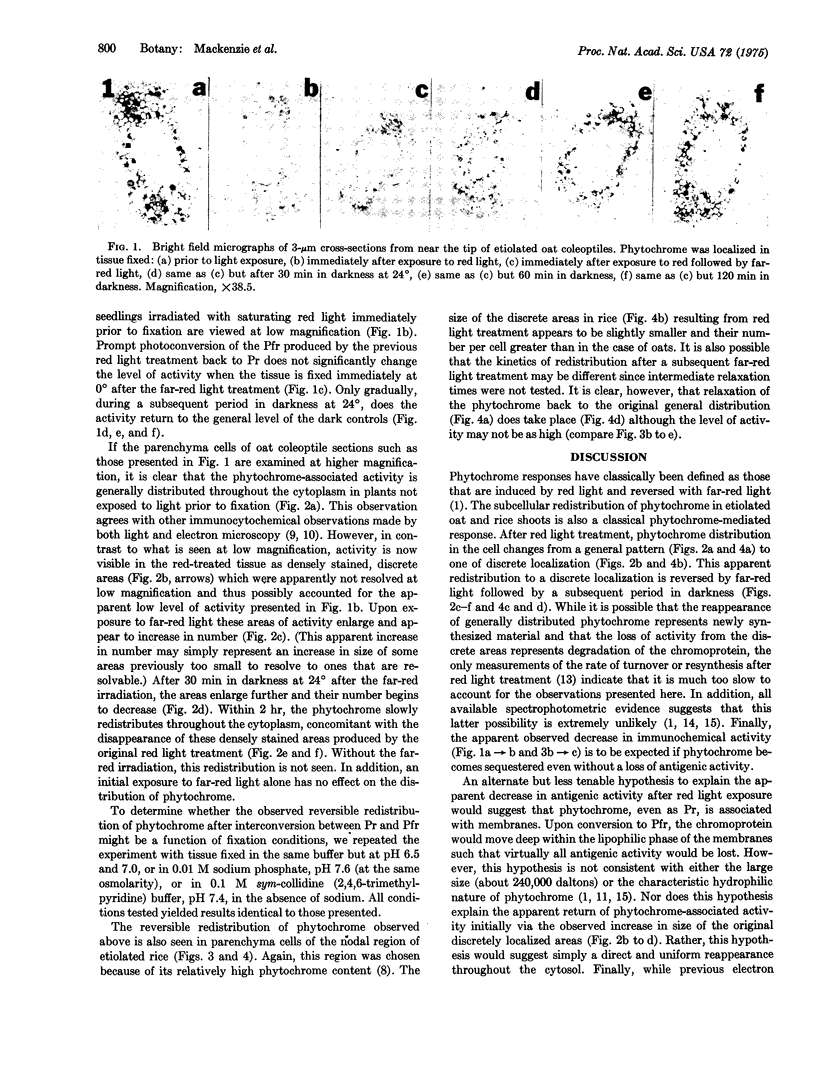
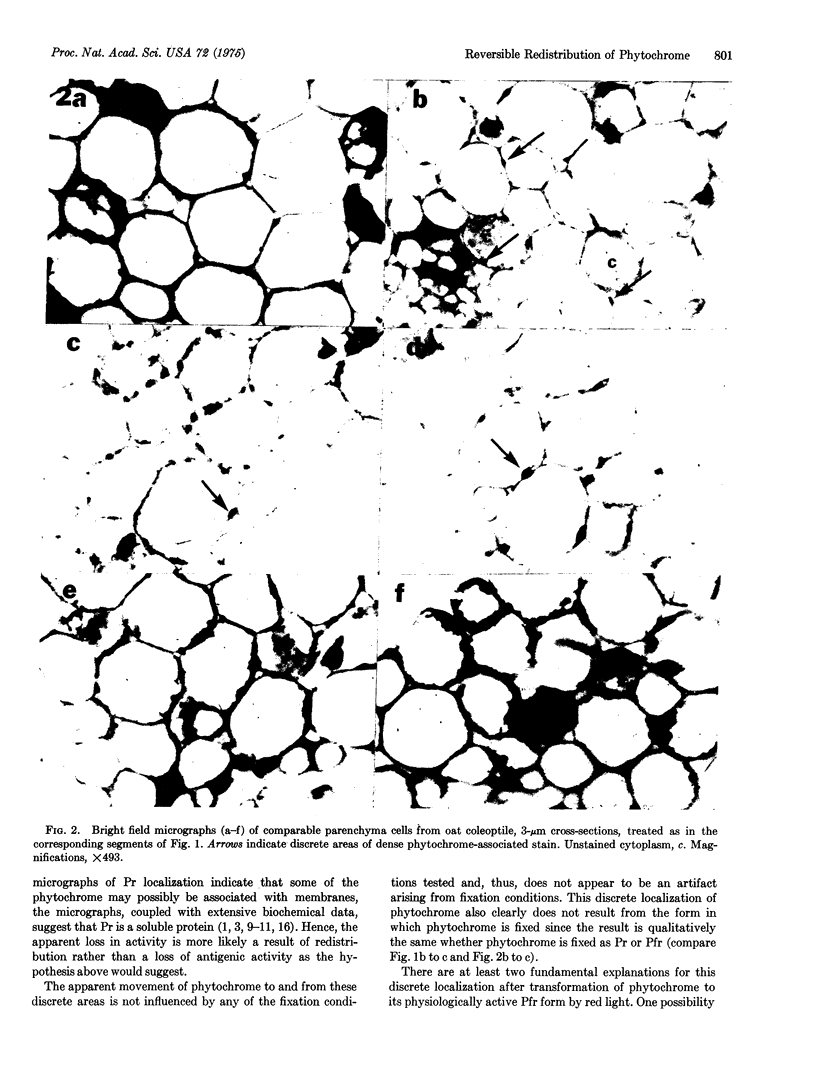
The intracellular localization of phytochrome was seen in dark-grown oat (Avena sativa L., cv. Garry) and rice (Oryza sativa L., cv. unknown) shoots after various light treatments using an indirect peroxidase-antiperoxidase antibody labeling method. Phytochrome is generally distributed throughout the cytoplasm in cells of tissue that had not been exposed to light prior to fixation. Within, at most, 8 min after the onset of saturating red irradiation, phytochrome, now present in the far-red-absorbing form, becomes associated with discrete regions of the cell. These regions do not appear to be nuclei, plastids, or mitochondria. After phototransformation back to the red-absorbing form originally present, phytochrome slowly resumes its general distribution. It is possible that this discrete localization of the far-red-absorbing form of phytochrome represents a physiologically significant binding with a receptor site in the cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boisard J., Marmé D., Briggs W. R. In Vivo Properties of Membrane-bound Phytochrome. Plant Physiol. 1974 Sep;54(3):272–276. doi: 10.1104/pp.54.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. A., Pratt L. H. Electron microscopic localization of phytochrome in plants using an indirect antibody-labeling method. J Histochem Cytochem. 1974 Nov;22(11):1039–1047. doi: 10.1177/22.11.1039. [DOI] [PubMed] [Google Scholar]
