Abstract
There are currently more than 600 diseases characterized as affecting the central nervous system (CNS) which inflict neural damage. Unfortunately, few of these conditions have effective treatments available. Although significant efforts have been put into developing new therapeutics, drugs which were promising in the developmental phase have high attrition rates in late stage clinical trials. These failures could be circumvented if current 2D in vitro and in vivo models were improved. 3D, tissue-engineered in vitro systems can address this need and enhance clinical translation through two approaches: (1) bottom-up, and (2) top-down (developmental/regenerative) strategies to reproduce the structure and function of human tissues. Critical challenges remain including biomaterials capable of matching the mechanical properties and extracellular matrix (ECM) composition of neural tissues, compartmentalized scaffolds that support heterogeneous tissue architectures reflective of brain organization and structure, and robust functional assays for in vitro tissue validation. The unique design parameters defined by the complex physiology of the CNS for construction and validation of 3D in vitro neural systems are reviewed here.
Keywords: Tissue engineering, Central nervous systems, Blood brain barrier, 3D in vitro model
1. Introduction
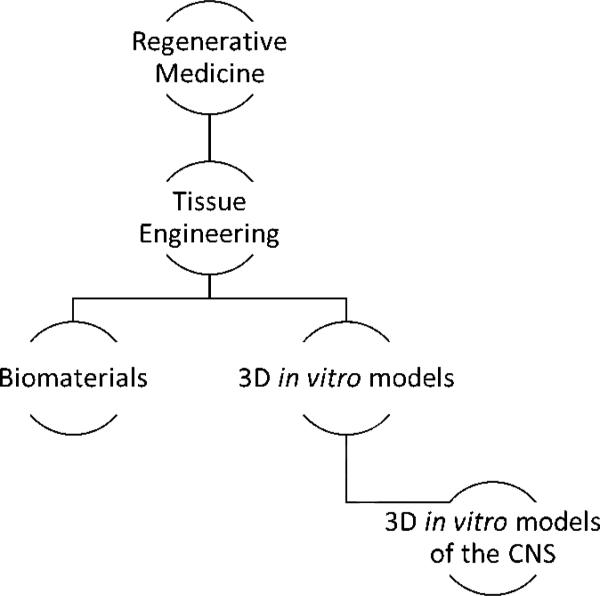
Tissue engineering and regenerative medicine are fields which have a unique tactic to solve clinical problems: combining the principles of engineering, clinical medicine, biology and materials science. Regenerative medicine, according to the National Institute of Health (NIH), is a broad field which involves “intervention to improve the self-healing capacity of the human body by use of either scaffolding materials, biologically active molecules and cellular components”, or some combination of these components. There are many approaches within regenerative medicine, including, but not limited to: genetic engineering and subsequent implantation of cells (Lee et al., 2012), bottom-up design and synthesis of tissue constructs (Kwon et al., 2014), construction of native decellularized extracellular matrix (ECM) (Wagner et al., 2014), and regenerative methods (Klar et al., 2014), Figure 1. Tissue engineering is a large subfield of regenerative medicine which refers to a combinatorial approach of the aforementioned components into a functional tissue or “unit” of tissue in vitro. Tissue engineering encompasses biomaterial development, which results in novel, biocompatible materials suitable for interfacing with living tissues. Subsequent utilization of the biomaterials as a scaffolding support for the cells during in vitro culture allows for development of 3D tissue models. Among these, several models aim for reconstruction of specific anatomical structures of CNS such as cortex, optic nerve, blood-brain barrier (BBB) or spinal cord tissue. This review will focus on tissue engineering as a tool applied to the development of in vitro models of the CNS.
Figure 1.
Graphical representation of 3D tissue modeling subfield. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
1.1 History of Clinical Neuroscience
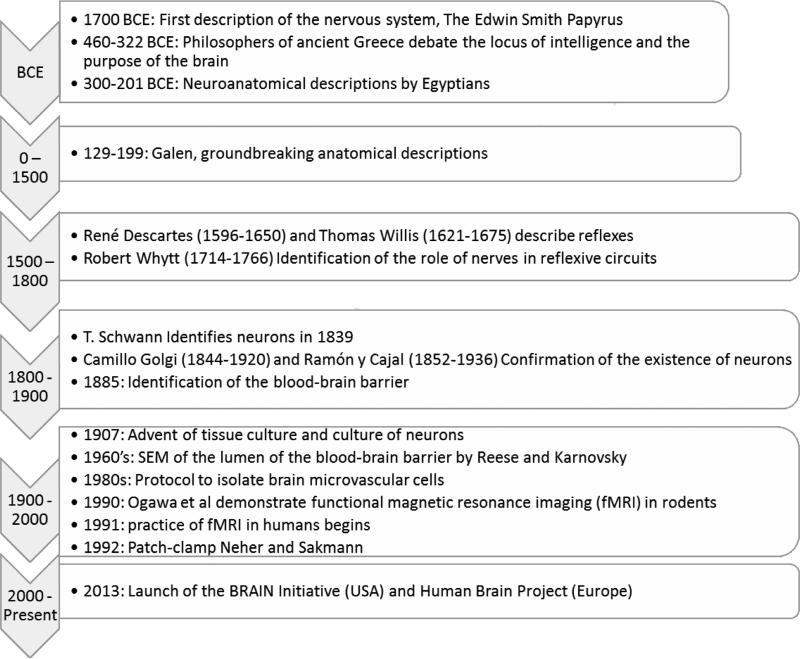
The brain and its substructures were identified early in human history, the nervous system being cited from the Babylonian period, 1700 BCE, with the Edwin Smith papyrus being the earliest known to describe surgical techniques to heal injuries (Wilkins, 1964). Since this primary discovery animal models were used in order to study the brain as well as the relationship between the brain and the rest of the body (i.e. behavioral studies). For ancient Greeks these studies were philosophical in form as they considered the human body sacred and did not dissect it. They focused on debates of the seat of intelligence; attempting to answer questions involving the locus of emotion and thought. The first documented which clearly defined the brain as a place where thoughts are generated, sensory information accepted and processed, and memories stored was in documents by Alemaeon of Croton (Crivellato and Ribatti, 2007). This view was shared by Hippocrates (460-375BCE) and Socrates (471-399BCE), but contradicted by Plato (427-347 BCE) and Aristotle (384-322 BCE). In contrast to the theoretical approach of the Greeks, Egyptians were conducting dissections of the human body. In the Alexandrian period, 3rd century BCE, groundbreaking neuroanatomical descriptions were produced (Gross, 1987). From this point until the 16th century, with the exception of Galen (129-199), there were almost no new contributions other than refinement of ancient practices in Islamic Middle East. In the 16th century studies on anatomy catalyzed the concept of structure-function relationships, bridging the previous gap between the Greeks’ observational science and the Egyptian's experimental science.
Early studies of structure-function relationships in the nervous system began with reflexes, for example the automatic withdrawal from a painful stimulus, beginning with René Descartes (1596-1650) and Thomas Willis (1621-1675). However, the neural mechanisms behind these circuits were not rigorously tested until Robert Whytt (1714-1766) conducted experiments on frogs. These studies were restricted to the tissue-level, and a valid hypothesis of the cellular constituents of nerves by Theodore Schwann did not occur until 1839. However, acceptance of the existence of neurons, the primary functional cell type of the CNS, was resisted until the studies using silver stain by Camillo Golgi (1844-1920) and Ramón y Cajal (1852-1936) revealed the structure of the neuron. Upon this discovery, many scientists became interested in the cellular-level contribution to the function of the brain.
The study of neurons in 2D culture first became possible in 1907 when Rose Harrison introduced tissue culture by maintaining tissue explants outside of the animal and observing the outgrowth of nerve cells (Harrison et al., 1907). Refer to Figure 2 for a timeline.
Figure 2.
A graphical summary of key milestones in clinical neuroscience. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
Since these early studies, in vitro tissue models of the CNS have advanced, however none are able to fully capture the functionalities and subtle mechanisms of the actual tissues. This is due to challenges of complexity (structure, number and flux of bioactive factors), physiological relevance (substrate stiffness, cell-cell interactions, and ultrastructure) and methods for functional evaluation (electrophysiology). For these reasons, researchers are invested in the development of tissue-like models through tissue engineering.
1.2 A guide to reading this review
Although tissue engineering of the nervous system is in its infancy, a number of important subfields have emerged. While the details of these are beyond the scope of this review, we direct readers to review papers in the fields of: (i) nerve guide conduits for peripheral nerve repair (Marquardt and Sakiyama-Elbert, 2013), (ii) in vitro models of the BBB (Naik and Cucullo, 2012; Wong et al., 2013), (iii) models of the brain (Brennand et al., 2012; D'Angelo et al., 2013; Morrison et al., 2011; Zaman, 2013) (iv) microfluidic systems (Harink et al., 2013; Millet et al., 2007; Morin et al., 2006; Taylor et al., 2003), (v) drug delivery to the nervous system (Pardridge, 2002; Pehlivan, 2013), (vi) brain-device interfaces (Aregueta-Robles et al., 2014; Cullen et al., 2011; Lebedev and Nicolelis, 2006), and (vii) prevention of adverse reactions to device implantation (Shain et al., 2003; Spataro et al., 2005; Zhong and Bellamkonda, 2007). This review will focus on in vitro tissue models of the brain and BBB.
Tissue engineering of functional neural systems for in vitro studies presents unique challenges arising from a limited understanding of neuronal cell network functions in vivo, the lack of readily available human cell sources, and the difficulty in capturing electrophysiological information. This review will provide a brief introduction on the relevant anatomy, physiology and biology with respect to each of these design challenges, the critical requirements which must be met for each challenge, as well as the current techniques available to meet these requirements. In particular, this review will focus on these topics as they apply to the development of in vitro tissue models for the study of the CNS. This review is organized so that each section is dedicated to each of the major categories of design criteria for in vitro tissue models. Each section begins with relevant background information, followed by highlights of the key qualities which must be captured by the in vitro tissue-models, and finally what the status is of current technologies and the present shortcomings based on these design requirements. The major sections will include: motivation and current technologies, designing the ECM, cellular sources, assembly of 3D structures, functional evaluation and a summary with conclusions and future perspectives.
List of acronyms used in this paper includes: ABC (ATP-binding cassette); AQP (aquaporin); BBB (blood-brain barrier); BMECs (brain microvascular endothelial cells); CNS (central nervous system); CSPGs (chondroitin sulfate proteoglycans); DRG (dorsal root ganglia); ECM (extracellular matrix); ECS (extracellular space); EEG (electroencephalography); ELISA (enzyme-linked immunosorbent assay); GABA (gamma-aminobutyric acid); GDNF (glial-derived neurotrophic factor); GLUT1 (glucose transporter 1); h (human); HA (hyaluronic acid); iPSCs (induced pluripotent stem cells); JAMs (junctional adhesion molecules); LAT1 (L-type amino acid transporter 1); MBP (myelin basic protein); multi-drug resistant protein 1 (MDR1); MCT1 (monocarboxylic transporter 1); mesenchymal stem cell (MSC); NCAM (neural cell adhesion molecule); NIH (National Institute of Health); NPC (neural progenitor cell); NSC (neural stem cell); OAT (organic anion transporter); PNN (perineuronal net); PNS (peripheral nervous system); SCI (spinal cord injury); SVZ (subventricular zone); TBI (traumatic brain injury); TEER (transepithelial electrical resistance); TJ (tight junctions); VEGF (vascular endothelial growth factor); ZO (zona occludens).
1.3 Tissue engineering in vitro models of the CNS
1.3.1 Motivation in perspective
Death is defined as the irreversible cessation of life, where in the clinic they will define this as the cessation of heart beat and breathing, or brain activity (Goila and Pawar, 2009; Keely et al., 1981). With the advent of life support, a patient's life can be maintained long after brain-death. However, when the brain fails to function properly, it significantly reduces the quality of life, and, depending on the extent of the injury or the stage of the disease, it can result in changes in personality or paralysis (spinal cord injury). To some philosophers, this state of being is such that life no longer becomes worth living (Greene, 2013), which has motivated CNS research toward healing the injured or diseased nervous system. However, there are ethical concerns with research that aims to change the structure and function of the nervous system: computational modeling resulting in artificial intelligence (Ashrafian, 2014), and physiological research resulting in technologies which could be used for mind control or mass monitoring (Ford and Deshpande, 2013). There is also the possibility for unintentional repercussions, for example identity crisis. However, most governments believe that the fear of abuse of knowledge should not outweigh the need for development in these areas and as such have proceeded to fund research. Fortunately, these initiatives have included branches to debate philosophy and ethics so that policy can stay up-to-speed with science.
In 2013 significant funding was been allocated into “brain mapping” projects, akin to the Human Genome Project. Funding was awarded by both European Commission (Human Brain Project) and American government (BRAIN Initiative) (Reardon, 2014). The approach of Human Brain Project is theoretical, using computing power to generate a model and the BRAIN Initiative seeks to develop technological methods (e.g. non-invasive imaging) to study the human brain in real time as well as methods for histological analysis post-mortem. The motivation of both initiatives to develop novel technologies include the well-being of societies, staying on the “cutting edge”, and to avoid losing scientists and clinicians to other countries.
There is a critical need for innovative therapeutic strategies for patients suffering from neurological disorders. Despite significant research efforts over the past several decades, there is still a vast amount unknown about the structure-function relationships of the nervous system. Though resources have been invested into identifying the different circuits of the brain through the study of primates and other animal models (Joshua and Lisberger, 2014) and imaging in humans (Craddock et al., 2013), these tend to be limited when trying to translate structure to function, particularly on the cellular or subcellular level. One of the most fascinating aspects to the brain are the neural circuits and their formation, which is typically studied in relatively simple organisms. However, even the most simple of the animal models used for studying neural circuits has proven complex to decipher. For example, the circuit for the somatogastric system of crustaceans, a common animal model which is used to study circuit dynamics and central pattern generators (neural networks which generate rhythmic behaviors, e.g. digestion) has only 26 neuron cell bodies (as opposed to the estimated 100 billion in the adult human CNS), and by comparison these neurons are large in size. Even this system has not been “solved” and continues to provide new questions and insight (Rodriguez et al., 2013; Stein, 2009; Tansey et al., 2012). More complex animals used to model pathological states often differ significantly from human systems biochemically (e.g. gene expression, metabolism) (Balaban, 2013; Ohtsuki et al., 2013; Wesseling et al., 2013). In addition, there are strong ethical concerns in some sectors regarding use of larger animal models (Levy, 2012). Therefore, simplified circuits that could be built neuron by neuron in vitro would help to elucidate cellular mechanisms of brain function.
In addition to cell biology, the effects of different ECM molecules on different disease states and progression should be elucidated both to improve prognosis and potential therapeutic targets. For example, some researchers hypothesize that the progress and prognosis of a brain tumor could be monitored by observations of changes in the ECM composition (Zamecnik, 2005). This hypothesis is being supported by work done with other types of cancer (Bergamaschi et al., 2008). The information derived in these experiments could also provide insight on the differentiation of stem cells into neural specific cells. Many CNS-targeted therapeutics fail in clinical trials, in part due to the insufficient understanding of the cellular biology involved, the interconnected nature of cells-ECM and function, and many of the issues raised above.
While there is no substitute for the low cost and simplicity of 2D cell culture studies or the complexity provided by in vivo systems, the goal of 3D in vitro tissue modeling is to fill in the knowledge gap that exists between the 2D in vitro and in vivo approaches. The 2D cell culture approaches lack the complexity and physiological relevance, but allow to use human-derived cellular and protein constituents. On the other hand, animal studies offer complexity which cannot be modeled in vitro, but which is also difficult to control and manipulate, and therefore results in data that must be extrapolated to human systems. The goal of 3D in vitro tissue modeling is to provide human-relevant studies that are highly reproducible with tight control over the finite variables within these simplified neural systems.
1.3.2 Existing technology
There are several approaches to modeling CNS functions, and they can be broadly categorized as in vivo, in vitro, ex vivo, and in silico. In vivo work includes studies typically conducted on rodents for the purpose of basic science and pharmacology studies (e.g. BBB, stroke, drug toxicity) (Hoehn et al., 2002), on primates for cognitive and behavioral neuroscience work, and other vertebrate and invertebrate species for other structural nervous system research (Shiba et al., 2014). In vivo models related to the brain include, but aren't limited to, behavioral studies for structure-function relationships (van der Staay et al., 2009), drug discovery (Markou et al., 2009), traumatic brain injury (TBI) (Xiong et al., 2013), Alzheimer's (Götz and Ittner, 2008), and sensory information processing (de Brito Sanchez and Giurfa, 2011). Ex vivo models utilize slices of brain tissue dissected form the animal body which preserves the complexity of the brain tissue's structure while allowing for modes of evaluation which are typically only permissive in 2D environments. However, it has been reported that there is a significant loss of brain function once removed from the body due to the rapid loss of viability, and there are limitations on the amount of tissue which is available (Doering, 2010). In vitro models are cell-based models which involve culturing cells in 2D on functionalized surfaces (tissue culture plastic, natural or synthetic polymer coatings), frequently co-cultured with other cell types utilizing transwells (Cecchelli et al., 2014; Xue et al., 2013). These traditional in vitro models have provided a wealth of information about neural cell types; examples include spontaneous network formation, cell attachment sites for adhesion and migration, axonal guidance mechanisms, molecules (soluble and insoluble) in synaptic targeting, and the resting membrane potential of different cell types (Kay and Wong, 1986; O'Shaughnessy et al., 2003). Another type of in vitro model, referred to as cell aggregates (Kato-Negishi et al., 2013), organoids (Lancaster et al., 2013) and neurospheres (Hogberg et al., 2013; Urich et al., 2013), is based on the growth of either stem cells or differentiated cells as a conglomerate (Jafari et al., 2013). These are advantageous as they allow for high cell density with spontaneous self-organization and cell guided assembly of 3D environment. However, they tend to suffer from high variability due to the stem cell clone-ability and commonly form necrotic cores due to the insufficient oxygen and nutrient diffusion, subsequently leading to size limitation (Lancaster et al., 2013). Results gained from the in vitro, ex vivo and in vitro models serve as a basis for in silico models which use the computing power to generate predictions based on experimental data sets. Common in silico models are used to observe the pharmacology of untested compounds in a specific physiological system (Broccatelli et al., 2012). For example, an in silico model might attempt to predict how long a drug at a particular dose would take to reach its target site by modeling the drug's transport from the plasma through relevant tissues. They can also include imitation of neural circuits, better known as “artificial intelligence” (Bluebrain, 2014). In addition, in silico modeling has been applied to the prognosis of brain cancer (Sanga et al., 2007). However, in silico models are highly limited in terms of number of components they can take under consideration and require huge computing resources. However, the goal of in silico models is quite similar to 3D in vitro models: in both approaches the objective is to connect what is seen in 2D cultures on the cellular/molecular scale with what is seen in vivo on the anatomical and systems scale.
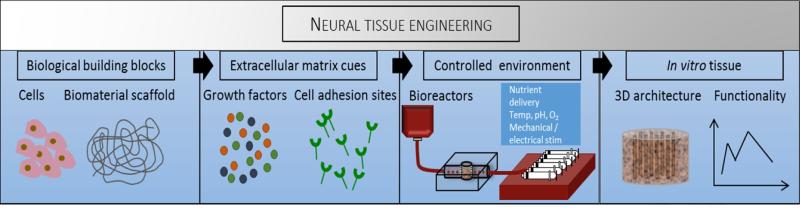
Although several animal models of neurological diseases offer phenotypically relevant models of human pathologies, knowledge gaps exist between information provided by 2D cultures and animal models, and the unsatisfactory correlation between animal and human physiology (Albers et al., 2001; Birmingham, 2002). In vitro cultures of neuronal cells on flat 2D surfaces offer simplified, high throughput systems to study disease, drug toxicity, and biological processes by controlling environmental factors and directly measuring cell responses (Blain et al., 2010; Hopkins et al., 2013). However, 2D cultures lack endogenous 3D cell-cell interactions and physiological cues provided by the ECM. Tissue engineering aims to recapitulate human tissue systems outside of the body for tissue replacement and regeneration or for the in vitro study of cellular mechanisms and therapeutic development. Traditional tissue engineering techniques involve the culture of cells on biomaterial scaffolds in a controlled environment established in bioreactor systems, Figure 3. These systems also have an advantage over in silico models in that they are not as subject to presumptions or theories: To design a mathematical model certain assumptions must be made, choosing the wrong mathematical model or making incorrect assumptions can result in oversimplification of the system or in error prorogation. In addition, in silico models are limited in complexity by current processing power, particularly once variability amongst individuals is considered. Refer to Table 1 for a summary of the advantages and disadvantages of each approach.
Figure 3.
Traditional tissue engineering techniques. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
Table 1.
Advantages and disadvantages of current approaches to modeling CNS. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
| Type of Model | Advantages | Disadvantages | References |
|---|---|---|---|
| In Vivo | • High complexity, particularly pathological modeling • Suitable for long-term studies • Anatomical relevance |
• Limited sample size • Low reproducibility • High volume and low control over variables • Limited relevance to humans • Difficult to quantify results |
(Cernak, 2005; Götz and Ittner, 2008) |
| Ex Vivo (Tissue-Slices) | • Complex architecture • Capability for functional tests used on 2D slices • Contains all cell types |
• Loss of tissue function • Short-term culture only • Low access to human tissue |
(Hanson et al., 2010; Morrison et al., 2006) |
| 2D In Vitro | • Inexpensive • Highly reproducible • Capable of high throughput studies • Quantifiable • Ability to carefully control environmental conditions • Numerous, wellcharacterized assays |
• Limited complexity • Short-term culture only • Limited cell sources available • Limited insight into biological mechanisms and functions, particularly on the whole-tissue scale |
(Abbott et al., 2010; Blain et al., 2010; Dubois-Dauphin et al., 2010; Fedoroff and Richardson, 2010) |
| In Silico | • Inexpensive • High-throughput • Highly quantitative • Highly reproducible |
• Currently, impossible to model all parameters • Input parameters limited to animal studies |
(Braitenberg, 2001; Liu et al., 2004) |
| 3D In Vitro: Cell Aggregate | • High throughput • Quantifiable • Simultaneous examination of biological mechanisms and functional outputs • 3D |
• Typically cannot have both high complexity and high variable control • Formation of necrotic core |
(Lancaster et al., 2013; Tieng et al., 2014) |
| 3D In Vitro: Tissue-Engineered Approaches | • Reproducible • Controlled complexity • Capability for long-term studies • Quantifiable • Capability to probe biological mechanisms • Possibility for human and physiological relevance |
• Lack of protocols and technology for functional evaluation • Limited human cell sources • Bioreactors required for longer cultures (≥ 6 months) and criticallysized constructs • Currently, limited to tissue-scale (cannot model whole-organ) |
(Cecchelli et al., 2007; Ogunshola, 2011) |
The goal of most current tissue models is to establish systems which can be used to study pharmacology, network formation, pathology, or combination thereof. There are two basic approaches to develop these models: bottom-up (compartmentalized) and top-down (developmental/regenerative). Bottom-up is the methodology of first designing the scaffolding structure and then seeding it with the relevant cell types and adding biochemical components into the preformed structures. That is, all of the factors are assembled into specific spatial coordinates at specific time points. This approach was used by our research group to develop novel complex functional 3D brain-like cortical tissues, maintained for months in vitro, formed from primary rat cortical neurons in modular 3D compartmentalized architecture with electrophysiological function (Tang-Schomer et al., 2014). Bottom-up approach was also used by Cucullo and co-workers who described the formation of preformed capillaries and venules kept in series through a systolic bioreactor system (Cucullo et al., 2013). These vessel-like structures were assembled using relevant cell types and shown to be functional through assays measuring TEER and permeability which will be discussed in Section 5.
In contrast, top-down, or regenerative approaches rely on the “intelligence” of the cells and involve encapsulation of cells within a substrate and allowing them to spontaneously form structures within the soft substrata which the cells are able to degrade and reconstruct. An example of this approach is an embryonic stem cell-based model developed by Dubois-Dauphin and co-workers depicting spatially organized nervous tissue resembling brain sub-ependymal nervous tissue maintained in vitro for at least 3.5 months (Dubois-Dauphin et al., 2010). Moreover, Lancaster and co-workers showed that human induced pluripotent stem cells (hiPSCs) encapsulated and grown in Matrigel result in spontaneous development of “cerebral organoids” which formed structures anatomically comparable to the organization of the progenitor zone in human brain (Lancaster et al., 2013). In this line, Kato-Negishi and co-workers presented the neural building blocks build by a combined culture of massive amount of rat cortical and hippocampal neurospheroids in a confined space (Kato-Negishi et al., 2013). Similarly, Urlich and co-workers proposed a 3D BBB model based on a self-assembling human heterocellular spheroid (Urich et al., 2013). Further details of these approaches will be discussed in Section 4. Independently from the applied strategy, all tissue-engineered in vitro models have common challenges: designing the ECM, choosing and maintaining CNS-derived cells, engineering 3D architecture, and validating function. These design challenges will be the focus of the rest of this review.
2. Designing the ECM
2.1 Molecular composition and function
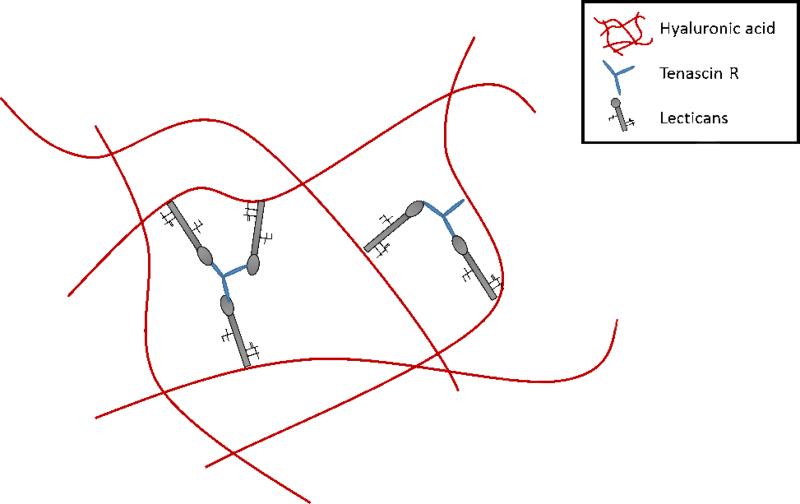
Due to the high density of cells in the brain, only about 20% of total brain volume is extracellular space, and 20-30% is comprised of ECM (Nicholson, 2001; Sykova and Nicholson, 2008). Unlike ECM of many other organ systems, adult CNS tissue lacks high concentrations of fibrillar collagen, fibronectin, and laminin (Bellail et al., 2004), although there is laminin and fibronectin present in the basal lamina (Liesi et al., 1983, 1986). The ECM of healthy brain tissue is comprised primarily of hyaluronan, proteoglycans (lecticans), and tenascins (Bellail et al., 2004; Bignami et al., 1993; Lander, 1993; Ruoslahti, 1996). Lecticans are a family of chondroitin sulfate proteoglycans (CSPGs) which are a primary organizational component of brain ECM. There are four types which have been identified: aggrecan, versican, neurocan and brevican, where the latter two are unique to the brain. These CSPGs all have a C-type lectin domain on the C terminus and a hyaluronan binding site on the N-terminus (Yamaguchi, 2000). These lecticans also all have sites to bind to tenascins. Tenascins are glycoproteins believed to act as linkers, connecting the different proteoglycans as well as providing cell binding sites. There are four members of the family, tenascin-W, C, X and R; in the CNS tenascin-C and tenascin-R are shown to play critical roles (Anlar and Gunel-Ozcan, 2012). Tenascin-R is a crosslinker for lecticans, and tenascin-C is associated with cell attachment and detachment (migration) (Anlar and Gunel-Ozcan, 2012; Jakovcevski et al., 2013). Hyaluronan, also known as hyaluronic acid (HA), is a glycosaminoglycan which carries a high negative charge, thereby attracting cations such as sodium (Na+) and charged proteins, consequently increasing hydration of brain tissue (Bignami et al., 1993). This high water content causes the volume of brain tissue to expand to 10 times greater than that of its molecular constituents and is hypothesized to be the primary mechanism behind the low stiffness of brain tissue (Bignami et al., 1993). HA is also the primary structural component of the ECM (Anlar and Gunel-Ozcan, 2012; Bignami et al., 1993). Insoluble fibronectin is a protein dimer which has a high density of integrin binding sites as well as binding sites to other ECM molecules, it is described by some as the glue of the human body (Pankov and Yamada, 2002). Laminin is a glycoprotein with a characteristic, asymmetric cross-shape which acts as a functional integrator between cells bound to ECM components (Aumailley, 2013).
These different components of ECM and their correlation to different functions of the brain is highly complex. Most of the ECM is a part of the perineuronal net (PNN), also referred to as the Golgi net, which is a dense, mesh-like matrix found around neural soma and concentrated around synapses. The PNN acts to prevent random synapse formation; degrading this matrix reestablishes high, experience-based plasticity seen in the developmental period (Celio et al., 1998; Wang and Fawcett, 2012). It is also shown to act as an ion buffer and to protect against oxidative stress (Suttkus et al., 2012). Although the structure of this mesh is highly varied, there is high spatial, temporal, and cell subtype specificity there is a general, hypothesized structure, refer to Figure 4 (Celio et al., 1998). This hypothesis states that HA is bound to neuronal bodies, lecticans are bound to HA, and the lecticans are linked together via tenascin R (Suttkus et al., 2014; Yamaguchi, 2000). A similar dense matrix structure known as the perisynaptic ECM, also referred to as axonal coats, also exists. It plays a role in brain homeostasis through growth factor sequestration, creation of diffusion barriers (due to high cell density and the highly tortuous ECS of the brain), and by providing substrate for cell stabilization and raw materials for signaling components (Frischknecht and Gundelfinger, 2012). The final, major ECM structure in the brain is referred to as “basal lamina-like”; this ECM is found at the BBB and within the neurogenic niche. The neurogenic niche refers to a space found near brain capillaries where there is a limited source of neural stem cells.
Figure 4.
Representation of the PNN. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
Neural tissues have low elastic moduli ranging from <1 kPa for brain slices (Elkin et al., 2007) and single brain cells (Lu et al., 2006) to ~90-230 kPa for spinal cord (Chang et al., 1988; Levental et al., 2007), which is similar to muscle fibers (~30-50 kPa, (Fridén and Lieber, 2003)) and skin (10-100 kPa, (Zheng and Mak, 1997)) and much softer than bone (106-107 kPa, (Rho et al., 1993)). Recent work has demonstrated that substrate stiffness significantly affects in vitro cell attachment, survival, and growth of many cell types including fibroblasts, myocytes, and neurons (Discher et al., 2005; Engler et al., 2006). Neural culture studies on substrates of different stiffness have reported changes in growth and axon extension. Neurites from chick dorsal root ganglia (DRG) explants grew significantly longer down the gradient of stiffness than up the gradient (Man et al., 2011). Interestingly, Koch and co-workers found that, in comparison, peripheral nerve outgrowths are more sensitive to changes in substrate stiffness than CNS hippocampal neural extensions (Koch et al., 2012). This sensitivity of peripheral neurite outgrowth to substrate stiffness may be due to the increased diversity in mechanical properties of the peripheral nervous system (PNS) as compared to the CNS where connective tissue consists largely of glial cells (Koch et al., 2012). Accordingly, the work of Balgude and co-workers on DRG outgrowth in agarose gels (Balgude et al., 2001) as well as our own studies of DRG neurite extension on silk hydrogels (Hopkins et al., 2013) resulted in varying growth rates on substrates of different stiffness. However, in general, hydrogel substrates that are <80 kPa have been most successful for neural cell culture (Balgude et al., 2001; Hopkins et al., 2013; Man et al., 2011). Of importance, it has been shown that stiffer substrates, as seen in pathologies such as Alzheimer's, can have a snowball effect, further perpetuating the pathological state (Franze et al., 2013). Therefore, careful adjustment of mechanical properties of the scaffolds is of highest importance to match the CNS tissue characteristics, ensure efficient neurite outgrowth and prevent cell differentiation.
2.2 Critical design requirements of polymers
Critical design requirements for polymers used for neural tissue engineering can be broadly categorized as physical (stiffness, degradation rate), biochemical (biological activity) and practical (cost, reproducibility). As described above, soft matrices are required because they significantly improve axon length, cell attachment, and survival. They are also more mimetic of the mechanical properties seen in vivo, which is necessary for any mechanical modeling (e.g. of a mechanical injury). A successful polymer has an elastic modulus of <1kPa, imitating the softness of brain and being shown to benefit neural cultures (Georges et al., 2006). Moreover, it needs to be slowly degradable to allow for material remodeling along the cell growth and morphogenesis. For example hiPSC need 20-30 days to develop a defined brain structures (Lancaster et al., 2013). Therefore, the polymer chosen for CNS modelling must persist for at least 30 days or more. These two physical requirements can be challenging to resolve; the low-protein content of the soft, natural polymer matrices results in hydrolysis occurring more rapidly in the aqueous environment of cell-culturing media. An additional requirement for support of neuron growth is that the 3D scaffolds must have proper pore size and porosity to allow for nutrient diffusion and cell infiltration throughout the gel. A successful material should exhibit similar diffusion properties to brain tissue which is 1.5-2.35 cm2/s for albumins (Tao and Nicholson, 1996). Materials must also be permissive to various forms of evaluation. This includes optical clarity allowing for imaging of high-density cell cultures and thick constructs, or free ion movement for electrical measurements (Aregueta-Robles et al., 2014; Dana et al., 2014). Another interesting requirement is electrical conductance to allow for the delivery of electrical current to cells or to electrically activate molecular structures (Balint et al., 2014). By including these structures it allows for selective opening of cryptic sites in the polymer which can include soluble or insoluble, bioactive compounds. The purpose of the development of electrically conductive polymers, within the context of neural tissue engineering, is to study electrical stimulation for axonal guidance and for incorporation into devices to attract or repel neuronal growth (Aregueta-Robles et al., 2014). In addition, these electrically active polymers could significantly refine the time-scale for presentation of bioactive molecules, which is highly regulated in vivo, furthering possibilities of in vitro cultures. However, a necessary requirement if there is to be functional evaluation of the model, is that the hydrogel permits ion movement. This requirement will be illustrated in Section 5.
In order to allow for the growth of neurons, materials must have binding sites recognized by the integrins or other adhesion receptors expressed by neural cell types in order to bind to the surface of the biomaterial. The substrates must also have biologically active sites which catalyze signaling cascades of interest. Due to the heterogeneity among cell types for these behaviors, it is typically necessary to include proper controls as it is difficult to directly compare to values obtained from other sources. Doing these control experiments can also provide a bridge between the in vitro model being investigated and well-established 2D experiments, further validating the model in question. Some parameters which are generally observed are axon length and myelination, functional synapse formation, and cell morphology.
Moreover, the polymer should also be highly reproducible and cost effective. Quality control of the performance and fabrication of the materials is critical in order to confirm results across research groups. Since significant amounts of materials are required to form 3D constructs, it is essential that the material is affordable. This can prove costly when including proper amount of replicates, controls, and various experimental groups. The matrix must also be statistically equivalent across replicates in order to be successful, as in any study.
2.3 Polymers utilized in neural tissue engineering
Due to their low stiffness, high water content, and ability to be functionalized with bioactive components, hydrogels have been the major class of biomaterials investigated for in vitro neural culture. Hydrogels can be of natural or synthetic origin and include agarose (Balgude et al., 2001; Bellamkonda et al., 1995a, 1995b; Cullen et al., 2007a; Dillon et al., 1998; O'Connor et al., 2001), collagen type I (Coates and Nathan, 1987; Coates et al., 1992; Krewson et al., 1994; O'Connor et al., 2000a; O'Shaughnessy et al., 2003), hyaluronic acid (Brännvall et al., 2007, 2007; Hou et al., 2006; Pedron et al., 2013; Tian et al., 2005), N-(2-hydroxypropyl) methacrylamide (HPMA) (Woerly et al., 1990, 1991), poly(ethylene glycol) (PEG) (Pedron et al., 2013; Wang et al., 2014; Zustiak et al., 2013), chitosan (Gao et al., 2014; Leipzig et al., 2011; McKay et al., 2014), alginate (Frampton et al., 2011; Kuo and Chang, 2013; Matyash et al., 2012; Mosahebi et al., 2003), silk fibroin (Benfenati et al., 2010, 2012; Hopkins et al., 2013; Hu et al., 2013; Kim et al., 2010; Tien et al., 2013; Zhang et al., 2012) and methylcellulose (Stabenfeldt and LaPlaca, 2011; Tate et al., 2001), Table 2.
Table 2.
Biomaterials investigated for neural culture systems. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
| Biomaterial | Design Features Investigated | References |
|---|---|---|
| Agarose | • Pore size • Laminin-derived peptides • Charge • Polymer concentration • 3D encapsulation • Acute mechanical insult |
(Balgude et al., 2001; Bellamkonda et al., 1995a, 1995b; Cullen et al., 2007a; Dillon et al., 1998; O'Connor et al., 2001) |
| Alginate | • Pore size • Charge • ECM molecule functionalization • Encapsulation of cells (Schwann, neurons, astrocytes, iPSCs, etc.) • Neural culture models (network formation) • Implantation and drug delivery |
(Frampton et al., 2011; Kuo and Chang, 2013; Matyash et al., 2012; Mosahebi et al., 2003) |
| Collagen type I | • Electrophysiology of cells at surface • Collagen concentration • ECM molecule (fibronectin, laminin) functionalization • Collagen-agarose composites • NPC differentiation |
(Coates and Nathan, 1987; Krewson et al., 1994; O'Connor et al., 2000b; O'Shaughnessy et al., 2003) |
| Hyaluronic acid | • Implantation response • NSC proliferation and differentiation • Cell attachment sites • Neurite growth and inhibition • Effects on pathological states |
(Hou et al., 2006; Pedron et al., 2013; Tian et al., 2005) |
| Chitosan | • NSC differentiation with immobilized bioactive factors • Injectable matrix for implantation • Gene delivery |
(Gao et al., 2014; Leipzig et al., 2011; McKay et al., 2014) |
| Methyl-cellulose | • Implantation in brain injury • Neural cell viability • Substrate stiffness • ECM molecule (laminin) functionalization |
(Stabenfeldt and LaPlaca, 2011; Tate et al., 2001) |
| PEG | • Matrix stiffness • Encapsulation of cells • ECM molecule functionalization • 3D glioma modeling |
(Pedron et al., 2013; Wang et al., 2014; Zhou et al., 2013) |
| p(HEMA), p(GMA), HPMA | • Implantation response • Implantation in SCI • Neural cell viability & morphology |
(Woerly et al., 1990, 1991, 2001) |
| Silk fibroin | • Substrate stiffness • ECM molecule functionalization (fibronectin, laminin) • Growth factor delivery • Neurite growth rate • Charge • Neurite alignment • Brain-device interface |
(Benfenati et al., 2010, 2012; Hopkins et al., 2013; Hu et al., 2013; Kim et al., 2010; Tien et al., 2013; Zhang et al., 2012) |
ECM = extracellular matrix; iPSC = induced pluripotent stem cell; NPC = neural precursor cell, NSC = neural stem cell, SCI = spinal cord injury
Most of these hydrogels meet the most basic requirements for successful neural cell culture such as low stiffness, efficient nutrient, oxygen and waste diffusion, and cell attachment sites which do not cause aberrations from neural phenotype. Reported stiffness values of hydrogels match those of neural tissues ranging from ~1 to 400 kPa (Ahearne et al., 2005, 2010; Hopkins et al., 2013; Man et al., 2011). Many natural hydrogels contain endogenous cell attachment sites such as the amino acid sequence arginine-glycine-aspartic acid (RGD) (Kardestuncer et al., 2006; Pierschbacher and Ruoslahti, 1984; Rice et al., 2013; Sakiyama et al., 1999) and synthetic hydrogels often offer chemical moieties for covalently incorporating cell-binding proteins (Patterson et al., 2010; Rice et al., 2013). Several groups have investigated neural cell growth on hydrogels functionalized with ECM proteins including laminin and fibronectin (Bellamkonda et al., 1995a, 1995b; Hopkins et al., 2013; Hou et al., 2006; Stabenfeldt and LaPlaca, 2011). A prevalent integrin subunit expressed by neurons is the β1 subunit; integrins α1-3,6β1 have been shown to bind laminin and collagen, α3-5β1 to fibronectin, and α7β1 to vitronectin mediating neurite outgrowth when cultured on laminin or collagen IV (Tomaselli, 1991). Diffusion of proteins through hydrogels is also similar to that of brain tissue (e.g. 2-9 × 10−6 cm2s−1 of bovine serum albumin through 1 to 4.5% collagen gels, (Ramanujan et al., 2002)). However, each of these biomaterials has particular advantages and disadvantages, which will be discussed here for the more commonly utilized materials.
Collagen type I has been employed in modeling in vitro systems of brain tissue (Al Ahmad et al., 2011; Bercu et al., 2013; Tang-Schomer et al., 2014) due to the fact that it promotes significantly higher neuron survival, cell attachment, and neurite outgrowth when compared to other ECM materials (Krewson et al., 1994; Zhou et al., 2013). Additionally, since collagen type I contains endogenous RGD motif, costly macromolecules such as laminin are not necessary to improve its biological activity. For example, collagen encapsulation of umbilical cord blood stem cells induced their spontaneous neuronal differentiation and allowed for their extended culture with high viability (Bercu et al., 2013). Moreover, neural stem cells were shown to differentiate into neurons, astrocytes, and oligodendrocytes when grown in a dense 3D collagen cultures (Watanabe et al., 2007). Similarly, collagen-entrapped stem and progenitors actively expanded and efficiently generated neurons, which developed neuronal polarity, neurotransmitters, ion channels/receptors, and excitability (Ma et al., 2004). It overall suggest, that collagen is a permissive environment allowing for spontaneous or guided stem cell differentiation toward desired neuronal and glial phenotypes. However, collagen type I is limited in its relevance to neural tissue engineering due to its relatively fast degradation rate and its absence in brain tissue. Consequently, collagen type I does not contribute to synaptic guidance; typical fabrication techniques do not allow for patterned presentation of cell receptors (Antoine et al., 2014). This decreases the reproducibility of the system, which is not desirable in the field of modeling. However, collagen type I holds promise in the subfield of glioblastoma modeling (East et al., 2009), where cells are shown to overproduce fibrous proteins allowing for permissive growth and a highly migratory environment (Kaufman et al., 2005). Moreover, collagen type I is frequently utilized in 3D endothelial cell culture and microfluidic applications to model the vascular structures (Al Ahmad et al., 2011; Chrobak et al., 2006), which could be translated to model BBB and vascular compartment of the brain. Nevertheless, much remains yet to be optimized in terms of vascular structures stability, permeability and collagen degradation. Similarly, HA has been used glioblastoma modeling (Pedron et al., 2013). HA is attractive for use in neural tissue engineering due to its prevalence in the brain ECM and its low cost. However, HA can prove complicated to work with due to the broad spectrum of molecular weights and the variation in chain sulfation. HA has been shown to guide differentiation of neural progenitors toward either neuronal or glial cells by the modulation of hydrogel stiffness (Seidlits et al., 2010). Additionally, the differentiation pattern was shown to be divergent in case of fetal vs. adult progenitor cells. Fetal cells had the tendency to differentiate into glial cells, and while adult cells preferentially differentiated into neurons (Aurand et al., 2014). However, due to the tendency to inhibit neurite outgrowth HA has not been successful in long-term cultures (Bignami et al., 1993). In this line, HA is enriched in the neural stem cell niches where it maintains the stem cell quiescence and prevents the differentiation (Preston and Sherman, 2011). Therefore, HA is increasingly being explored as a composite material in terms of 3D guided neural stem cell differentiation towards complex constructs of CNS.