- Kidd G. H., Pratt L. H. Phytochrome destruction: an apparent requirement for protein synthesis in the induction of the destruction mechanism. Plant Physiol. 1973 Oct;52(4):309–311. doi: 10.1104/pp.52.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe K., Furuya M. Phytochrome-dependent Reduction of Nicotinamide Nucleotides in the Mitochondrial Fraction Isolated from Etiolated Pea Epicotyls. Plant Physiol. 1974 Mar;53(3):343–347. doi: 10.1104/pp.53.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Coleman R. A. Immunocytochemical localization of phytochrome. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2431–2435. doi: 10.1073/pnas.68.10.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H. Comparative immunochemistry of phytochrome. Plant Physiol. 1973 Jan;51(1):203–209. doi: 10.1104/pp.51.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Kidd G. H., Coleman R. A. An immunochemical characterization of the phytochrome destruction reaction. Biochim Biophys Acta. 1974 Sep 13;365(1):93–107. doi: 10.1016/0005-2795(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Marmé D., Schäfer E. Particle-bound phytochrome from maize and pumpkin. Nat New Biol. 1973 Oct 10;245(145):189–191. doi: 10.1038/newbio245189a0. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Schäfer E., Marmé D. Turnover of phytochrome in pumpkin cotyledons. Plant Physiol. 1973 Aug;52(2):128–131. doi: 10.1104/pp.52.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]