Finally, agarose and PEG gels have been investigated for in vitro culture as an inert biomaterial with tunable stiffness. These hydrogels can be functionalized through covalent coupling of bioactive molecules such as ECM-derived peptides or growth factors, which makes them advantageous for observing the decoupled effects of different parameters on cell fate (Balgude et al., 2001; Bellamkonda et al., 1995a; Seidlits et al., 2010; Tsurkan et al., 2013; Wang et al., 2014). However, both polymers cannot be easily remodeled by cells, unless modified with degradable peptide moieties or cross-linked with hydrolysis-prone chemistry (Chwalek et al., 2011).
Silk protein has also received recent attention as a favorable candidate for neural tissue applications due to its tunable stiffness, functionalization capability, and versatility in forming a myriad of scaffold platforms, Box 1 (Rockwood et al., 2011; Tang-Schomer et al., 2014; Vepari and Kaplan, 2007). These early works of hydrogel characterization for 3D neural cultures has begun to lay down the foundation for construction of more complex in vitro neural systems through engineering of the neural extracellular space with regard to stiffness, diffusivity, and cell attachment sites.
3. Use of cells in the in vitro modeling of the CNS
3.1 Cells types of the CNS
There is variety of cell types found in the brain which broad role and function, and recent progress in the field is being discussed in detail during semiannual European Glial Cell meeting. However, at this stage of neural tissue engineering six types are typically considered: neurons, astrocytes, microglia, oligodendrocytes, endothelial cells and pericytes. Neurons are the primary functional unit of the brain and are responsible for electrical transmissions which allow for both conscious and autonomic behaviors. Glial cells are present in the CNS in nearly a one to one ratio to neurons and are thought of as a supportive cell type (Azevedo et al., 2009; Herculano-Houzel, 2014). The classification ‘glia’ encompasses astrocytes, microglia, oligodendrocytes, and ependymal cells (ventricle liners and cerebrospinal fluid secretors) (Iacovetta et al., 2012; Verkhratsky and Kettenmann, 1996). Vascular endothelial cells of the CNS are specialized with tight junctions (TJs) comprising the highly selective BBB. Pericytes reinforce this barrier function and are influenced by surrounding neurons and glia controlling cerebrovascular tension (intracranial pressure), angiogenesis (wound healing), and permeability (inflammatory response) (Wong et al., 2013).
Neurons are polarized cells with processes that extend from their soma (dendrites) and an axon that extends away from the cell body to conduct the action potential (Amadoro et al., 2014). Any processes extending from a neuron are generically termed a neurite. Neurons are also functionally polarized: dendrites and the soma receive electrical and chemical signals, and axons pass them on. Generally there are many, highly arborized dendrites and one axon for each neuron cell body, although axons may also branch. There are two basic types: projection neurons (principal cells) and intrinsic neurons (interneurons). The soma and dendrites of principal neurons reside in one brain region while their axons form synapses with neurons in other regions (Bota and Swanson, 2007). Interneurons have shorter axons which connect locally with neurons in the same brain region (Bota and Swanson, 2007). The basic neuron cell types include: pseudo-unipolar (sensory neuron), bipolar (interneuron), multipolar (motor neuron), and pyramidal (Takács and Metzger, 2002). There are many more specific classifications of cells which is beyond the scope of this review, for example Purkinje cells of the cerebellum (Albergaria and Carey, 2014). Within the spinal cord there tends to be less variety of neural cell types. It is composed primarily of afferent (sensory information to the brain) and efferent (motor output from the brain) neurons which are pseudo-unipolar and multipolar, respectively, with fewer interneurons and simple circuits. Neurons transmit electrical signals by movement of ion species. When neurotransmitter or electricity reaches the dendrite of a neuron it results in the depolarization of the membrane by opening of voltage-gated cation channels. Once a particular voltage threshold is reached, depending on the neuron, an action potential is fired down the axon in an “all-or-none” fashion in which there is no loss in amplitude from one action potential to the next.
In culture, neural cells derived from the CNS grow well on stiff, 2D surfaces such as tissue culture plastic, if coated with cell-adhesion-promoting factors such as poly-L-lysine or laminin. Neuronal cells express cell adhesion molecules (e.g. neural cell adhesion molecule (NCAM), L1, and N-cadherin) and integrins which require these functionalized surfaces to adhere to (Rutishauser et al., 1988). Cell-cell interactions are also critical enabling post-mitotic neurons to migrate along radial glial cell guidance fibers from the ventricular zone to the appropriate level in the layered cortex during development (Anton et al., 1999). Cerebral cortex formation was disrupted in α3β1 and α6β1 knock-out mice, supporting the notion that cell-cell and cell-matrix interactions are critical during embryogenesis and tissue development (De Arcangelis and Georges-Labouesse, 2000; Danen and Sonnenberg, 2003).
Historically, glial cells were considered a part of the extracellular matrix as the structural ‘glue’ physically supporting neural networks. It is now well known, however, that glial cells are functional entities, essential to maintaining homeostasis for normal function of the entire nervous system. Currently there are around eleven distinct morphologies of astrocytes identified, of these distinct subtypes eight are associated with the cerebral vasculature (Abbott, 2002). Astrocytes (or astroglia) are also the greatest in number and perform a myriad of functions including BBB homeostasis (Hawkins and Davis, 2005), volume regulation (Iacovetta et al., 2012), neuronal metabolism assistance through lactate shuttling (Schurr et al., 1997), neurotransmitter and ion buffering (Wong et al., 2013), disease (Schlachetzki et al., 2013; Sturrock, 1976), and secretion of basal lamina (Liesi et al., 1983). Astrocytes have also been shown to engage in slow-wave calcium signaling, and are hypothesized to contribute to the electrical behavior of neurons (Carlsen and Perrier, 2014). The other glial cell types are less diverse in subtype, however tend to be less well-understood. Microglia are specialized macrophages in the nervous system that participate in phagocytosis and act at the first line of defense upon insult to the nervous system. They are found throughout the brain and spinal cord of the CNS and are one of the few mobile cell types in the CNS, which can proliferate readily at the sign of injury (Ransohoff and Brown, 2012). Oligodendrocytes are responsible for myelination of axons facilitating rapid conduction of the action potential from one node of Ranvier to the next (Huang et al., 2014).
3.1.2 Cell types of the BBB
The brain endothelium is vital in maintaining homeostasis of the brain by allowing essential nutrients to enter, balancing ions and pH, and keeping out harmful toxins. A gross description of the unique qualities these endothelial cells possess include presence of TJs between the cells, expression of highly selective, polarized transporter systems, and specialized enzymatic systems. These assets allow for the maintenance of brain homeostasis by defense from peripheral substances, regulation of metabolism, immune response and acting as an interface for endocrine processes (Eigenmann et al., 2013; Hawkins and Davis, 2005; Wong et al., 2013).
TJs are protein complexes found between cells in monolayers which prevent paracellular movement of soluble compounds. Due to the presence of highly organized TJs between brain microvascular endothelial cells (BMECs), these cells have been described as “epithelial-like”, as this is more commonly seen in epithelial tissues. Transmembrane proteins in TJ complexes include claudins (in particular claudin-5), occludins, junctional adhesion molecules (JAMs) and nectin. Intracellular proteins involved in this complex are zona occludens 1 and 2 (ZO1 and ZO2, associated with TJ proteins), afadin, α-catenin (associated with nectin), and β-catenin (associated with claudin-5).
Polarized transporter systems expressed by BMECs function for nutrient delivery, waste and neurotoxin removal, ion balance, and macromolecule movement. Transporters can be qualified as follows: (1) active or passive and (2) efflux or bidirectional transport. Passive transporters allow certain molecules to diffuse down their concentration gradient and active transporters, such as the ATP-binding cassette transporter (ABC transporter), require ATP to transport up a concentration gradient. Bidirectional transporters mobilize their substrates to both sides of the lumen depending on the physiological state whereas efflux transporters move their substrate in one direction. Transporters are polarized between the lumen (apical) and the albuminal side (basal), depending on its function. For example, some systems which are expressed in high concentration in the lumen are glucose transporter-1 (GLUT1) for glucose monocarboxylate transporter-1 (MCT1) for lactate, L-type amino acid transporter (LAT1) for amino acids and L-dopamine (Ohtsuki et al., 2013). Some these are highly expressed on the ablumenal side include and ABC transporter called multidrug resistant protein-1 or permeability-glycoprotein 1 (MDR1 or P-gp, respectively), an efflux transporter associated with high drug resistance of the BBB, and organic anion transporters (OATs). The expression of these transporters is remarkably different among species; readers are referred to Uchida et al. for an example study which compares human to mouse transporter expression using proteomic techniques (Uchida et al., 2011).
Pericytes take the leading edge of blood vessel formation in brain capillaries, making them critical for normal development of the vascular tree and angiogenesis in response to various stimuli (e.g. TBI, hypoxia) (Engelhardt and Liebner, 2014; Hawkins and Davis, 2005). Studies have also indicated that they are critical in regulation of immune response, production of basal lamina and phagocytic activity (Lu and Sood, 2008). Pericytes are of diverse development origin, potentially having the same progenitor as neurons, so they also provide a limited source for neural stem cells; pericytes require cell-cell anchorage to endothelial cells to remain in the differentiated state (Lu and Sood, 2008).
Astrocytes, neurons and microglia are also considered to be active in the function of the BBB. The astrocytes which are the most intimate with the BMECs have been shown through freeze-fracture of tissue slices to have end-feet which encompass about 60% of the capillary length (Wilhelm et al., 2011). These end-feet contain a high density of aquaporin 4 (AQP4), a transporter which regulates ion balance by movement of solvent. By secretion of pro-inflammatory factors, astrocytes also contribute to the regulation of BBB permeability. Astrocytes have been shown in vitro to induce BBB properties in BMECs through secretion of soluble growth factors, for example glial-derived neurotrophic factor (GDNF) (paracrine signaling), through gap junctions, and indirectly through cell-ECM bioactivity (Isobe et al., 1996). Neurons specifically are more important to normal BBB physiology by acting as a functional regulator (e.g. blood flow, permeability) (Abbott, 2002). Microglia, on the other hand, are not directly involved in common BBB functions (Jin et al., 2010; Ransohoff and Brown, 2012). In addition, neurons and microglia induce certain BBB-specific behaviors of the endothelium such as expression of γ-glutamyl transpeptidase, an important enzyme system for aminoacid transport (Tontsch and Bauer, 1991).
3.2 Critical design requirements for neural cell culture
Cells used for in vitro modeling of the CNS have requirements which can be classified as morphological, biochemical, functional and practical. Morphological requirements include basic cell morphology and proper growth patterns and behavior. Biochemical requirements include human-relevant gene expression, and responsiveness to different soluble and insoluble factors. Functional requirements include that proteins produced are functional, the cells exhibit normal behaviors, and the cells work cohesively to provide functions associated with ultrastructure (e.g. nerves and circuits). Details on functional requirements will be pursued in greater detail in Section 5.
In general, cells used in 3D cultures must be able to grow robustly and in high densities. Depending on the cell type, certain morphologies are expected, refer to previous background discussion. The neurons should express specific markers such as NCAM and βIII-tubulin and depending on the specific neuronal subtype, other proteins should be present, for example gamma-aminobutyric acid (GABA) receptor. These markers indicate the proper cell phenotype and implicate that the cells are functional. Depending on the neuron type, they must have a particular resting potential (typically −70 mV) and fire an action potential in response to depolarizing inputs, once the threshold for action potential is reached. In addition, the chosen neurons must be tailored to the application, for example if attempting to recapitulate an inhibitory circuit a neuron must be inhibitory in nature such as a GABAergic neuron (Ohmori, 2014). It is also important to consider neural cell type for certain pathological modeling. For example, in Alzheimer's the first type of neuron to degenerate is acetylcholinergic neurons found in the nucleus basalis of Meynert (Wahlberg et al., 2012).
Astrocytes and other glial cells used for CNS tissue models must be able to grow in a controlled manner without overgrowing the neural cultures in order to maintain the proper cell to cell ratio. They must also play critical supportive roles, both for neural tissue and BBB modeling. This requires the patterned expression of functional AQP-4 for solvent regulation. In pathological states, astrocytes must be able to go into reproducible reactive states and form glial scars. Moreover, oligodendrocytes are necessary for studying myelination events such as white matter tract formation or repair and multiple sclerosis. Successful oligodendrocytes will improve myelination of neurons, which can be monitored by expression of myelin basic protein (MBP). In addition, there should be an improvement of electrical output of the neural cultures if oligodendrocytes are present. Microglia must show active role in brain modeling and immune-related disease modeling. Microglia used in modeling of innate immune response should secrete pro-inflammatory factors and increased vesicle formation (phagocytosis).
Endothelial cells which are used to model the BBB must have the following qualities: TJ formation, polarized transporter expression, and functional transporter expression. Endothelial cells should express TJ proteins such as ZO-1 and claudin, as well as transporters such as MDR1 and GLUT1. Pericytes used in BBB models must play critical supportive roles and secrete soluble factors (vascular endothelial growth factor [VEGF]) as well as insoluble factors (basal lamina) conducive to angiogenesis.
Already 25 years ago it was observed that 2D cultures do not replicate the complex environment of CNS in terms of axonal regeneration (Fawcett et al., 1989) which was later supported by variety of publications (Cullen et al., 2007b; East et al., 2009, 2013). Based on this phenomena, there has been an expansion of tissue modeling research to include the most relevant cell types in the model design. Moreover, 3D co-culture generally employs higher cell density, resulting in intimate contact of various cell types. This better imitates the complex cell-cell interaction as compared to 2D cultures. For example, neural-astrocyte interactions can be employed in tissue engineering for controlling neuronal cell growth. Neural and glial cells are capable of physically binding to each other through cell adhesion molecules such as L1 (L1CAM, CD171) and NCAM (Rutishauser et al., 1988). Astrocytes have also been credited with mediating many homeostatic mechanisms of the brain including vascular regulation, water homeostasis (Simard and Nedergaard, 2004; Thrane et al., 2011), synaptic transmission and ion buffering (Halassa and Haydon, 2010; Haydon, 2001; Iadecola and Nedergaard, 2007; Kuo and Lu, 2011; Perea et al., 2009; Saito et al., 2011). Astrocytes are also robust in cellular culture making them a favorable tissue engineering tool for culturing neurons in vitro as they provide both structural and biochemical support. For example, several studies have reported the increase in neuronal cell survival in the presence of astrocytes or astrocyte-conditioned media (Gaillard et al., 2000). During development, neurons rely on tracts of astrocytes (radial glial fibers) for migration to their destinations for maturation (Lois et al., 1996; Recknor et al., 2004). Frequently, however, even with these co-cultures neurons do not exhibit human-relevant biochemical profiles. Astrocytes are also frequently co-cultured with endothelial cells in the models of neurovascular unit (Al Ahmad et al., 2011; Ferreri et al., 2010). Another co-culture example is focused on the process of axonal myelination utilizing neurons and oligodendrocytes (Gardner et al., 2012). Although frequently studied in 2D (Donoghue et al., 2013; Sorensen et al., 2008), this aspect has been explored in 3D cultures only to a limited extent. There is one particularly elegant example of a 3D model of CNS based on embryonic stem cells, cultured for over 3 months, which showed the presence of mature synapses and myelinated axons, suggesting functional maturation (Dubois-Dauphin et al., 2010). Another model evaluated the influences of gravity, topography, fluid flow, and scaffold dimension on myelinating cultures (Donoghue et al., 2014). As myelination is involved in several disease states such as Alzheimer and multiple sclerosis, modeling these diseases with 3D CNS tissues will require further development of co-culture methods, in particular to including oligodendrocytes.
3.3 Types and sources of neural cells
The major categories of cell sources available are: primary cells, immortalized cell lines and stem cells, Table 3. Primary cells are isolated from the dissected and dissociated tissues. Brain-derived primary cells are usually used at passage 0, directly after the isolation due to their tendency to differentiate and weak proliferation potency. Immortalized cell lines are primary cells which have been genetically transformed so that they can be passaged for prolonged number of divisions in culture. Stem cells, such as embryonic-derived neural stem cells, neural progenitors or iPSCs are cells which have the capacity to become neurons, as well as glial cells (Dubois-Dauphin et al., 2010; Gage, 2000).
Table 3.
Cell Sources for 3D Neural Cultures. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
| System | Tissue Source | Cell Type | References |
|---|---|---|---|
| General Protocols | (Doering, 2010) | ||
| Primary Cells | |||
| CNS | Whole brain (mouse, rat, human) | Astrocytes | (Emery and Dugas, 2013; Foo, 2013; Olah et al., 2012; Schildge et al., 2013) |
| Oligodendrocytes | |||
| Microglia | |||
| Pericytes and BMVECs | (Carson and Haudenschild, 1986) | ||
| Cortex | NPCs | (Schwartz et al., 2003) | |
| BMVECs | (Bernas et al., 2010) | ||
| Hippocampus | Neurons | (Brewer, 1997; Giordano and Costa, 2011) | |
| PNS | Neurons | Spinal motoneurons | (Juurlink, 2001) |
| Explant Culture | |||
| CNS | Brain slices | Neurons, glia, vascular cells | (Murray et al., 1999) |
| Cortex | (Elkin et al., 2007) | ||
| Hippocampus | (Murray et al., 1999; Stoppini et al., 1991) | ||
| Spinal cord | (Krassioukov et al., 2002; Stavridis et al., 2005) | ||
| PNS | Dorsal root ganglia | Sensory neurons | (Friedel et al., 1997) |
| Immortalized Cell Lines | |||
| CNS | Whole brain | Oligodendrocytes | (Bottenstein, 1986) |
| MVECs | (Sano et al., 2007; Stins et al., 1997) | ||
| Human brain tissue | MVECs (hCMEC/D3, hBMEC, TY10, BB19) | (Eigenmann et al., 2013; Poller et al., 2010) | |
| Hippocampus | Neurons | (Gingerich et al., 2010) | |
| Neuroblastoma | NPCs (SH-SY5Y, IMR-32, SMS-KCNR) | (Carmeliet et al., 1994; Jia et al., 2011; Matsuo and Thiele, 1998; Sugimoto et al., 2013) | |
| Hypothalamus | Neurons | (Mayer et al., 2009) | |
| Pheochromocytoma | NPCs (PC12) | (Giordano and Costa, 2011; Krewson et al., 1994) | |
| Human spinal cord | Motoneurons | (Cashman et al., 1992; Zschuntzsch et al., 2013) | |
| Astrocytes (C6) | (Whittemore et al., 1994) | ||
| Mouse embryonic teratoma | NPCs (P19) | (Negraes et al., 2012) | |
| PNS | Peripheral nerves | Schwann cells | (Hai et al., 2002) |
| Stem Cells | |||
| CNS | Mouse embryonic | Neurons Astrocytes | (Kothapalli and Kamm, 2013) |
| Human neuroepithelium | Neural stem cells | (Uchida et al., 2000) | |
| Human bone marrow and dental pulp | (Karaöz et al., 2011) | ||
| Human adipose tissue | (Carelli et al., 2014) | ||
| Fibroblasts (Mouse, Human) | iPSCs | (Takahashi and Yamanaka, 2006; Takahashi et al., 2007) | |
| Adult somatic cells | (Compagnucci et al., 2014) | ||
CNS = central nervous system; PNS = peripheral nervous system; NPCs = neural progenitor cells; BMVECs = brain microvascular endothelial cells; iPSCs = induced pluripotent stem cells
Human nervous system tissues are not usually considered as feasible source of primary cells for in vitro culture due to limited, ethically permissible sources (Cecchelli et al., 2007). On a practical level, unlike other tissues, most adult neural cells are terminally differentiated without regenerative capabilities and therefore cannot be easily expanded in vitro. However, 3D cultures require very high seeding densities in the range of several million cells per cubic centimeter (Miller, 2014), thus we need to look for other solutions to build relevant tissue models. The most common mammalian source of primary cells are rodents, which however exhibit significant differences in the organization and function of their nervous systems as compared to humans such as different ratio of neurons to glial cells (Herculano-Houzel, 2014). However, these cells are readily available and provide valuable information, therefore rat and mouse hippocampal and cortical neural and glial cell isolations are frequently employed for in vitro culture (Devon, 2001; Doering, 2010; Giordano and Costa, 2011). Neurons from these sources are easy to acquire due to low cost and ease of isolation and have provided a wealth of information for basic cell biology (Balgude et al., 2001; Hopkins et al., 2013). However, the primary neurons may experience a loss of function from the isolation process. In addition, the long time required to form neural networks in vitro makes the neurons frequently quiescent before the networks are fully formed, in particular if the cell density is below optimum. Since primary cells reach senescence after a limited number of population doublings, immortalized cell lines are often employed. Neural cell lines are derived either from brain tumors (e.g. neuroblastoma or glioblastoma) or by genetically modifying healthy isolated cells (Anderson and Ferreira, 2004; Carmeliet et al., 1994; Sugimoto et al., 2013). PC-12 cells (a rat pheochromocytoma-derived cell line) and P19 cells (from a mouse embryo-derived teratocarcinoma) differentiated to neural cells have also been widely employed to study neuronal processes and differentiation in vitro (Giordano and Costa, 2011; Krewson et al., 1994; Negraes et al., 2012; Nojehdehian et al., 2010). Cell lines of other neuronal cell types such as astrocytes, for example C6, (Whittemore et al., 1994), are also frequently employed. It is also common to use endothelial cell lines in the development of BBB models, for example hBMEC-3. Cell lines are advantageous in that they can be passaged multiple times and grow robustly in culture. However, they are disadvantageous in that the biochemical characteristics of the cells are altered by the immortalization process or tumor origin.
For this reason researchers have turned to stem cells and iPSCs of human origin to overcome challenges associated with the use of primary and immortalized cell sources. Transitioning from animal to human cell sources is a major challenge for neural tissue engineers; adult stem or stem-like cells may be the most feasible option. Since ethical issues surround the use of human embryonic stem cells, hematopoietic stem cells, and iPSCs raised as an option since they are capable of differentiating to neural phenotypes, Box 2 (Robertson et al., 2008; Selvaraj, 2012).
A culture with pluripotent cell populations provides flexibility for differentiation down several lineages (neuronal, astrocytic, oligodendrocytic) within the same construct for a self-assembled tissue system. In addition, stem cells can be passaged to high numbers and proliferate readily, making them convenient for high density, multiple-replicate cultures. For producing neural cell types, the most promising tissue sources seem to be bone marrow for mesenchymal stem cells and fibroblasts for iPSCs, however many other tissue sources have been used successfully, Table 3. However, these cells are still poorly characterized compared to primary cultures and cell lines: they are expensive and time-consuming to maintain in the undifferentiated state. These cultures are costly from this culture maintenance, and the differentiation process takes several weeks and requires numerous, specific media components. Assuming that differentiation is successful, cultures often contain impurities of other, less stable adult somatic cells, thus requiring thorough characterization. Moreover, differentiation potential differs between the clones and between the donors, perpetuating the variability in the results. Therefore, specific protocols for cell maintenance and differentiation need to be followed through the lengthy process.
Conversely, in favor their use, stem cells can be derived from the disease affected patients such as Alzheimer or Parkinson, which allows for modeling of disease-specific neuronal tissues. It has been recently shown that iPSC-based cerebral organoid model can recapitulate the distinct characteristics of microcephaly, a disorder that has been previously challenging to obtain with in vivo rodent models (Lancaster et al., 2013). Moreover, iPSC-derived cells were used to develop fully human BBB model comprising of hPSC-derived BMECs, brain pericyte, astrocytes and neurons derived from human neural progenitor cells (NPCs) (Lippmann et al., 2014). Increasing number of publications including iPSCs indicates that the stem cells have the potential to become the major cell source utilized in the future.
Neural stem cells (NSCs) represent yet another cell source for CNS engineering. NSCs can be derived from embryos, fetal tissues or adults, however, the first two sources raise ethical concerns, reviewed in (Jakel et al., 2004). Adult NSCs are found in neurogenic regions of the adult brain, the hippocampus and subventricular zone (SVZ), as well as the spinal cord. They are able to differentiate into the major cell types (neurons, oligodendrocytes, astrocytes), thus NSCs are of interest for 3D CNS engineering. Although studied mainly in 2D setting, their differentiation potential directly in 3D culture has been also evaluated in biomaterial scaffolds such as methacrylamide chitosan (Li et al., 2012), type I collagen (Watanabe et al., 2007), graphene foam (Li et al., 2013) further confirming that the composition and the structure of 3D scaffolds seem to play a significant functional role.
4. Tissue engineering the 3D architecture
4.1 The anatomy of brain tissue
When anatomically describing the brain it is typically said that there are five main brain regions, and within these regions there are high-operative structures which are further categorized based on function (Giedd, 2004). Several of these brain structures have implications in neurological disease that may benefit from modeling in vitro. For example, Alzheimer's disease affects the memory forming and recall capabilities of the hippocampus and cortex (Anderson and Ferreira, 2004; Arnold et al., 1991; DeKosky and Scheff, 1990; Laakso et al., 1995; de Leon et al., 1989); Parkinson's and Huntington's diseases selectively destroy high-level motor and learning neurons of basal ganglia particularly the substantia nigra and the striatum, respectively (Bø et al., 2003; Dragunow et al., 1995); multiple sclerosis attacks myelinated axon tracts both in the brain and spinal cord (Asahi et al., 2001; Goebels, 2007); and cerebrovascular regulation by the neurovascular unit (i.e. the BBB functionally coupled with neurons) is affected by nearly all CNS conditions (Asahi et al., 2001; Hawkins and Davis, 2005; Haydon and Carmignoto, 2006; Ujiie et al., 2003). Although all of these structures are incredibly complex and unique, there are shared architectural features found throughout the brain. These include aligned neural tracts (e.g. lamination of the cortex, white matter tracts, optic radiations, and hippocampal circuits), dense clusters of neuronal cell bodies (e.g. basal ganglia, basal forebrain), and blood vessels regulated by surrounding neurons and astroglial endfeet (i.e. the neurovascular unit).
4.2 Critical design requirements for engineering in 3D
The lack of appropriate fabrication methods and across-scale knowledge makes it impossible to faithfully design and construct whole brain regions in vitro. Therefore, current goal of tissue engineering is to develop functional CNS tissue units on the micro- and millimeter scale which maintain common architectural features found in all the brain regions such as neuro-vascular unit or neuronal networks. This process is guided by the basic requirements and assumptions of tissue engineering which include faithful reconstruction of architectural features of the native tissue, utilization of appropriate fabrication techniques and reproducibility of the system.
Moreover, the architectural requirements of models, are also important from the point of analysis of cell morphology, biochemistry and function. Therefore, the models mimicking spinal cord are frequently utilizing arrays of aligned fibers (Chow et al., 2007; Weightman et al., 2014) or gels (East et al., 2010; Phillips, 2014) to achieve directional growth of axons and nerve regeneration. Architectural requirements include high cell density and cell compartmentalization enabling development of vascularized features and neuronal networks. It has been suggested that neural stem cells favor higher cell concentration during growth and differentiation in 3D cultures (Watanabe et al., 2007). However, high cell density in 3D cultures carries a high risk of cell necrosis due to the limited diffusion of oxygen, nutrients and wastes, and has been observed in organoid cultures of CNS (Lancaster et al., 2013). Therefore, means increasing the transport rate of metabolic products and byproducts to and from the 3D culture need to be developed in order to diminish the cell death. As mentioned in Section 2.3, when constructs are critically sized passive diffusion of nutrients and oxygen is not sufficient to meet metabolic demands. Therefore, to prevent the formation of a necrotic core in critically-sized constructs, structural elements such as channels (Chrobak et al., 2006; Wray et al., 2012), or solutions such as bioreactors (Bellas et al., 2013; Lancaster et al., 2013) must be implemented. Several existing 3D CNS models included the dynamic aspect of fluid flow such as spinner flask culture (Hogberg et al., 2013; Lancaster et al., 2013) or microfluidic perfusion (Cucullo et al., 2013; Griep et al., 2013), however majority of them is still based on the static culture due to the high cost and tedious work connected with bioreactor cultures.
Requirements of fabrication techniques include: the technique does not harm cellular constituents, the process is highly repeatable, and the technique does not alter the bioactivity of the hydrogel. There are many potential modes of cell damage from polymerization reactions. For example, unmodified chitosan requires lowering the pH to cell-damaging levels in order to polymerize, therefore, encapsulation in chitosan requires modification of the polymer (Wang et al., 2010). In order to appropriately recapitulate what is seen in vivo neurons should grow in alignment in fasciculated bundles, preferably guided along radial glial-like tracts. This is also important for extracellular recordings, which will be discussed in Section 5. It is also important that any fabrication process does not negatively affect the bioactivity of a macromolecule. For example, an approach to functionalizing synthetic polymers is to attach peptide sequences to increase the number of cell binding sites. It is important that the covalent bonding technique does not result in the peptide losing its bioactivity.
Practical requirements include that the process is repeatable and manageable in cost. The process must be highly repeatable in order to draw significant conclusions. The 3D system must also be sustainable for long term culture; there cannot be ultrastructure collapse. Overall the greatest challenge with designing the structure is to match complexity with practicality; complex fabrication techniques often involve cytotoxic components and expensive materials.
4.3 Current strategies for assembly of 3D structures
In spite of significant evidence behind the value of 3D cultures (Kofron et al., 2009), there are currently few 3D architectures inspired by in vivo structures for CNS tissue modeling. However, techniques from other fields can be borrowed for assembling complex 3D neural tissue systems, Table 4. For example, microfabrication of physical or chemically printed 2D patterned substrates are often employed for guidance of neurite extension (Delivopoulos et al., 2009; James et al., 2000; Sørensen et al., 2007). This technique can be translated to 3D structures of aligned neural tracts by bonding the patterned material to glass forming hollow channels that can be functionalized for cell growth (Shin et al., 2004), or by stacking several layers of patterned cell-laden gels made from a positive mold (Tan and Desai, 2005). Some notable approaches to aligning neural tracts which have already been demonstrated in 3D are the use of electrospun fibers, a technique also shown to be highly effective at aligning neural growth in 2D culture (Puschmann et al., 2014) and the use of mechanical gradients (Sundararaghavan et al., 2009). We have also observed self-assembled dense clusters of neurons separated from the neuronal tracts by the scaffold structure mimicking white/gray matter of the cortex (Tang-Schomer et al., 2014). For top-down approaches, 3D rapid prototyping techniques have been applied in which porous polymer scaffolds are machined to exhibit defined macroscopic geometries (Lin et al., 2004; Sherwood et al., 2002). Our group has recently characterized a porous silk-based scaffold platform for such top-down tissue engineering of the neurovascular unit, Box 3. Another powerful approach is to encapsulate cells in a soft matrix and allow them to spontaneously form structures as demonstrated by the examples of iPSC-derived cerebral organoids (Lancaster et al., 2013) or self-assembled BBB (Al Ahmad et al., 2011; Urich et al., 2013). This approach is especially useful for stem cell-based models as it explores the natural property of cells to self-assemble during the long-term differentiation process allowing for development of unique niches and structures (Dubois-Dauphin et al., 2010; Hogberg et al., 2013; Lancaster et al., 2013). Each of these approaches has advantages and disadvantages, refer to Table 4.
Table 4.
Approaches for assembly of heterogeneous 3D tissue structures. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
| Fabrication Techniques | Biomaterials | Cell Sources | Bioreactors | |
|---|---|---|---|---|
| Bottom-up | • Cell aggregates • Microfabrication • Cell sheets • 3D printing |
• 2D patterned substrates (e.g. thin films, hydrogels) | • Low passage adult somatic cells • Stem cells • iPSCs |
• Perfusion • Spinning / rotating • Stretching • Dynamic compression • Tension / Torsion • Pulsatile flow • Electrical stimulation |
| Top-down | • Machined scaffold • Compartmental cell seeding • Molding |
• Solid degradable polymers (e.g. sponges, hydrogels) | • Primary cells • Adult somatic cells |
|
Tissue engineering of 3D constructs often requires control of environmental factors by bioreactors. Simple bioreactor designs include spinner flasks or perfusion through culture chambers, refer to Table 4. There are also many advanced designs of bioreactors capable of delivering physiological stimuli experienced by different tissue types such as sheer stress or stretching (Freshney et al., 2007). In general, bioreactors aim to regulate many facets of the cellular environment such as directing cell-seeding into scaffold compartments, improving mass transfer of nutrients/waste, insuring adequate gas exchange to the bulk culture, replenishing media, controlling temperature/pH, and delivering physiological stimuli (Freshney et al., 2007). Neural cells do not readily proliferate and are not subjected to flow stresses under normal conditions, which may contribute to why there are few bioreactors designed for long-term culture of 3D neural tissues. However, thick cultures (≥500 μm) of neurons and glia in hydrogels experience poor viability at high densities (≥6,275 cells/μL) (Iii et al., 2011). The simplest bioreactors employed for neural tissues consist of a silicone or porous membrane that is rocked gently within a media bath (Morrison et al., 2000, 2006; Stoppini et al., 1991) or a spinner-flask (Lancaster et al., 2013). More recent bioreactor designs for neural cultures include custom-built bioreactors to support higher cell density 3D cultures (Braitenberg, 2001; Gabbott and Stewart, 1987; Vukasinovic et al., 2009) as well as those equipped with mechanical functions and sensors for testing TBI (Iii et al., 2011; LaPlaca et al., 2005; Morrison et al., 1998, 2011, 2000, 2006).
Other specialized bioreactor systems frequently employed for neural tissue systems are employed for BBB studies. Traditional culture platforms for constructing tight microvascular endothelial cell layers for BBB modeling consist of a porous membrane (e.g. Transwell, Corning Inc.) suspended in a well representing the BBB lumen (Transwell insert) and ab-luminal (lower well) compartments of the blood vessel (Cecchelli et al., 1999; Dehouck et al., 1990; Di et al., 2003; Hatherell et al., 2011; Megard et al., 2002). Perfusion systems employed for dynamic BBB models have also been constructed with bundles of hollow fibers where endothelial cells can be cultured within the lumen and support cells can be seeded ablumen with sample ports for accessing permeability of the flow-through (Cucullo et al., 2002, 2011). For more extensive reviews on BBB models, readers are referred to (Grant et al., 1998; Naik and Cucullo, 2012; Wong et al., 2013).
5. Validating function
In order to be useful for gaining new knowledge about human systems, in vitro 3D models should provide a physiological response to treatments and stimuli. With the exception of in vitro BBB models, characterization of 3D in vitro neural systems was characterized through viability and morphology studies, as well as electrophysiology. Up to date most of the 3D tissue engineering research is using microscopy as a major mode of evaluation. Although significant information can be obtained through visual analysis of neuronal outgrowth, it is clear that understanding cell-cell interactions, complex mechanisms, and specific electrical activity of neural systems is critical to developing effective therapeutics for CNS conditions. Additionally, the existing 3D models are based on low cell density resulting in poor axon alignment, or axon fasciculation which affects their physiological functionality. To address these needs, there have been few attempts to increase the cell density by using the spheroid and organoid approaches (Kato-Negishi et al., 2013; Lancaster et al., 2013). Both approaches showed spontaneous neuronal activity as demonstrated with calcium imaging. However, there is an urgent need for new methods to validate the function of 3D models and provide convincing translational evidence.
Due to the hurdles connected to evaluation of 3D in vitro cultures this section will describe the principles of these assays in their 2D configuration and then highlight the challenges of translation to 3D. In addition, the few currently existing technologies to evaluate function of 3D cultures will be highlighted and described. Table 5 outlines assays available for 3D in vitro analysis including viability and morphology analysis (not discussed here) and functional testing (discussed below).
Table 5.
Major methods used to assess in vitro neurological tissues. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
| Technique | Description | 2D or 3D | Functional Assay? | Challenges in 3D cultures | Reference |
|---|---|---|---|---|---|
| Live/Dead fluorescent assays | Indicates intracellular esterase activity (live cells) vs. loss of plasma membrane integrity | Both | No | Diffusion through 3D constructs; Optical clarity of biomaterial substrates; Loss of viability | (O'Connor et al., 2001) |
| Immuno-histological / cytological fluorescent assays | Phenotypic characterization of cultures by targeting antigens of interest with fluorescent-labeled antibodies | Both | Indirect; Cells will synthesize certain proteins or sugars if they are serving a particular function | Diffusion through 3D constructs; Non-specific fluorescence; Optical clarity of biomaterial substrates; Loss of viability | (Krassioukov et al., 2002; Stavridis et al., 2005; Whittemore et al., 1994) |
| Flow cytometry | Phenotypic characterization of cultures by targeting antigens of interest with fluorescent-labeled antibodies; Allows for cell counting, sorting and purification | Both | Indirect; Cells will synthesize certain proteins or sugars if they are serving a particular function | Destruction of 3D architecture; Interference of biomaterials with cell counting / sorting | (Emery and Dugas, 2013; Foo, 2013) |
| Molecular techniques (e.g. qPCR, ELISA, proteomics) | Phenotypic or genotypic characterization based on detection of genomic DNA, mRNA, or protein | Both | Indirect; Cells will synthesize certain proteins or sugars if they are serving a particular function | Destruction of 3D architecture; Interference of biomaterials with assay; Low cell density does not yield sufficient titers of protein or genetic material | (Maximino et al., 2014; Noda et al., 2014; Wesseling et al., 2013) |
| Patch-clamping | Single cell recordings of membrane potential in real time | 2D | Yes | Micro-electrodes too delicate to penetrate biomaterials; Poor optical clarity of biomaterials hinders identification of single cells during patching; Electrical insulation properties of biomaterials may affect recordings; Lack of knowledge of signal processing of 3D in vitro | (Kolaj et al., 2014; Neher and Sakmann, 1992; Ward, 1997) |
| Fluorescent calcium indicators | Fluorescent markers that bind calcium and that bind calcium and allow for measurement of calcium flux indicating neurotransmitter release and other intracellular processes | Both | Yes | Diffusion through 3D constructs; Non-specific fluorescence; Optical clarity of biomaterial substrates; Loss of viability; Highspeed capture camera required in case of action potential propagation | (Catterall et al., 2013; Frank, 2014; Kim and Jun, 2013) |
| Permeability studies | Continuous flow through cultures to measure permeability of epithelial/endothelial barrier | Both | Yes | Bioreactor required to ensure equal flow through entire construct; Reproducibility of biomaterial scaffold to eliminate variability among samples | (Cucullo et al., 2013; Deli et al., 2005; Sun and Pang, 2008) |
| TEER | Measurement of electrical resistance of epithelial/endothelial barrier | Both | Yes | Placement of electrodes on either side of cell layer requires custom culture chamber design which depends on 3D architecture; Reproducibility of biomaterial scaffold critical to eliminate variability among samples | (Cucullo et al., 2013) |
| Multi-electrode arrays | Extracellular recordings; Small (~1 μm) arrays implanted in brain tissue to individually record single neurons present in a particular locale; May be flexible and conform to complex surfaces | 3D | Yes | Significant spike sorting required; Reproducibility difficult; Limited to recordings of groups of single cells = may be missing significance of whole network | (Claverol-Tinture et al., 2005; Izhikevich, 2004) |
| Flexible electrodes | Extracellular recordings; Biomaterial reinforcements of thin electrodes for penetration of dense tissue and prevention of glial scar formation | 3D | Yes | Significant spike sorting required; Reproducibility difficult; Must take into account biomaterial degradation effects on signal processing | (Aregueta-Robles et al., 2014; Kim et al., 2010) |
qPCR = Quantitative polymerase chain reaction; ELISA = enzyme-linked immunosorbent assay; BBB = blood-brain barrier; TEER = trans-endothelial electrical resistance
5.1 Assays and their principles
5.1.1 Evaluation of neuronal function
Functionality of neural cells is most reliably detected through electrophysiological measurements. Although neurons are often considered the only electrically active cells in the nervous system, transmission of ionic current through other cell types, for example astrocytes has been shown to directly contribute to neuronal action potential transmission via ‘gliotransmission’ of compounds at the synaptic cleft (Perea et al., 2009). In neurons, action potential generation requires that the membrane potential depolarizes from −70 mV to −55 mV. Both membrane potential and action potential propagation are generated by ion flux through voltage-gated ion channels (Kolaj et al., 2014). Due to the role of ionic current in the generation and transmission of action potentials, neuronal firing can be assessed both indirectly through monitoring of ion flux and directly through measurement of electrical conductance. This gave rise to three basic methods: patch clamping (ion-based measurements) (Ward, 1997), calcium imaging (ion-based measurements) (Kim and Jun, 2013) and extracellular electrical recordings (electrical measurements) (Churchland et al., 2007).
The patch clamp technique, pioneered by Nobel Prize-winners Erwin Neher and Bert Sakmann, is the evaluation of the electrical potential of a single or a small group of membrane ion channels on a single cell (Neher and Sakmann, 1992). The electrode used in this technique consists of a 1 μm-diameter, glass micropipette “patching” a piece of the cell surface. By using microscopy as a guide to touch the cell membrane, negative pressure is applied resulting in a high resistance (GΩ) seal, referred to as a cell-attachment patch. Other variations include inside-out patch, whole cell clamp, and the outside-out patch (Neher and Sakmann, 1992; Ward, 1997). Independent of the set-up, the intracellular membrane is exposed to various environments as controlled by fluid delivered by the micropipette or the external bathing solution. Therefore, the effects of ion flux under various test conditions while in voltage-clamp mode, for example at what threshold voltage a particular ion channel will open, can be elucidated. Patch clamp technique has been successfully used to demonstrate neuronal activity in a 3D CNS models showing that values measured in 3D are similar to 2D (Coates and Nathan, 1987; Irons et al., 2008; Xu et al., 2009). Moreover, it allowed to determine whether neurons growing in 3D developed functional synapses by detecting postsynaptic current, thus proving that the method is suitable method of neuronal evaluation for tissue engineering approach. However, utilization of this method poses certain requirements on the side of the biomaterial, which needs to be relatively transparent to allow visual cell allocation under the microscope. Moreover, it has to be relatively soft to enable pipette penetration toward the cell body, but at the same time do not cause the pipette occlusion. From the perspective of cell culture, the method is relatively time consuming and does not allow for high throughput measurements. It could benefit from high cell density cultures which increase the chance of patching a cell, however, on the other hand measurements performed by this method can be performed mostly close to the surface of the 3D construct as deeper levels lack the optical clarity, especially in dense cultures or cell spheroids. Due to the variety of drawbacks connected to patch clamping calcium imaging became the method of choice for characterization of electrically (and non-electrically) active cells in 3D. Intracellular calcium flux is responsible for many cell physiological processes including slow cell-cell signaling, vesicular neurotransmitter packaging, transport, and release at the neuronal synapse (Catterall et al., 2013; Frank, 2014). Fluorescent calcium indicators such as Fura and Fluo series (as well as several others) and genetically encoded calcium indicators (GECI) allow for quantification of intracellular calcium over time through image-sequence or video capture during fluorescence microscopy (Grienberger and Konnerth, 2012). Calcium imaging requires optical clarity of biomaterial cultures and a high-speed capture camera, in the case of action potential propagation. Calcium sensitive dyes must be loaded at the beginning of each imaging session, making chronic neural activity recordings problematic especially in 3D, where the diffusion of a dye is affected by the density of the biomaterial. By contrast, GECI can be cell-type specific and exhibit persistent expression, enabling monitoring of the same neural population over weeks. Due to its robust character calcium imaging dyes are widely used in CNS engineering allowing to record the activity of whole neuronal populations and networks (Kato-Negishi et al., 2013; Lancaster et al., 2013). On the contrary, GECI, as a very recent method, have not yet been used in a tissue engineered setting. However, due to the several advantages which they offer over the dyes, GECI seem to be a method of choice in the future.
Another method for characterizing electrical activity of a neuronal culture is a direct measurement of electrical current through extracellular electrical recordings (Csicsvari, 1998). For characterizing neuronal networks in healthy brain tissue, signal processing and spike sorting is necessary and requires computational modeling due to the extensive variety of spikes encountered (Izhikevich, 2004). Multi-electrode arrays (MEAs) of ~1 μm in diameter inserted into the brain may detect that activity of at most one surrounding neuron (Claverol-Tinturé et al., 2005). Extracellular recording were successfully utilized by our group in the CNS 3D model (Tang-Schomer et al., 2014) as well as by others, (Dubois-Dauphin et al., 2010; Frampton et al., 2011). Although these recordings enable to record the activity neurons in response to stimuli, the signals are very low and requires exact coordinate matching among samples to reproduce measurements.
5.1.2 Evaluation of endothelial cell function
Transepithelial electrical resistance (TEER) is a gold standard method utilized to measure the tightness of BBB-mimicking models. It is based on measurement of the electrical resistance of an endothelial cell monolayer which is measured by generating an electrical field across the cell monolayer. As the monolayer forms and TJs develop, it becomes increasingly difficult for ionic charge carriers to migrate through the biological membrane. Additionally, in the case of matured monolayers, there are transporter systems which work to maintain a particular ion balance, which can result in a resting current which is oppositional to the applied current. TEER measurement was incorporated in various models of neurovascular unit (Griep et al., 2013; Lippmann et al., 2014) including one 3D model (Cucullo et al., 2013). As the measurement of TEER in 3D models is a highly sophisticated and requires advanced engineering skills to design electrode system compatible with the given 3D model of neurovascular unit it is not frequently used in 3D tissue engineering setup. Finally, TEER is limited to the measurement of paracellular tightness. Therefore, to measure the activity of transporters, permeability tests must be conducted alongside.
Apparent permeability (Papp) is observed by sampling how much a compound moves from the lumen compartment (known input) to the brain compartment (observed output) over a defined period of time, and normalize this to the area which separates the lumen compartment from the brain compartment, Eqn 1 (Sun and Pang, 2008). CR refers to the concentration of the drug imparted into the receiving compartment, Vs refers to the sampling volume, CD,0 refers to the concentration into the donor compartment at time zero, t refers to the time when sampling occurs, and A refers to the area of the membrane.
| Eq. 1 |
There are three basic routes by which a compound can enter the brain (1) paracellular, (2) active transport, (3) passive transport. Though not impossible, diffusion through the cell membrane is rarely considered as a possible route due to the low pinocytic activity of the endothelial cells of the BBB (Deli et al., 2005). The qualities that determine which pathway the drug takes includes but is not limited to size, charge, polarity and solubility. To measure the activity of a particular transporter, one must use its substrate (or an analogue), usually fluorescently-labelled to enable trace it by the means of microscopy. The most frequently utilized molecules include sucrose, BSA, dextran (Cucullo et al., 2013; Price and Tien, 2011; Winfried Neuhaus, 2006).
5.2 Critical design requirements for development of technology for validation of 3D in vitro tissue-model function
There are many difficult requirements to fulfill when attempting to measure electrophysiology of 3D in vitro tissue cultures, refer to Table 6. For patch-clamping this includes optical clarity of hydrogels, a reliable method to fix the substrate in place, a robotic system with high control in x, y and z directions, and a heated stage to maintain culture viability during prolonged testing periods. Calcium imaging requires all of these factors as well, except for the robotic system. Instead a high-speed capture microscope is needed to observe action potential propagation. As some performance benchmarks, the resting membrane potential of most neurons should be around −70 mV and action potentials should occur around −55 mV (Kolaj et al., 2014).
Table 6.
Functional validation of 3D in vitro neural systems and associated challenges. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
| Physiological Process | Analysis Method | Challenges | 3D in vitro Approaches |
|---|---|---|---|
| BBB integrity | Compound permeability | • Robust perfusion cultures • Reproducible measurements |
• Perfusion bioreactor consisting of bundles of hollow channels, (Cecchelli et al., 2007; Cucullo et al., 2002, 2013) |
| TEER | • Placement of electrodes within vessels • Reproducible electrode placement & readings |
• Custom-built chambers, electrodes, and power/recording systems, (Cucullo et al., 2002, 2013; McAllister et al., 2001; Santaguida et al., 2006) | |
| Neural electrical activity | Patch-clamping | • Penetration of glass micropipette through scaffold • Visualization through scaffold |
• Increase strength of small electrodes (Tien et al., 2013) • Optically transparent biomaterials (Balint et al., 2014) • Slice preparations (Hanson et al., 2010; Kapfhammer, 2010; Stavridis et al., 2005) |
| Extracellular recordings | • Culturing high neural cell densities • Construction of polarized neural populations |
• Optimization of 3D in vitro culture systems in regards to cell density and 3D architecture (Cullen et al., 2006; East et al., 2010; Miller, 2014) | |
| Neuronal and glial signaling | Calcium imaging, VSDs | • Visualization through 3D biomaterials • Identification of different cell types • Diffusion of dyes through 3D tissues • Signal-to-noise ratios |
• Optically transparent biomaterials (Dana et al., 2014) • Ratiometric dyes (Homma et al., 2009) • Slice preparations (Dana et al., 2014) |
BBB = blood-brain barrier; TEER = transendothelial electrical resistance; VSD = voltage-sensitive dye
The main requirement for permeability testing, both for TEER and Papp measurements, is that there are two compartments where the only semi-permeable surface is the cell monolayer. This usually translates as design requirements for the hydrogel and tissue assembly, however, it also requires that the sampling ports do not disturb the monolayer. TEER also requires that the electric field remains stable and constant, and that the device is tuned to the particular electrical field geometry. For TEER, a model using primary animal endothelial cell lines usually requires a minimum of 200 Ωcm2 to be considered suitable for permeability testing, this value being associated with healthy, confluent monolayers. For cells to be considered as TJ forming values should be ≥ 500-1000 Ωcm2. Models using human cell lines have shown to be only partially restrictive with values ranging from 20-200 Ωcm2 (Eigenmann et al., 2013; Hatherell et al., 2011; Wong et al., 2013). Primary human BMECs normally have values closer to that of primary animal cells, with values >150 Ωcm2 and can also exceed 1000 Ωcm2 (Wong et al., 2013). Values for apparent permeability testing depends on the tracer. Some common tracers include sucrose (apparent permeability <10−7cm s−1), sodium-fluorescein, rhodamine-123 and different molecular weight dextran (Wong et al., 2013).
5.3 Current techniques for evaluating neural cell culture
There are many challenges of patch clamping or field potential recordings in vitro including presence of exogenous biomaterial hydrogels and scaffolds and the inevitable lower cell density than present in vivo. Even in vivo patch-clamping often results in “blind patching”, the patching of unknown neurons, or requires the use the use of confocal microscopy, which also has disadvantages (Dana et al., 2014; Homma et al., 2009). Efforts have been made toward increasing the usability and success of 3D patch clamping through automated processes (Yang et al., 2013). Recently, robots for patch clamping in vivo have been developed allowing for automated blind patching of neurons (Kodandaramaiah et al., 2012). Although a first step, this robot has yet to become commercially available. Additionally, efforts to patch clamp neural cells have not been successful: embedded in a collagen matrix (mechanical damage to electrode and hydrogel), on the surface of brain slices (Nashmi et al., 2002) and cell-laden collagen hydrogels (Coates and Nathan, 1987; Coates et al., 1992; Krewson et al., 1994). It is also difficult to immobilize soft 3D constructs such as hydrogels for simultaneous perfusion of test compounds and recording of response (O'Shaughnessy et al., 2003). These challenges have limited recording of tissue-engineered constructs mainly to extracellular recordings.
Extracellular electrical recordings measure groups of neurons as opposed to single neurons. For example, excitatory post-synaptic potentials (EPSPs) are well established within neural tracts in the hippocampus where the structure and directionality of signal transmission is well defined (Andersen et al., 1969). Electroencephalography (EEG) is also regularly employed to measure brain waveforms during various levels of consciousness, for example to understand memory consolidation during sleep (Moroni et al., 2012) and to test novel anesthetics for anesthesia awareness (Hayashi et al., 2014; Khan et al., 2014; Schneider et al., 2014). Therefore, to employ meaningful extracellular stimulation and recordings in vitro, culturing techniques, technology and data processing techniques must all be advanced and standardized. In order to produce appreciable signals, neural cultures must be robust, high density and have a polarized structure (isotropic signals may cancel each other out). In order to interpret data, electrodes and devices that are sensitive in this lower amplitude range must be developed. Additionally, cultures must be well-characterized enough to understand the biological mechanism behind waveforms. For example frequency of action potential firing, calcium activity, and neuron geometry (Buzsáki et al., 2012). For these reasons, this type of recording could not be biologically conclusive on its own, and would need input from biochemical assays, a disadvantage when compared with patch-clamping.
Indirect analysis of membrane voltage changes and action potential firing can be performed through fluorescence imaging of Ca2+ indicators or voltage-sensitive dyes (VSDs). Fluorescence Ca2+ indicators and VSDs do not require physical placement of electrodes and provide opportunity to record signals ranging from intracellular structures to entire populations of cells simultaneously. However, optical techniques require visualization and image capture of cells, which is challenging in biomaterial scaffolds with poor optical transparency, especially in thicker constructs. Poor magnitude and repeatability of diffusion of the dyes through connective tissue (or biomaterial) to cell membrane targets results in poor signal-to-noise ratio and presents a significant challenge. It may also be difficult to discern neuronal signals from those of other cell types in mixed cultures since Ca2+ signaling is responsible for a myriad of cellular physiological processes. For example in astrocytes, Ca2+ flux has been shown to account for gliotransmission (Martineau, 2013) as well as long-distance communication in astrocyte syncytium (Volterra and Meldolesi, 2005). Pre-staining cell types with viable cell tracer dyes may allow for identification of neurons in mixed cultures. Overall such optical imaging techniques offer an alternative to electrophysiology for capturing electrical events in vitro (Tang-Schomer et al., 2014).
To our knowledge, there is only one system which exists that has successfully measured TEER in 3D, this is from the work of 3D BBB modeling utilizing parallel bundles of hollow conduits previously described by Cucullo and co-workers (Cucullo et al., 2013). The lack of available systems is due to the fact that it is difficult to build-in electrodes without disturbing the culture, to acquire stable readings (difficult to form full monolayers in 3D, more variability in ion-path through polymers), and to reliably place the electrodes in the same 3D coordinates (locamotor, mechanical damage to electrode or monolayer). There are, however, many existing systems for measuring permeability of tracers in 3D. These systems typically have two configurations: one where there is an inlet and outlet through a unidirectional pump system. The other where there is a sampling port to the abluminal compartment, allowing for direct measurement of the concentration difference between the two compartments (Naik and Cucullo, 2012). These systems are well-characterized, and simple to employ in perfused cultures.
6. Conclusions
Throughout history scientists and philosophers have questioned the source of human intelligence and consciousness. Over time the field of neuroscience evolved from philosophical debate and gross anatomy to connecting anatomical structures to behavior, and the isolation of single neurons to discern their electrophysiological properties. Today, researchers are interested in how the function of a network of individual neurons adds up to the complexities of brain function, and how this knowledge can be applied to medicine. Neural tissue engineering approaches to nervous system injury, disease, diagnostics, and in vitro modeling offers great promise for therapeutic innovation. The development of neural systems for recapitulation of human neural physiology in vitro can be accomplished through careful engineering of the extracellular space, cellular components, and greater hierarchical 3D structure. Current state of the art 3D neural systems employ hydrogel biomaterials for cell encapsulation and in vitro culture. These first generation tissue models have revealed many critical challenges associated with 3D neural tissue culture including the maintenance of high cell density cultures within soft materials, organization of 3D heterogeneous structures, and functional assessment of in vitro neural tissues. The next generation of in vitro neural model systems should strive to address these challenges, refer to Table 7.
Table 7.
Critical requirements which have not yet been met by current in vitro tissue models. Amy Hopkins, Elise DeSimone, Karolina Chwalek and David Kaplan, Progress in Neurobiology.
| Biomaterials | ||
| Requirement | Justification | Remaining Challenges |
| Development of “smart” biomaterials which maintain previous biochemical characteristics (e.g. bioactivity, optical clarity, electrical conductance, etc.) without introducing cytotoxicity | To make the platform more compatible with functional assays such as stimulation with light (photo-catalyzed release of compounds, live-culture imaging) and electricity (electrical stimulation of neurons) while maintaining viability | Introducing favorable biochemical characteristics in “smart” polymers that frequently exhibit cytotoxic fabrication techniques; or, Optimizing physical characteristics of existing biopolymers for functional testing |
| Cellular Sources | ||
| Requirement | Justification | Remaining Challenges |
| Use of stem cells and iPSCs | The expandability of stem or stem-like cells combined with high control over genotype and phenotype is required to reach high cell densities, achieve appropriate number of replicates and imitate human phenotype | Verified protocols for expansion, differentiation, and maintaining differentiated state in long-term cultures; Optimized growth and differentiation in 3D |
| Designing 3D Space | ||
| Requirement | Justification | Remaining Challenges |
| High spatial control combined with gentle processing methods | Neural processes such as synaptic targeting are high resolution and high sensitivity | Optimizing seeding protocols to be compatible with fragile cells and proteins |
| Functional Evaluation | ||
| Requirement | Justification | Remaining Challenges |
| Development of methods of functional evaluation which are compatible with 3D in vitro systems including electrophysiological and imaging | Relevant functional outputs are necessary for validation of tissue model | Resolving and interpreting extracellular recordings to the low density, 3D cultures; High spatial control for probing electrodes; Ability to measure through thick tissue constructs (e.g. penetration of electrodes) |
iPSCs = induced pluripotent stem cells
Box 1. Versatility of Bombyx mori silk fibroin protein as a biomaterial.
Raw silk fibroin protein is extracted from the cocoons of the Bombyx mori silkworm through aqueous-based processing yielding large volumes of protein solution per gram of starting silk fibroin (Vepari and Kaplan, 2007). Over 400,000 tons of dry silkworm cocoons are available per year at low cost for the textile industry, which can be used for biomaterial development. Silk proteins are large polymers comprised of repeating sequences of large hydrophobic domains interspaced with smaller hydrophilic domains (Vepari and Kaplan, 2007). This structure promotes β-sheet formation of tightly stacked anti-parallel sheets supported by hydrogen bonding allowing for stabilization into a variety of structures including fibers, films, gels, sponges, and electrospun mats without the use of chemical cross-linkers or organic solvents (Rockwood et al., 2011; Vepari and Kaplan, 2007).
Silk is a favorable candidate for biomaterial platforms due to its biocompatibility, aqueous-based processing, and robust mechanical and degradation properties. Although silk is inert on its own, it can be functionalized through chemical modification, or physical adsorption or encapsulation. For example, silk materials have been chemically modified with RGD to promote cell adhesion, designed to slowly release encapsulated drugs and morphogens over time and decorated with inorganic compounds such as hydroxyapetite (Furuzono et al., 2004; Kardestuncer et al., 2006; Li et al., 2006; Pritchard and Kaplan, 2011).
We recently examined silk hydrogels as soft biomaterials for neural tissue engineering applications (Hopkins et al., 2013). We found that silk hydrogels exhibit a range of low stiffness comparable to neural tissues and other hydrogel platforms such as collagen, fibrin, and agarose, and maintains its structural integrity significantly longer than these gels; <50% by mass degraded within 25 days (Hopkins et al., 2013). Differential DRG explant neurite outgrowth was observed on silk gels of different stiffness, growth factor content, and ECM molecular functionalization illustrating control of environmental conditions for in vitro study of neuronal growth mechanisms (Hopkins et al., 2013).
Several other silk-based biomaterial platforms have proven useful for neural system applications. For example, bioactive silk nerve guide conduits have been employed for peripheral nerve repair (Lin et al., 2011). Silk films loaded with chondroitinase ABC show promise for reduced gliotic scarring at the neuron-implant interface and ultrathin, resorbable electronics (Benfenati et al., 2010, 2012; Kim et al., 2010; Tien et al., 2013). Currently, efforts are focused on development of 3D neuronal cell cultures supported by silk sponge scaffolds for construction of complex architectures mimicking form and function of brain regions (Tang-Schomer et al., 2014), Box 3.
Box 2. Promise of iPSCs as a reliable human cell source for neural tissue engineering.
Although animal models have been employed for neurological studies for many years, the primate cerebral cortex has many different features than a rodent's including significantly larger size and complexity, and increase in diversity of cell types (Hill and Walsh, 2005; Rakic, 2009). Human neural cell sources are challenging to come by prompting researchers to explore stem cell options for in vitro neural cultures.
Human induced pluripotent stem cells (hiPSCs) have gained a lot of recent attention since their production in 2007. iPSCs are generated from adult somatic cells (i.e. skin, blood, liver, etc.) through induction of genes that make them pluripotent (Gianotti-Sommer et al., 2008). These genetic alterations turn back the clock, effectively reprogramming cells to resemble (at least phenotypically) embryonic stem cells (Gianotti-Sommer et al., 2008). There are many benefits of using iPSCs as a human cell source for tissue engineering including lack of ethical issues present concerning the use of embryonic stem cells, readily available iPSCs and differentiation protocols, and the ability to create patient-specific tissues that may better represent pathophysiological events lacking in animal models (Gunaseeli et al., 2010).
Recently, Lie et al., 2012 outlined a detailed protocol for the differentiation, culture, and characterization of hiPSCs to all classes of mature cortical projection neurons (Lie et al., 2012). Interestingly, these methods appear to temporally recapitulate the stages of human cortical development including differentiation of hiPSCs to neuroepithelial cells, primary and secondary progenitor cells, and finally early-born deep-layer as well as later-born upper-layer excitatory neurons and astrocytes ADDIN ZOTERO_ITEM CSL_CITATION {“citationID”:“25pcms0uen”,“properties”:{“formattedCitation”:“(Lie et al., 2012)”,“plainCitation’:“(Lie et al., 2012)”},“citationItems”:[{“id”:812,“uris”:[“http://zotero.org/users/local/JzGdbClU/items/MC
Box 3. Silk sponge scaffolds for top-down approaches to forming heterogeneous 3D tissues in vitro.
As discussed in Box 1, silk fibroin from silkworm cocoons can be used to form a variety of biomaterial platforms. Recently, porous silk sponge scaffolds have been investigated as a platform for critically sized, 3D in vitro tissues (Wray et al., 2013). This platform aims to address the need for improved nutrient and oxygen delivery and waste removal from large 3D constructs by including arrays of hollow channels throughout the bulk of the scaffold.
Our group developed silk-based platform for use as an in vitro model of the brain-like tissue by seeding primary rat neurons in the silk sponge scaffold filling the void space with collagen (Tang-Schomer et al., 2014). We found that these scaffolds matched the stiffness of endogenous rat brain tissue and that constructs remained viable in culture for at least 2 months. Furthermore, immunofluorescence staining showed formation of axonal tract within the bulk of the collagen matrix and clustering of neuronal bodies on the silk sponge, thus mimicking white/gray matter architecture of the cortex. In response to injury our engineered CNS tissues showed similar biochemical and electrophysiological response to in vivo cases.
The addition of hollow channels to the silk sponge scaffolds will allow for localized functionalization and compartmentalized seeding of the vasculature and bulk neural tissue. These features are well suited for construction of complex heterogeneous structures such as those found in the brain. For example, channels can be coated with endothelial-promoting factors such as collagen or fibronectin to promote endothelial cell attachment and monolayer formation and the bulk scaffold can be impregnated with collagen gel for neural cell growth.
Analysis of critical design inputs of 3D, in vitro tissue models of the CNS
Summary and explanation of critical design requirements for CNS tissue models
Summary of the critical, unmet challenges for existing CNS tissue models
Acknowledgments
We would like to thank our funding sources for studies referenced (NIH P41 EB002520, R01 AR061988) as well as the other members of the Neurological Tissue Group in the Kaplan lab for their support: Min Tang-Schomer, Jimmy White, Lee Tien, Disha Sood, Si Ran Wang, and Nurdan Ozkucur. K.C. was supported by the German Research Foundation (DFG) (CH 1400/2-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearne M, Yang Y, El Haj AJ, Then KY, Liu K-K. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface R. Soc. 2005;2:455–463. doi: 10.1098/rsif.2005.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearne M, Liu K-K, El Haj AJ, Then KY, Rauz S, Yang Y. Online monitoring of the mechanical behavior of collagen hydrogels: influence of corneal fibroblasts on elastic modulus. Tissue Eng. Part C Methods. 2010;16:319–327. doi: 10.1089/ten.TEC.2008.0650. [DOI] [PubMed] [Google Scholar]
- Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2011;31:693–705. doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albergaria C, Carey MR. All Purkinje cells are not created equal. eLife. 2014;3 doi: 10.7554/eLife.03285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers GW, Goldstein LB, Hall D, Lesko LM, Aptiganel Acute Stroke Investigators Aptiganel hydrochloride in acute ischemic stroke: A randomized controlled trial. JAMA. 2001;286:2673–2682. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- Amadoro G, Corsetti V, Florenzano F, Atlante A, Bobba A, Nicolin V, Nori SL, Calissano P. Morphological and bioenergetic demands underlying the mitophagy in post-mitotic neurons: the pink-parkin pathway. Front. Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Bliss TV, Lomo T, Olsen LI, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Acta Physiol. Scand. 1969;76:4A–5A. doi: 10.1111/j.1748-1716.1969.tb04499.x. [DOI] [PubMed] [Google Scholar]
- Anderson KL, Ferreira A. Alpha1 Integrin activation: a link between beta-amyloid deposition and neuronal death in aging hippocampal neurons. J. Neurosci. Res. 2004;75:688–697. doi: 10.1002/jnr.20018. [DOI] [PubMed] [Google Scholar]
- Anlar B, Gunel-Ozcan A. Tenascin-R: role in the central nervous system. Int. J. Biochem. Cell Biol. 2012;44:1385–1389. doi: 10.1016/j.biocel.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Antoine EE, Vlachos PP, Rylander MN. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014 doi: 10.1089/ten.teb.2014.0086. doi:10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. TIG. 2000;16:389–395. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- Aregueta-Robles UA, Woolley AJ, Poole-Warren LA, Lovell NH, Green RA. Organic electrode coatings for next-generation neural interfaces. Front. Neuroengineering. 2014;7:15. doi: 10.3389/fneng.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb. Cortex N. Y. N. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. Off. J. Soc. Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H. Artificial Intelligence and Robot Responsibilities: Innovating Beyond Rights. Sci. Eng. Ethics. 2014:1–10. doi: 10.1007/s11948-014-9541-0. [DOI] [PubMed] [Google Scholar]
- Aumailley M. The laminin family. Cell Adhes. Migr. 2013;7:48–55. doi: 10.4161/cam.22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurand ER, Wagner JL, Shandas R, Bjugstad KB. Hydrogel formulation determines cell fate of fetal and adult neural progenitor cells. Stem Cell Res. 2014;12:11–23. doi: 10.1016/j.scr.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Balaban RS. Allometry of brain metabolism. Proc. Natl. Acad. Sci. 2013;110:3216–3217. doi: 10.1073/pnas.1221313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10:2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int. J. Biochem. Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Bellamkonda R, Ranieri JP, Aebischer P. Laminin oligopeptide derivatized agarose gels allow three-dimensional neurite extension in vitro. J. Neurosci. Res. 1995a;41:501–509. doi: 10.1002/jnr.490410409. [DOI] [PubMed] [Google Scholar]
- Bellamkonda R, Ranieri JP, Bouche N, Aebischer P. Hydrogel-based three-dimensional matrix for neural cells. J. Biomed. Mater. Res. 1995b;29:663–671. doi: 10.1002/jbm.820290514. [DOI] [PubMed] [Google Scholar]
- Bellas E, Marra KG, Kaplan DL. Sustainable three-dimensional tissue model of human adipose tissue. Tissue Eng. Part C Methods. 2013;19:745–754. doi: 10.1089/ten.tec.2012.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati V, Toffanin S, Capelli R, Camassa LMA, Ferroni S, Kaplan DL, Omenetto FG, Muccini M, Zamboni R. A silk platform that enables electrophysiology and targeted drug delivery in brain astroglial cells. Biomaterials. 2010;31:7883–7891. doi: 10.1016/j.biomaterials.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati V, Stahl K, Gomis-Perez C, Toffanin S, Sagnella A, Torp R, Kaplan DL, Ruani G, Omenetto FG, Zamboni R, et al. Biofunctional silk/neuron interfaces. Adv. Funct. Mater. 2012;22:1871–1884. [Google Scholar]
- Bercu MM, Arien-Zakay H, Stoler D, Lecht S, Lelkes PI, Samuel S, Or R, Nagler A, Lazarovici P, Elchalal U. Enhanced survival and neurite network formation of human umbilical cord blood neuronal progenitors in three-dimensional collagen constructs. J. Mol. Neurosci. MN. 2013;51:249–261. doi: 10.1007/s12031-012-9933-z. [DOI] [PubMed] [Google Scholar]
- Bergamaschi A, Tagliabue E, Sørlie T, Naume B, Triulzi T, Orlandi R, Russnes HG, Nesland JM, Tammi R, Auvinen P, et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J. Pathol. 2008;214:357–367. doi: 10.1002/path.2278. [DOI] [PubMed] [Google Scholar]
- Bernas MJ, Cardoso FL, Daley SK, Weinand ME, Campos AR, Ferreira AJG, Hoying JB, Witte MH, Brites D, Persidsky Y, et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat. Protoc. 2010;5:1265–1272. doi: 10.1038/nprot.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A, Hosley M, Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat. Embryol. (Berl.) 1993;188:419–433. doi: 10.1007/BF00190136. [DOI] [PubMed] [Google Scholar]
- Birmingham K. Future of neuroprotective drugs in doubt. Nat. Med. 2002;8:5–5. doi: 10.1038/nm0102-5a. [DOI] [PubMed] [Google Scholar]
- Blain M, Miron VE, Lambert C, Darlington PJ, Cui Q-L, Saikali P, Yong VW, Antel JP. Isolation and culture of primary human CNS neural cells. In: Doering LC, editor. Protocols for Neural Cell Culture. Humana Press; 2010. pp. 87–104. [Google Scholar]
- Bluebrain The Blue Brain Project | EPFL. 2014.
- Bø L, Vedeler CA, Nyland HI, Trapp BD, Mørk SJ. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J. Neuropathol. Exp. Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- Bota M, Swanson LW. The neuron classification problem. Brain Res. Rev. 2007;56:79–88. doi: 10.1016/j.brainresrev.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein JE. Growth requirements in vitro of oligodendrocyte cell lines and neonatal rat brain oligodendrocytes. Proc. Natl. Acad. Sci. U. S. A. 1986;83:1955–1959. doi: 10.1073/pnas.83.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V. Brain size and number of neurons: an exercise in synthetic neuroanatomy. J. Comput. Neurosci. 2001;10:71–77. doi: 10.1023/a:1008920127052. [DOI] [PubMed] [Google Scholar]
- Brännvall K, Bergman K, Wallenquist U, Svahn S, Bowden T, Hilborn J, Forsberg-Nilsson K. Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix. J. Neurosci. Res. 2007;85:2138–2146. doi: 10.1002/jnr.21358. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Tran N, Gage FH. Modeling psychiatric disorders at the cellular and network levels. Mol. Psychiatry. 2012;17:1239–1253. doi: 10.1038/mp.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J. Neurosci. Methods. 1997;71:143–155. doi: 10.1016/s0165-0270(96)00136-7. [DOI] [PubMed] [Google Scholar]
- De Brito Sanchez G, Giurfa M. A comparative analysis of neural taste processing in animals. Philos. Trans. R. Soc. B Biol. Sci. 2011;366:2171–2180. doi: 10.1098/rstb.2010.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccatelli F, Larregieu CA, Cruciani G, Oprea TI, Benet LZ. Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv. Drug Deliv. Rev. 2012;64:95–109. doi: 10.1016/j.addr.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli S, Messaggio F, Canazza A, Hebda DM, Caremoli F, Latorre E, Grimoldi MG, Colli M, Bulfamante G, Tremolada C, et al. Characteristics and properties of mesenchymal stem cells derived from micro-fragmented adipose tissue. Cell Transplant. 2014 doi: 10.3727/096368914X681603. [DOI] [PubMed] [Google Scholar]
- Carlsen EM, Perrier J-F. Purines released from astrocytes inhibit excitatory synaptic transmission in the ventral horn of the spinal cord. Front. Neural Circuits. 2014;8:60. doi: 10.3389/fncir.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet G, Himpens B, Cassiman JJ. Selective increase in the binding of the alpha 1 beta 1 integrin for collagen type IV during neurite outgrowth of human neuroblastoma TR 14 cells. J. Cell Sci. 1994;107(Pt 12):3379–3392. doi: 10.1242/jcs.107.12.3379. [DOI] [PubMed] [Google Scholar]
- Carson MP, Haudenschild CC. Microvascular endothelium and pericytes: high yield, low passage cultures. Vitro Cell. Dev. Biol. J. Tissue Cult. Assoc. 1986;22:344–354. doi: 10.1007/BF02623409. [DOI] [PubMed] [Google Scholar]
- Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Leal K, Nanou E. Calcium channels and short-term synaptic plasticity. J. Biol. Chem. 2013;288:10742–10749. doi: 10.1074/jbc.R112.411645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchelli R, Dehouck B, Descamps L, Fenart L, Buée-Scherrer VV, Duhem C, Lundquist S, Rentfel M, Torpier G, Dehouck MP. In vitro model for evaluating drug transport across the blood-brain barrier. Adv. Drug Deliv. Rev. 1999;36:165–178. doi: 10.1016/s0169-409x(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck M-P, Fenart L. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007;6:650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- Cecchelli R, Aday S, Sevin E, Almeida C, Culot M, Dehouck L, Coisne C, Engelhardt B, Dehouck M-P, Ferreira L. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PloS One. 2014;9:e99733. doi: 10.1371/journal.pone.0099733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- Chang GL, Hung TK, Feng WW. An in-vivo measurement and analysis of viscoelastic properties of the spinal cord of cats. J. Biomech. Eng. 1988;110:115–122. doi: 10.1115/1.3108415. [DOI] [PubMed] [Google Scholar]
- Chow WN, Simpson DG, Bigbee JW, Colello RJ. Evaluating neuronal and glial growth on electrospun polarized matrices: bridging the gap in percussive spinal cord injuries. Neuron Glia Biol. 2007;3:119–126. doi: 10.1017/S1740925X07000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Sahani M, Shenoy KV. Techniques for extracting single-trial activity patterns from large-scale neural recordings. Curr. Opin. Neurobiol. 2007;17:609–618. doi: 10.1016/j.conb.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalek K, Levental KR, Tsurkan MV, Zieris A, Freudenberg U, Werner C. Two-tier hydrogel degradation to boost endothelial cell morphogenesis. Biomaterials. 2011;32:9649–9657. doi: 10.1016/j.biomaterials.2011.08.078. [DOI] [PubMed] [Google Scholar]
- Claverol-Tinturé E, Ghirardi M, Fiumara F, Rosell X, Cabestany J. Multielectrode arrays with elastomeric microstructured overlays for extracellular recordings from patterned neurons. J. Neural Eng. 2005;2:L1–L7. doi: 10.1088/1741-2560/2/2/L01. [DOI] [PubMed] [Google Scholar]
- Coates PW, Nathan RD. Feasibility of electrical recordings from unconnected vertebrate CNS neurons cultured in a three-dimensional extracellular matrix. J. Neurosci. Methods. 1987;20:203–210. doi: 10.1016/0165-0270(87)90052-5. [DOI] [PubMed] [Google Scholar]
- Coates PW, Fermini B, Strahlendorf JC, Strahlendorf HK. Utilization of three-dimensional culture for early morphometric and electrophysiological analyses of solitary cerebellar neurons. Dev. Neurosci. 1992;14:35–43. doi: 10.1159/000111645. [DOI] [PubMed] [Google Scholar]
- Compagnucci C, Nizzardo M, Corti S, Zanni G, Bertini E. In vitro neurogenesis: development and functional implications of iPSC technology. Cell. Mol. Life Sci. CMLS. 2014;71:1623–1639. doi: 10.1007/s00018-013-1511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, Jbabdi S, Yan C-G, Vogelstein JT, Castellanos FX, Di Martino A, Kelly C, Heberlein K, Colcombe S, Milham MP. Imaging human connectomes at the macroscale. Nat. Methods. 2013;10:524–539. doi: 10.1038/nmeth.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E, Ribatti D. Soul, mind, brain: Greek philosophy and the birth of neuroscience. Brain Res. Bull. 2007;71:327–336. doi: 10.1016/j.brainresbull.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Cucullo L, McAllister MS, Kight K, Krizanac-Bengez L, Marroni M, Mayberg MR, Stanness KA, Janigro D. A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res. 2002;951:243–254. doi: 10.1016/s0006-8993(02)03167-0. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2011;31:767–777. doi: 10.1038/jcbfm.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucullo L, Hossain M, Tierney W, Janigro D. A new dynamic in vitro modular capillaries-venules modular system: Cerebrovascular physiology in a box. BMC Neurosci. 2013;14:18. doi: 10.1186/1471-2202-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen DK, Lessing MC, LaPlaca MC. Collagen-dependent neurite outgrowth and response to dynamic deformation in three-dimensional neuronal cultures. Ann. Biomed. Eng. 2007a;35:835–846. doi: 10.1007/s10439-007-9292-z. [DOI] [PubMed] [Google Scholar]
- Cullen DK, Simon CM, LaPlaca MC. Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures. Brain Res. 2007b;1158:103–115. doi: 10.1016/j.brainres.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen DK, Wolf JA, Smith DH, Pfister BJ. Neural tissue engineering for neuroregeneration and biohybridized interface microsystems in vivo (Part 2). Crit. Rev. Biomed. Eng. 2011;39:241–259. doi: 10.1615/critrevbiomedeng.v39.i3.40. [DOI] [PubMed] [Google Scholar]
- Dana H, Marom A, Paluch S, Dvorkin R, Brosh I, Shoham S. Hybrid multiphoton volumetric functional imaging of large-scale bioengineered neuronal networks. Nat. Commun. 2014;5:3997. doi: 10.1038/ncomms4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen EHJ, Sonnenberg A. Integrins in regulation of tissue development and function. J. Pathol. 2003;200:471–480. doi: 10.1002/path.1416. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Solinas S, Garrido J, Casellato C, Pedrocchi A, Mapelli J, Gandolfi D, Prestori F. Realistic modeling of neurons and networks: towards brain simulation. Funct. Neurol. 2013;28:153–166. [PMC free article] [PubMed] [Google Scholar]
- Dehouck MP, Méresse S, Delorme P, Fruchart JC, Cecchelli R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J. Neurochem. 1990;54:1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann. Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Deli MA, Abrahám CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell. Mol. Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delivopoulos E, Murray AF, MacLeod NK, Curtis JC. Guided growth of neurons and glia using microfabricated patterns of parylene-C on a SiO2 background. Biomaterials. 2009;30:2048–2058. doi: 10.1016/j.biomaterials.2008.12.049. [DOI] [PubMed] [Google Scholar]
- Devon RM. Elimination of Cell Types from Mixed Neural Cell Cultures. In: Fedoroff S, Richardson A, editors. Protocols for Neural Cell Culture. Humana Press; 2001. pp. 325–332. [Google Scholar]
- Di L, Kerns EH, Fan K, McConnell OJ, Carter GT. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003;38:223–232. doi: 10.1016/s0223-5234(03)00012-6. [DOI] [PubMed] [Google Scholar]
- Dillon GP, Yu X, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J. Biomater. Sci. Polym. Ed. 1998;9:1049–1069. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Doering LC. Protocols for Neural Cell Culture. J. Neuropathol. Exp. Neurol. 2010 [Google Scholar]
- Donoghue PS, Lamond R, Boomkamp SD, Sun T, Gadegaard N, Riehle MO, Barnett SC. The development of a ε-polycaprolactone scaffold for central nervous system repair. Tissue Eng. Part A. 2013;19:497–507. doi: 10.1089/ten.TEA.2012.0382. [DOI] [PubMed] [Google Scholar]
- Donoghue PS, Sun T, Gadegaard N, Riehle MO, Barnett SC. Development of a novel 3D culture system for screening features of a complex implantable device for CNS repair. Mol. Pharm. 2014;11:2143–2150. doi: 10.1021/mp400526n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull RL, Lawlor P, Beilharz EJ, Singleton K, Walker EB, Mee E. In situ evidence for DNA fragmentation in Huntington's disease striatum and Alzheimer's disease temporal lobes. Neuroreport. 1995;6:1053–1057. doi: 10.1097/00001756-199505090-00026. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin ML, Toni N, Julien SD, Charvet I, Sundstrom LE, Stoppini L. The long-term survival of in vitro engineered nervous tissue derived from the specific neural differentiation of mouse embryonic stem cells. Biomaterials. 2010;31:7032–7042. doi: 10.1016/j.biomaterials.2010.06.017. [DOI] [PubMed] [Google Scholar]
- East E, Golding JP, Phillips JB. A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis. J. Tissue Eng. Regen. Med. 2009;3:634–646. doi: 10.1002/term.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East E, de Oliveira DB, Golding JP, Phillips JB. Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng. Part A. 2010;16:3173–3184. doi: 10.1089/ten.tea.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East E, Johns N, Georgiou M, Golding JP, Loughlin AJ, Kingham PJ, Phillips JB. A 3D in vitro model reveals differences in the astrocyte response elicited by potential stem cell therapies for CNS injury. Regen. Med. 2013;8:739–746. doi: 10.2217/rme.13.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenmann DE, Xue G, Kim KS, Moses AV, Hamburger M, Oufir M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood–brain barrier model for drug permeability studies. Fluids Barriers CNS. 2013;10:33. doi: 10.1186/2045-8118-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin BS, Azeloglu EU, Costa KD, Morrison B., 3rd Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J. Neurotrauma. 2007;24:812–822. doi: 10.1089/neu.2006.0169. [DOI] [PubMed] [Google Scholar]
- Emery B, Dugas JC. Purification of oligodendrocyte lineage cells from mouse cortices by immunopanning. Cold Spring Harb. Protoc. 2013;2013:854–868. doi: 10.1101/pdb.prot073973. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 2014;355:687–699. doi: 10.1007/s00441-014-1811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Housden E, Smith-Thomas L, Meyer RL. The growth of axons in three-dimensional astrocyte cultures. Dev. Biol. 1989;135:449–458. doi: 10.1016/0012-1606(89)90193-0. [DOI] [PubMed] [Google Scholar]
- Ferreri AJM, Illerhaus G, Zucca E, Cavalli F, International Extranodal Lymphoma Study Group Flows and flaws in primary central nervous system lymphoma. Nat. Rev. Clin. Oncol. 2010;7 doi: 10.1038/nrclinonc.2010.9-c1. doi:10.1038/nrclinonc.2010.9–c1; author reply doi:10:1038/nrclinonc.2010.9–c2. [DOI] [PubMed] [Google Scholar]
- Foo LC. Purification of rat and mouse astrocytes by immunopanning. Cold Spring Harb. Protoc. 2013;2013:421–432. doi: 10.1101/pdb.prot074211. [DOI] [PubMed] [Google Scholar]
- Ford PJ, Deshpande A. The ethics of surgically invasive neuroscience research. Handb. Clin. Neurol. 2013;118:315–321. doi: 10.1016/B978-0-444-53501-6.00026-3. [DOI] [PubMed] [Google Scholar]
- Frampton JP, Hynd MR, Shuler ML, Shain W. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed. Mater. Bristol Engl. 2011;6:015002. doi: 10.1088/1748-6041/6/1/015002. [DOI] [PubMed] [Google Scholar]
- Frank CA. How voltage-gated calcium channels gate forms of homeostatic synaptic plasticity. Front. Cell. Neurosci. 2014;8 doi: 10.3389/fncel.2014.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze K, Janmey PA, Guck J. Mechanics in neuronal development and repair. Annu. Rev. Biomed. Eng. 2013;15:227–251. doi: 10.1146/annurev-bioeng-071811-150045. [DOI] [PubMed] [Google Scholar]
- Freshney RI, Obradovic W, Grayson W, Cannizzaro C, Vunjak-Novakovic G. Principles of Tissue Engineering. Elsevier; 2007. Principles of tissue culture and bioreactor design. [Google Scholar]
- Fridén J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27:157–164. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- Friedel RH, Schnürch H, Stubbusch J, Barde YA. Identification of genes differentially expressed by nerve growth factor- and neurotrophin-3-dependent sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12670–12675. doi: 10.1073/pnas.94.23.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht R, Gundelfinger ED. The brain's extracellular matrix and its role in synaptic plasticity. Adv. Exp. Med. Biol. 2012;970:153–171. doi: 10.1007/978-3-7091-0932-8_7. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Stewart MG. Distribution of neurons and glia in the visual cortex (area 17) of the adult albino rat: a quantitative description. Neuroscience. 1987;21:833–845. doi: 10.1016/0306-4522(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gaillard PJ, van der Sandt IC, Voorwinden LH, Vu D, Nielsen JL, de Boer AG, Breimer DD. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood-brain barrier. Pharm. Res. 2000;17:1198–1205. doi: 10.1023/a:1026406528530. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang Z-Y, Zhang J, Zhang Y, Huo H, Wang T, Jiang T, Wang S. RVG-peptide-linked trimethylated chitosan for delivery of siRNA to the brain. Biomacromolecules. 2014;15:1010–1018. doi: 10.1021/bm401906p. [DOI] [PubMed] [Google Scholar]
- Gardner A, Jukkola P, Gu C. Myelination of rodent hippocampal neurons in culture. Nat. Protoc. 2012;7:1774–1782. doi: 10.1038/nprot.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, Belsham DD. Estrogen receptor α and G-protein coupled receptor 30 mediate the neuroprotective effects of 17β-estradiol in novel murine hippocampal cell models. Neuroscience. 2010;170:54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- Giordano G, Costa LG. Primary neurons in culture and neuronal cell lines for in vitro neurotoxicological studies. Methods Mol. Biol. Clifton NJ. 2011;758:13–27. doi: 10.1007/978-1-61779-170-3_2. [DOI] [PubMed] [Google Scholar]
- Goebels N. Organotypic CNS slice cultures as an in vitro model for immune mediated tissue damage and repair in multiple sclerosis. ALTEX. 2007;24:85–86. Spec No. [PubMed] [Google Scholar]
- Goila AK, Pawar M. The diagnosis of brain death. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2009;13:7–11. doi: 10.4103/0972-5229.53108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Grant GA, Abbott NJ, Janigro D. Understanding the physiology of the blood-brain barrier: in vitro models. News Physiol. Sci. Int. J. Physiol. Prod. Jointly Int. Union Physiol. Sci. Am. Physiol. Soc. 1998;13:287–293. doi: 10.1152/physiologyonline.1998.13.6.287. [DOI] [PubMed] [Google Scholar]
- Greene M. Saving a life but losing the patient. Theor. Med. Bioeth. 2013;34:479–498. doi: 10.1007/s11017-013-9273-1. [DOI] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Griep LM, Wolbers F, de Wagenaar B, ter Braak PM, Weksler BB, Romero IA, Couraud PO, Vermes I, van der Meer AD, van den Berg A. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed. Microdevices. 2013;15:145–150. doi: 10.1007/s10544-012-9699-7. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neuroscience, early history of. Encycl. Neurosci. 1987:843–847. [Google Scholar]
- Hai M, Muja N, DeVries GH, Quarles RH, Patel PI. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J. Neurosci. Res. 2002;69:497–508. doi: 10.1002/jnr.10327. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010;72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harink B, Gac SL, Truckenmüller R, Blitterswijk C. van, Habibovic P. Regeneration-on-a-chip? The perspectives on use of microfluidics in regenerative medicine. Lab. Chip. 2013;13:3512–3528. doi: 10.1039/c3lc50293g. [DOI] [PubMed] [Google Scholar]
- Harrison RG, Greenman MJ, Mall FP, Jackson CM. Observations of the living developing nerve fiber. Anat. Rec. 1907;1:116–128. [Google Scholar]
- Hatherell K, Couraud P-O, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods. 2011;199:223–229. doi: 10.1016/j.jneumeth.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Mukai N, Sawa T. Poincaré analysis of the electroencephalogram during sevoflurane anesthesia. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. pii. 2014:S1388–2457(14)00268-5. doi: 10.1016/j.clinph.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Haydon PG. Glia: listening and talking to the synapse. Nat. Rev. Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia. 2014;62:1377–1391. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Küstermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Föcking M, Arnold H, Hescheler J, Fleischmann BK, et al. Monitoring of implanted stem cell migration in vivo: A highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc. Natl. Acad. Sci. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogberg HT, Bressler J, Christian KM, Harris G, Makri G, O'Driscoll C, Pamies D, Smirnova L, Wen Z, Hartung T. Toward a 3D model of human brain development for studying gene/environment interactions. Stem Cell Res. Ther. 2013;4(Suppl 1):S4. doi: 10.1186/scrt365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma R, Baker BJ, Jin L, Garaschuk O, Konnerth A, Cohen LB, Zecevic D. Wide-field and two-photon imaging of brain activity with voltage- and calcium-sensitive dyes. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:2453–2467. doi: 10.1098/rstb.2009.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AM, De Laporte L, Tortelli F, Spedden E, Staii C, Atherton TJ, Hubbell JA, Kaplan DL. Silk hydrogels as soft substrates for neural tissue engineering. Adv. Funct. Mater. 2013;23:5140–5149. [Google Scholar]
- Hou S, Tian W, Xu Q, Cui F, Zhang J, Lu Q, Zhao C. The enhancement of cell adherence and inducement of neurite outgrowth of dorsal root ganglia co-cultured with hyaluronic acid hydrogels modified with Nogo-66 receptor antagonist in vitro. Neuroscience. 2006;137:519–529. doi: 10.1016/j.neuroscience.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Hu X, Tang-Schomer MD, Huang W, Xia X-X, Weiss AS, Kaplan DL. Charge-tunable autoclaved silk-tropoelastin protein alloys that control neuron cell responses. Adv. Funct. Mater. 2013;23:3875–3884. doi: 10.1002/adfm.201202685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Xu Y, Cheng Q, Yu S, Gao Y, Shu Q, Yang C, Sun Y, Wang J, Xu F, et al. The expression changes of myelin and lymphocyte protein (MAL) following optic nerve crush in adult rats retinal ganglion cells. J. Mol. Neurosci. MN. 2014 doi: 10.1007/s12031-014-0332-5. doi.10.1007/s12031-014-0332-5. [DOI] [PubMed] [Google Scholar]
- Iacovetta C, Rudloff E, Kirby R. The role of aquaporin 4 in the brain. Vet. Clin. Pathol. Am. Soc. Vet. Clin. Pathol. 2012;41:32–44. doi: 10.1111/j.1939-165X.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iii BM, Cullen DK, LaPlaca MC. In vitro models for biomechanical studies of neural tissues. In: Bilston LE, editor. Neural Tissue Biomechanics. Springer Berlin Heidelberg; 2011. pp. 247–285. [Google Scholar]
- Irons HR, Cullen DK, Shapiro NP, Lambert NA, Lee RH, Laplaca MC. Three-dimensional neural constructs: a novel platform for neurophysiological investigation. J. Neural Eng. 2008;5:333–341. doi: 10.1088/1741-2560/5/3/006. [DOI] [PubMed] [Google Scholar]
- Isobe I, Watanabe T, Yotsuyanagi T, Hazemoto N, Yamagata K, Ueki T, Nakanishi K, Asai K, Kato T. Astrocytic contributions to blood-brain barrier (BBB) formation by endothelial cells: A possible use of aortic endothelial cell for in vitro BBB model. Neurochem. Int. 1996;28:523–533. doi: 10.1016/0197-0186(95)00142-5. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Which model to use for cortical spiking neurons? IEEE Trans. Neural Netw. Publ. IEEE Neural Netw. Counc. 2004;15:1063–1070. doi: 10.1109/TNN.2004.832719. [DOI] [PubMed] [Google Scholar]
- Jafari P, Braissant O, Zavadakova P, Henry H, Bonafé L, Ballhausen D. Ammonium accumulation and cell death in a rat 3D brain cell model of glutaric aciduria type I. PloS One. 2013;8:e53735. doi: 10.1371/journal.pone.0053735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel RJ, Schneider BL, Svendsen CN. Using human neural stem cells to model neurological disease. Nat. Rev. Genet. 2004;5:136–144. doi: 10.1038/nrg1268. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Miljkovic D, Schachner M, Andjus P. Tenascins and inflammation in disorders of the nervous system. Amino Acids. 2013;44:1115–1127. doi: 10.1007/s00726-012-1446-0. [DOI] [PubMed] [Google Scholar]
- James CD, Davis R, Meyer M, Turner A, Turner S, Withers G, Kam L, Banker G, Craighead H, Isaacson M, et al. Aligned microcontact printing of micrometer-scale poly-L-lysine structures for controlled growth of cultured neurons on planar microelectrode arrays. IEEE Trans. Biomed. Eng. 2000;47:17–21. doi: 10.1109/10.817614. [DOI] [PubMed] [Google Scholar]
- J Csicsvari MP. Extracellular recording and analysis of neuronal activity: from single cells to ensembles. 1998.
- Jia X, Gharibyan AL, Öhman A, Liu Y, Olofsson A, Morozova-Roche LA. Neuroprotective and nootropic drug noopept rescues α-synuclein amyloid cytotoxicity. J. Mol. Biol. 2011;414:699–712. doi: 10.1016/j.jmb.2011.09.044. [DOI] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Lisberger SG. A tale of two species: neural integration in zebrafish and monkeys. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.04.048. pii: S0306-4522(14)00360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink BHJ. Chick spinal somatic motoneurons in culture. In: Fedoroff S, Richardson A, editors. Protocols for Neural Cell Culture. Humana Press; 2001. pp. 59–69. [Google Scholar]
- Karaöz E, Demircan PC, Sağlam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell Biol. 2011;136:455–473. doi: 10.1007/s00418-011-0858-3. [DOI] [PubMed] [Google Scholar]
- Kardestuncer T, McCarthy MB, Karageorgiou V, Kaplan D, Gronowicz G. RGD-tethered silk substrate stimulates the differentiation of human tendon cells. Clin. Orthop. 2006;448:234–239. doi: 10.1097/01.blo.0000205879.50834.fe. [DOI] [PubMed] [Google Scholar]
- Kato-Negishi M, Morimoto Y, Onoe H, Takeuchi S. Millimeter-sized neural building blocks for 3D heterogeneous neural network assembly. Adv. Healthc. Mater. 2013;2:1564–1570. doi: 10.1002/adhm.201300052. [DOI] [PubMed] [Google Scholar]
- Kaufman LJ, Brangwynne CP, Kasza KE, Filippidi E, Gordon VD, Deisboeck TS, Weitz DA. Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys. J. 2005;89:635–650. doi: 10.1529/biophysj.105.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR, Wong RK. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J. Neurosci. Methods. 1986;16:227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Keely GC, Gorsuch AM, McCabe JM, Wood WH, Deacon JC, Hill MKJ, Pierce WJ, Langrock PF. Uniform determination of death Act. Natl. Conf. Comm. Unif. State Laws. 1981 [Google Scholar]
- Khan MS, Zetterlund E-L, Gréen H, Oscarsson A, Zackrisson A-L, Svanborg E, Lindholm M-L, Persson H, Eintrei C. Pharmacogenetics, plasma concentrations, clinical signs and EEG during propofol treatment. Basic Clin. Pharmacol. Toxicol. 2014 doi: 10.1111/bcpt.12277. doi: 10.1111/bcpt.12277. [DOI] [PubMed] [Google Scholar]
- Kim SA, Jun SB. In-vivo optical measurement of neural activity in the brain. Exp. Neurobiol. 2013;22:158–166. doi: 10.5607/en.2013.22.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-H, Viventi J, Amsden JJ, Xiao J, Vigeland L, Kim Y-S, Blanco JA, Panilaitis B, Frechette ES, Contreras D, et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010;9:511–517. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AS, Güven S, Biedermann T, Luginbühl J, Böttcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials. 2014;35:5065–5078. doi: 10.1016/j.biomaterials.2014.02.049. [DOI] [PubMed] [Google Scholar]
- Koch D, Rosoff WJ, Jiang J, Geller HM, Urbach JS. Strength in the periphery: growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys. J. 2012;102:452–460. doi: 10.1016/j.bpj.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodandaramaiah SB, Franzesi GT, Chow BY, Boyden ES, Forest CR. Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat. Methods. 2012;9:585–587. doi: 10.1038/nmeth.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron CM, Fong VJ, Hoffman-Kim D. Neurite outgrowth at the interface of 2D and 3D growth environments. J. Neural Eng. 2009;6:016002. doi: 10.1088/1741-2560/6/1/016002. [DOI] [PubMed] [Google Scholar]
- Kolaj M, Zhang L, Hermes MLHJ, Renaud LP. Intrinsic properties and neuropharmacology of midline paraventricular thalamic nucleus neurons. Front. Behav. Neurosci. 2014;8:132. doi: 10.3389/fnbeh.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothapalli CR, Kamm RD. 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials. 2013;34:5995–6007. doi: 10.1016/j.biomaterials.2013.04.042. [DOI] [PubMed] [Google Scholar]
- Krassioukov AV, Ackery A, Schwartz G, Adamchik Y, Liu Y, Fehlings MG. An in vitro model of neurotrauma in organotypic spinal cord cultures from adult mice. Brain Res. Brain Res. Protoc. 2002;10:60–68. doi: 10.1016/s1385-299x(02)00180-0. [DOI] [PubMed] [Google Scholar]
- Krewson CE, Chung SW, Dai W, Mark Saltzman W. Cell aggregation and neurite growth in gels of extracellular matrix molecules. Biotechnol. Bioeng. 1994;43:555–562. doi: 10.1002/bit.260430704. [DOI] [PubMed] [Google Scholar]
- Kuo Y-C, Chang Y-H. Differentiation of induced pluripotent stem cells toward neurons in hydrogel biomaterials. Colloids Surf. B Biointerfaces. 2013;102:405–411. doi: 10.1016/j.colsurfb.2012.08.061. [DOI] [PubMed] [Google Scholar]
- Kuo Y-C, Lu C-H. Effect of human astrocytes on the characteristics of human brain-microvascular endothelial cells in the blood-brain barrier. Colloids Surf. B Biointerfaces. 2011;86:225–231. doi: 10.1016/j.colsurfb.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Kwon H, Rainbow RS, Sun L, Hui CK, Cairns DM, Preda RC, Kaplan DL, Zeng L. Scaffold structure and fabrication method affect proinflammatory milieu in three-dimensional-cultured chondrocytes. J. Biomed. Mater. Res. A. 2014 doi: 10.1002/jbm.a.35203. doi: 10.1002/jbm.a.35203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso MP, Soininen H, Partanen K, Helkala EL, Hartikainen P, Vainio P, Hallikainen M, Hänninen T, Riekkinen PJ., Sr Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: correlation with memory functions. J. Neural Transm. Park. Dis. Dement. Sect. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD. Proteoglycans in the nervous system. Curr. Opin. Neurobiol. 1993;3:716–723. doi: 10.1016/0959-4388(93)90143-m. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Cullen DK, McLoughlin JJ, Cargill RS., 2nd High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. J. Biomech. 2005;38:1093–1105. doi: 10.1016/j.jbiomech.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Nicolelis MAL. Brain–machine interfaces: past, present and future. Trends Neurosci. 2006;29:536–546. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin. Cancer Res. 2012;18:2780–2790. doi: 10.1158/1078-0432.CCR-11-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipzig ND, Wylie RG, Kim H, Shoichet MS. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials. 2011;32:57–64. doi: 10.1016/j.biomaterials.2010.09.031. [DOI] [PubMed] [Google Scholar]
- De Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer's disease: the atrophic hippocampus. Lancet. 1989;2:672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- Levy N. The use of animal as models: ethical considerations. Int. J. Stroke Off. J. Int. Stroke Soc. 2012;7:440–442. doi: 10.1111/j.1747-4949.2012.00772.x. [DOI] [PubMed] [Google Scholar]
- Li H, Wijekoon A, Leipzig ND. 3D differentiation of neural stem cells in macroporous photopolymerizable hydrogel scaffolds. PloS One. 2012;7:e48824. doi: 10.1371/journal.pone.0048824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang Q, Gao S, Song Q, Huang R, Wang L, Liu L, Dai J, Tang M, Cheng G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013;3:1604. doi: 10.1038/srep01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Dahl D, Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J. Cell Biol. 1983;96:920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Kirkwood T, Vaheri A. Fibronectin is expressed by astrocytes cultured from embryonic and early postnatal rat brain. Exp. Cell Res. 1986;163:175–185. doi: 10.1016/0014-4827(86)90570-7. [DOI] [PubMed] [Google Scholar]
- Lin CY, Kikuchi N, Hollister SJ. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J. Biomech. 2004;37:623–636. doi: 10.1016/j.jbiomech.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Lippmann ES, Al-Ahmad A, Azarin SM, Palecek SP, Shusta EV. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014;4:4160. doi: 10.1038/srep04160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, García-Verdugo J-M, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Lu C, Sood AK. Role of pericytes in angiogenesis. In: Teicher BA, L.M.E. MD, editors. Antiangiogenic Agents in Cancer Therapy. Humana Press; 2008. pp. 117–132. [Google Scholar]
- Lu Y-B, Franze K, Seifert G, Steinhäuser C, Kirchhoff F, Wolburg H, Guck J, Janmey P, Wei E-Q, Käs J, et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Fitzgerald W, Liu Q-Y, O'Shaughnessy TJ, Maric D, Lin HJ, Alkon DL, Barker JL. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp. Neurol. 2004;190:276–288. doi: 10.1016/j.expneurol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Man AJ, Davis HE, Itoh A, Leach JK, Bannerman P. Neurite outgrowth in fibrin gels is regulated by substrate stiffness. Tissue Eng. Part A. 2011;17:2931–2942. doi: 10.1089/ten.tea.2011.0030. [DOI] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: The future path for animal models. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt LM, Sakiyama-Elbert SE. Engineering peripheral nerve repair. Curr. Opin. Biotechnol. 2013;24:887–892. doi: 10.1016/j.copbio.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau M. Gliotransmission: focus on exocytotic release of L-glutamate and D-serine from astrocytes. Biochem. Soc. Trans. 2013;41:1557–1561. doi: 10.1042/BST20130195. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Thiele CJ. p27Kip1: a key mediator of retinoic acid induced growth arrest in the SMS-KCNR human neuroblastoma cell line. Oncogene. 1998;16:3337–3343. doi: 10.1038/sj.onc.1201830. [DOI] [PubMed] [Google Scholar]
- Matyash M, Despang F, Mandal R, Fiore D, Gelinsky M, Ikonomidou C. Novel soft alginate hydrogel strongly supports neurite growth and protects neurons against oxidative stress. Tissue Eng. Part A. 2012;18:55–66. doi: 10.1089/ten.TEA.2011.0097. [DOI] [PubMed] [Google Scholar]
- Maximino JR, de Oliveira GP, Alves CJ, Chadi G. Deregulated expression of cytoskeleton related genes in the spinal cord and sciatic nerve of presymptomatic SOD1(G93A) Amyotrophic Lateral Sclerosis mouse model. Front. Cell. Neurosci. 2014;8:148. doi: 10.3389/fncel.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CM, Fick LJ, Gingerich S, Belsham DD. Hypothalamic cell lines to investigate neuroendocrine control mechanisms. Front. Neuroendocrinol. 2009;30:405–423. doi: 10.1016/j.yfrne.2009.03.005. [DOI] [PubMed] [Google Scholar]
- McAllister MS, Krizanac-Bengez L, Macchia F, Naftalin RJ, Pedley KC, Mayberg MR, Marroni M, Leaman S, Stanness KA, Janigro D. Mechanisms of glucose transport at the blood-brain barrier: an in vitro study. Brain Res. 2001;904:20–30. doi: 10.1016/s0006-8993(01)02418-0. [DOI] [PubMed] [Google Scholar]
- McKay CA, Pomrenke RD, McLane JS, Schaub NJ, DeSimone EK, Ligon LA, Gilbert RJ. An injectable, calcium responsive composite hydrogel for the treatment of acute spinal cord injury. ACS Appl. Mater. Interfaces. 2014;6:1424–1438. doi: 10.1021/am4027423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megard I, Garrigues A, Orlowski S, Jorajuria S, Clayette P, Ezan E, Mabondzo A. A co-culture-based model of human blood-brain barrier: application to active transport of indinavir and in vivo-in vitro correlation. Brain Res. 2002;927:153–167. doi: 10.1016/s0006-8993(01)03337-6. [DOI] [PubMed] [Google Scholar]
- Miller JS. The billion cell construct: will three-dimensional printing get us there? PLoS Biol. 2014;12:e1001882. doi: 10.1371/journal.pbio.1001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet LJ, Stewart ME, Sweedler JV, Nuzzo RG, Gillette MU. Microfluidic devices for culturing primary mammalian neurons at low densities. Lab. Chip. 2007;7:987–994. doi: 10.1039/b705266a. [DOI] [PubMed] [Google Scholar]
- Morin F, Nishimura N, Griscom L, Lepioufle B, Fujita H, Takamura Y, Tamiya E. Constraining the connectivity of neuronal networks cultured on microelectrode arrays with microfluidic techniques: a step towards neuron-based functional chips. Biosens. Bioelectron. 2006;21:1093–1100. doi: 10.1016/j.bios.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Moroni F, Nobili L, De Carli F, Massimini M, Francione S, Marzano C, Proserpio P, Cipolli C, De Gennaro L, Ferrara M. Slow EEG rhythms and inter-hemispheric synchronization across sleep and wakefulness in the human hippocampus. NeuroImage. 2012;60:497–504. doi: 10.1016/j.neuroimage.2011.11.093. [DOI] [PubMed] [Google Scholar]
- Morrison B, Meaney DF, McIntosh TK. Mechanical characterization of an in vitro device designed to quantitatively injure living brain tissue. Ann. Biomed. Eng. 1998;26:381–390. doi: 10.1114/1.61. [DOI] [PubMed] [Google Scholar]
- Morrison B, Elkin BS, Dollé J-P, Yarmush ML. In vitro models of traumatic brain injury. Annu. Rev. Biomed. Eng. 2011;13:91–126. doi: 10.1146/annurev-bioeng-071910-124706. [DOI] [PubMed] [Google Scholar]
- Morrison B, 3rd, Eberwine JH, Meaney DF, McIntosh TK. Traumatic injury induces differential expression of cell death genes in organotypic brain slice cultures determined by complementary DNA array hybridization. Neuroscience. 2000;96:131–139. doi: 10.1016/s0306-4522(99)00537-0. [DOI] [PubMed] [Google Scholar]
- Morrison B, 3rd, Cater HL, Benham CD, Sundstrom LE. An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. J. Neurosci. Methods. 2006;150:192–201. doi: 10.1016/j.jneumeth.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Mosahebi A, Wiberg M, Terenghi G. Addition of fibronectin to alginate matrix improves peripheral nerve regeneration in tissue-engineered conduits. Tissue Eng. 2003;9:209–218. doi: 10.1089/107632703764664684. [DOI] [PubMed] [Google Scholar]
- Murray KD, McQuillin A, Stewart L, Etheridge CJ, Cooper RG, Miller AD, Gurling HM. Cationic liposome-mediated DNA transfection in organotypic explant cultures of the ventral mesencephalon. Gene Ther. 1999;6:190–197. doi: 10.1038/sj.gt.3300743. [DOI] [PubMed] [Google Scholar]
- Naik P, Cucullo L. In vitro blood–brain barrier models: Current and perspective technologies. J. Pharm. Sci. 2012;101:1337–1354. doi: 10.1002/jps.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Velumian AA, Chung I, Zhang L, Agrawal SK, Fehlings MG. Patch-clamp recordings from white matter glia in thin longitudinal slices of adult rat spinal cord. J. Neurosci. Methods. 2002;117:159–166. doi: 10.1016/s0165-0270(02)00096-1. [DOI] [PubMed] [Google Scholar]
- Negraes PD, Schwindt TT, Trujillo CA, Ulrich H. Neural differentiation of P19 carcinoma cells and primary neurospheres: cell morphology, proliferation, viability, and functionality. Curr. Protoc. Stem Cell Biol. 2012 doi: 10.1002/9780470151808.sc02d09s20. Chapter 2, Unit 2D.9. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakmann B. The patch clamp technique. Sci. Am. 1992;266:44–51. doi: 10.1038/scientificamerican0392-44. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion and related transport mechanisms in brain tissue. Rep. Prog. Phys. 2001;64:815. [Google Scholar]
- Noda M, Takii K, Parajuli B, Kawanokuchi J, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. FGF-2 released from degenerating neurons exerts microglial-induced neuroprotection via FGFR3-ERK signaling pathway. J. Neuroinflammation. 2014;11:76. doi: 10.1186/1742-2094-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojehdehian H, Moztarzadeh F, Baharvand H, Mehrjerdi NZ, Nazarian H, Tahriri M. Effect of poly-L-lysine coating on retinoic acid-loaded PLGA microspheres in the differentiation of carcinoma stem cells into neural cells. Int. J. Artif. Organs. 2010;33:721–730. doi: 10.5301/ijao.2010.5981. [DOI] [PubMed] [Google Scholar]
- O'Connor SM, Andreadis JD, Shaffer KM, Ma W, Pancrazio JJ, Stenger DA. Immobilization of neural cells in three-dimensional matrices for biosensor applications. Biosens. Bioelectron. 2000a;14:871–881. doi: 10.1016/s0956-5663(99)00055-x. [DOI] [PubMed] [Google Scholar]
- O'Connor SM, Stenger DA, Shaffer KM, Maric D, Barker JL, Ma W. Primary neural precursor cell expansion, differentiation and cytosolic Ca(2+) response in three-dimensional collagen gel. J. Neurosci. Methods. 2000b;102:187–195. doi: 10.1016/s0165-0270(00)00303-4. [DOI] [PubMed] [Google Scholar]
- O'Connor SM, Stenger DA, Shaffer KM, Ma W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci. Lett. 2001;304:189–193. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Neuronal specializations for the processing of interaural difference cues in the chick. Front. Neural Circuits. 2014;8 doi: 10.3389/fncir.2014.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Ikeda C, Uchida Y, Sakamoto Y, Miller F, Glacial F, Decleves X, Scherrmann J-M, Couraud P-O, Kubo Y, et al. Quantitative targeted absolute proteomic analysis of transporters, receptors and junction proteins for validation of human cerebral microvascular endothelial cell line hCMEC/D3 as a human blood-brain barrier model. Mol. Pharm. 2013;10:289–296. doi: 10.1021/mp3004308. [DOI] [PubMed] [Google Scholar]
- Olah M, Raj D, Brouwer N, De Haas AH, Eggen BJL, Den Dunnen WFA, Biber KPH, Boddeke HWGM. An optimized protocol for the acute isolation of human microglia from autopsy brain samples. Glia. 2012;60:96–111. doi: 10.1002/glia.21251. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy TJ, Lin HJ, Ma W. Functional synapse formation among rat cortical neurons grown on three-dimensional collagen gels. Neurosci. Lett. 2003;340:169–172. doi: 10.1016/s0304-3940(03)00083-1. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J. Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- Patterson J, Martino MM, Hubbell JA. Biomimetic materials in tissue engineering. Mater. Today. 2010;13:14–22. [Google Scholar]
- Pedron S, Becka E, Harley BAC. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials. 2013;34:7408–7417. doi: 10.1016/j.biomaterials.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Pehlivan SB. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm. Res. 2013;30:2499–2511. doi: 10.1007/s11095-013-1156-7. [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Phillips JB. Building stable anisotropic tissues using cellular collagen gels. Organogenesis. 2014;10:6–8. doi: 10.4161/org.27487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Poller B, Drewe J, Krähenbühl S, Huwyler J, Gutmann H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell. Mol. Neurobiol. 2010;30:63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M, Sherman LS. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front. Biosci. Sch. Ed. 2011;3:1165–1179. doi: 10.2741/218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GM, Tien J. Methods for forming human microvascular tubes in vitro and measuring their macromolecular permeability. Methods Mol. Biol. Clifton NJ. 2011;671:281–293. doi: 10.1007/978-1-59745-551-0_17. [DOI] [PubMed] [Google Scholar]
- Puschmann TB, de Pablo Y, Zandén C, Liu J, Pekny M. A novel method for three-dimensional culture of central nervous system neurons. Tissue Eng. Part C Methods. 2014;20:485–492. doi: 10.1089/ten.TEC.2013.0445. [DOI] [PubMed] [Google Scholar]
- Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys. J. 2002;83:1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J. Clin. Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon S. Brain-mapping projects to join forces. Nature. 2014 doi:10.1038/nature.2014.14871. [Google Scholar]
- Recknor JB, Recknor JC, Sakaguchi DS, Mallapragada SK. Oriented astroglial cell growth on micropatterned polystyrene substrates. Biomaterials. 2004;25:2753–2767. doi: 10.1016/j.biomaterials.2003.11.045. [DOI] [PubMed] [Google Scholar]
- Rho JY, Ashman RB, Turner CH. Young's modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993;26:111–119. doi: 10.1016/0021-9290(93)90042-d. [DOI] [PubMed] [Google Scholar]
- Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the Regenerative Microenvironment with Biomaterials. Adv. Healthc. Mater. 2013;2:57–71. doi: 10.1002/adhm.201200197. [DOI] [PubMed] [Google Scholar]
- Robertson MJ, Gip P, Schaffer DV. Neural stem cell engineering: directed differentiation of adult and embryonic stem cells into neurons. Front. Biosci. J. Virtual Libr. 2008;13:21–50. doi: 10.2741/2558. [DOI] [PubMed] [Google Scholar]
- Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JC, Blitz DM, Nusbaum MP. Convergent rhythm generation from divergent cellular mechanisms. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:18047–18064. doi: 10.1523/JNEUROSCI.3217-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Acheson A, Hall AK, Mann DM, Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988;240:53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- Saito T, Shibasaki K, Kurachi M, Puentes S, Mikuni M, Ishizaki Y. Cerebral capillary endothelial cells are covered by the VEGF-expressing foot processes of astrocytes. Neurosci. Lett. 2011;497:116–121. doi: 10.1016/j.neulet.2011.04.043. [DOI] [PubMed] [Google Scholar]
- Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999;13:2214–2224. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- Sanga S, Frieboes HB, Zheng X, Gatenby R, Bearer EL, Cristini V. Predictive oncology: multidisciplinary, multi-scale in-silico modeling linking phenotype, morphology and growth. NeuroImage. 2007;37:S120–S134. doi: 10.1016/j.neuroimage.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y, Shimizu F, Nakayama H, Abe M, Maeda T, Ohtsuki S, Terasaki T, Obinata M, Ueda M, Takahashi R, et al. Endothelial cells constituting blood-nerve barrier have highly specialized characteristics as barrier-forming cells. Cell Struct. Funct. 2007;32:139–147. doi: 10.1247/csf.07015. [DOI] [PubMed] [Google Scholar]
- Santaguida S, Janigro D, Hossain M, Oby E, Rapp E, Cucullo L. Side by side comparison between dynamic versus static models of blood-brain barrier in vitro: a permeability study. Brain Res. 2006;1109:1–13. doi: 10.1016/j.brainres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Schildge S, Bohrer C, Beck K, Schachtrup C. Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. JoVE. 2013 doi: 10.3791/50079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlachetzki JCM, Saliba SW, Oliveira ACP. Studying neurodegenerative diseases in culture models. Rev. Bras. Psiquiatr. São Paulo Braz. 1999. 2013;35(Suppl 2):S92–S100. doi: 10.1590/1516-4446-2013-1159. [DOI] [PubMed] [Google Scholar]
- Schneider G, Jordan D, Schwarz G, Bischoff P, Kalkman CJ, Kuppe H, Rundshagen I, Omerovic A, Kreuzer M, Stockmanns G, et al. Monitoring depth of anesthesia utilizing a combination of electroencephalographic and standard measures. Anesthesiology. 2014;120:819–828. doi: 10.1097/ALN.0000000000000151. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ, Rigor BM. Glia are the main source of lactate utilized by neurons for recovery of function posthypoxia. Brain Res. 1997;774:221–224. doi: 10.1016/s0006-8993(97)81708-8. [DOI] [PubMed] [Google Scholar]
- Schwartz PH, Bryant PJ, Fuja TJ, Su H, O'Dowd DK, Klassen H. Isolation and characterization of neural progenitor cells from post-mortem human cortex. J. Neurosci. Res. 2003;74:838–851. doi: 10.1002/jnr.10854. [DOI] [PubMed] [Google Scholar]
- Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- Selvaraj V. Differentiating human stem cells into neurons and glial cells for neural repair. Front. Biosci. 2012;17:65. doi: 10.2741/3916. [DOI] [PubMed] [Google Scholar]
- Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltzman M, Turner JN. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2003;11:186–188. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- Sherwood JK, Riley SL, Palazzolo R, Brown SC, Monkhouse DC, Coates M, Griffith LG, Landeen LK, Ratcliffe A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–4751. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Santangelo AM, Braesicke K, Agustín-Pavón C, Cockcroft G, Haggard M, Roberts AC. Individual differences in behavioral and cardiovascular reactivity to emotive stimuli and their relationship to cognitive flexibility in a primate model of trait anxiety. Front. Behav. Neurosci. 2014;8:137. doi: 10.3389/fnbeh.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Matsuda K, Ishii O, Terai H, Kaazempur-Mofrad M, Borenstein J, Detmar M, Vacanti JP. Endothelialized networks with a vascular geometry in microfabricated poly(dimethyl siloxane). Biomed. Microdevices. 2004;6:269–278. doi: 10.1023/B:BMMD.0000048559.29932.27. [DOI] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Sørensen A, Alekseeva T, Katechia K, Robertson M, Riehle MO, Barnett SC. Long-term neurite orientation on astrocyte monolayers aligned by microtopography. Biomaterials. 2007;28:5498–5508. doi: 10.1016/j.biomaterials.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Sorensen A, Moffat K, Thomson C, Barnett SC. Astrocytes, but not olfactory ensheathing cells or Schwann cells, promote myelination of CNS axons in vitro. Glia. 2008;56:750–763. doi: 10.1002/glia.20650. [DOI] [PubMed] [Google Scholar]
- Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W. Dexamethasone treatment reduces astroglia responses to inserted neuroprosthetic devices in rat neocortex. Exp. Neurol. 2005;194:289–300. doi: 10.1016/j.expneurol.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Van der Staay FJ, Arndt SS, Nordquist RE. Evaluation of animal models of neurobehavioral disorders. Behav. Brain Funct. BBF. 2009;5:11. doi: 10.1186/1744-9081-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenfeldt SE, LaPlaca MC. Variations in rigidity and ligand density influence neuronal response in methylcellulose-laminin hydrogels. Acta Biomater. 2011;7:4102–4108. doi: 10.1016/j.actbio.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavridis SI, Dehghani F, Korf H-W, Hailer NP. Characterisation of transverse slice culture preparations of postnatal rat spinal cord: preservation of defined neuronal populations. Histochem. Cell Biol. 2005;123:377–392. doi: 10.1007/s00418-004-0743-4. [DOI] [PubMed] [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J. Comp. Physiol. A. 2009;195:989–1009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- Stins MF, Prasadarao NV, Zhou J, Arditi M, Kim KS. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell. Dev. Biol. Anim. 1997;33:243–247. doi: 10.1007/s11626-997-0042-1. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sturrock RR. Quantitative changes in neuroglia in the white matter of the mouse brain following hypoxic stress. J. Anat. 1976;121:7–13. [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Gotoh T, Yagyu S, Kuroda H, Iehara T, Hosoi H, Ohta S, Ohira M, Nakagawara A. A MYCN-amplified cell line derived from a long-term event-free survivor among our sixteen established neuroblastoma cell lines. Cancer Lett. 2013;331:115–121. doi: 10.1016/j.canlet.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Sun H, Pang KS. Permeability, transport, and metabolism of solutes in Caco-2 cell monolayers: a theoretical study. Drug Metab. Dispos. Biol. Fate Chem. 2008;36:102–123. doi: 10.1124/dmd.107.015321. [DOI] [PubMed] [Google Scholar]
- Sundararaghavan HG, Monteiro GA, Firestein BL, Shreiber DI. Neurite growth in 3D collagen gels with gradients of mechanical properties. Biotechnol. Bioeng. 2009;102:632–643. doi: 10.1002/bit.22074. [DOI] [PubMed] [Google Scholar]
- Suttkus A, Rohn S, Jager C, Arendt T, Morawski M. Neuroprotection against iron-induced cell death by perineuronal nets - an in vivo analysis of oxidative stress. Am. J. Neurodegener. Dis. 2012;1:122–129. [PMC free article] [PubMed] [Google Scholar]
- Suttkus A, Rohn S, Weigel S, Glockner P, Arendt T, Morawski M. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death Dis. 2014;5:e1119. doi: 10.1038/cddis.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E, Nicholson C. Diffusion in Brain Extracellular Space. Physiol. Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács J, Metzger F. Morphological study of organotypic cerebellar cultures. Acta Biol. Hung. 2002;53:187–204. doi: 10.1556/ABiol.53.2002.1-2.18. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tan W, Desai TA. Microscale multilayer cocultures for biomimetic blood vessels. J. Biomed. Mater. Res. A. 2005;72:146–160. doi: 10.1002/jbm.a.30182. [DOI] [PubMed] [Google Scholar]
- Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydon PG, Kaplan DL. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13811–13816. doi: 10.1073/pnas.1324214111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey KE, McKay WB, Kakulas BA. Restorative neurology: consideration of the new anatomy and physiology of the injured nervous system. Clin. Neurol. Neurosurg. 2012;114:436–440. doi: 10.1016/j.clineuro.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Tao L, Nicholson C. Diffusion of albumins in rat cortical slices and relevance to volume transmission. Neuroscience. 1996;75:839–847. doi: 10.1016/0306-4522(96)00303-x. [DOI] [PubMed] [Google Scholar]
- Tate MC, Shear DA, Hoffman SW, Stein DG, LaPlaca MC. Biocompatibility of methylcellulose-based constructs designed for intracerebral gelation following experimental traumatic brain injury. Biomaterials. 2001;22:1113–1123. doi: 10.1016/s0142-9612(00)00348-3. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Rhee SW, Tu CH, Cribbs DH, Cotman CW, Jeon NL. Microfluidic multicompartment device for neuroscience research. Langmuir ACS J. Surf. Colloids. 2003;19:1551–1556. doi: 10.1021/la026417v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane AS, Rappold PM, Fujita T, Torres A, Bekar LK, Takano T, Peng W, Wang F, Rangroo Thrane V, Enger R, et al. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc. Natl. Acad. Sci. U. S. A. 2011;108:846–851. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian WM, Hou SP, Ma J, Zhang CL, Xu QY, Lee IS, Li HD, Spector M, Cui FZ. Hyaluronic acid-poly-D-lysine-based three-dimensional hydrogel for traumatic brain injury. Tissue Eng. 2005;11:513–525. doi: 10.1089/ten.2005.11.513. [DOI] [PubMed] [Google Scholar]
- Tien LW, Wu F, Tang-Schomer MD, Yoon E, Omenetto FG, Kaplan DL. Silk as a multifunctional biomaterial substrate for reduced glial scarring around brain-penetrating electrodes. Adv. Funct. Mater. 2013;23:3185–3193. [Google Scholar]
- Tomaselli KJ. Beta 1-integrin-mediated neuronal responses to extracellular matrix proteins. Ann. N. Y. Acad. Sci. 1991;633:100–104. doi: 10.1111/j.1749-6632.1991.tb15600.x. [DOI] [PubMed] [Google Scholar]
- Tontsch U, Bauer H-C. Glial cells and neurons induce blood-brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- Tsurkan MV, Chwalek K, Prokoph S, Zieris A, Levental KR, Freudenberg U, Werner C. Defined polymer-peptide conjugates to form cell-instructive starPEG-heparin matrices in situ. Adv. Mater. 2013;25:2606–2610. doi: 10.1002/adma.201300691. [DOI] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirc. N. Y. N 1994. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- Urich E, Patsch C, Aigner S, Graf M, Iacone R, Freskgård P-O. Multicellular self-assembled spheroidal model of the blood brain barrier. Sci. Rep. 2013;3:1500. doi: 10.1038/srep01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vepari C, Kaplan DL. Silk as a biomaterial. Prog. Polym. Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Vukasinovic J, Cullen DK, LaPlaca MC, Glezer A. A microperfused incubator for tissue mimetic 3D cultures. Biomed. Microdevices. 2009;11:1155–1165. doi: 10.1007/s10544-009-9332-6. [DOI] [PubMed] [Google Scholar]
- Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, Lathrop MJ, Wallis JD, Daly AB, Lam YW, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials. 2014;35:3281–3297. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg LU, Lind G, Almqvist PM, Kusk P, Tornøe J, Juliusson B, Söderman M, Selldén E, Seiger Å, Eriksdotter-Jönhagen M, et al. Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: a technology platform for restorative neurosurgery. J. Neurosurg. 2012;117:340–347. doi: 10.3171/2012.2.JNS11714. [DOI] [PubMed] [Google Scholar]
- Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- Wang C, Tong X, Yang F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol. Pharm. 2014;11:2115–25. doi: 10.1021/mp5000828. [DOI] [PubMed] [Google Scholar]
- Wang G, Ao Q, Gong K, Wang A, Zheng L, Gong Y, Zhang X. The effect of topology of chitosan biomaterials on the differentiation and proliferation of neural stem cells. Acta Biomater. 2010;6:3630–3639. doi: 10.1016/j.actbio.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Ward JM. Patch-clamping and other molecular approaches for the study of plasma membrane transporters demystified. Plant Physiol. 1997;114:1151–1159. doi: 10.1104/pp.114.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Nakamura M, Okano H, Toyama Y. Establishment of three-dimensional culture of neural stem/progenitor cells in collagen type-1 gel. Restor. Neurol. Neurosci. 2007;25:109–117. [PubMed] [Google Scholar]
- Weightman AP, Pickard MR, Yang Y, Chari DM. An in vitro spinal cord injury model to screen neuroregenerative materials. Biomaterials. 2014;35:3756–3765. doi: 10.1016/j.biomaterials.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Wesseling H, Chan MK, Tsang TM, Ernst A, Peters F, Guest PC, Holmes E, Bahn S. A combined metabonomic and proteomic approach identifies frontal cortex changes in a chronic phencyclidine rat model in relation to human schizophrenia brain pathology. Neuropsychopharmacology. 2013;38:2532–2544. doi: 10.1038/npp.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore SR, Neary JT, Kleitman N, Sanon HR, Benigno A, Donahue RP, Norenberg MD. Isolation and characterization of conditionally immortalized astrocyte cell lines derived from adult human spinal cord. Glia. 1994;10:211–226. doi: 10.1002/glia.440100308. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Fazakas C, Krizbai IA. In vitro models of the blood-brain barrier. Acta Neurobiol. Exp. (Warsz.) 2011;71:113–128. doi: 10.55782/ane-2011-1828. [DOI] [PubMed] [Google Scholar]
- Wilkins RH. NEUROSURGICAL CLASSIC. XVII. J. Neurosurg. 1964;21:240–244. doi: 10.3171/jns.1964.21.3.0240. [DOI] [PubMed] [Google Scholar]
- Neuhaus W, Lauer R, Oelzant S, Fringeli UP, Ecker GF, Noe CR. A novel flow based hollow-fiber blood-brain barrier in vitro model with immortalised cell line PBMEC/C1-2. J. Biotechnol. 2006;125:127–141. doi: 10.1016/j.jbiotec.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Woerly S, Marchand R, Lavallée C. Intracerebral implantation of synthetic polymer/biopolymer matrix: a new perspective for brain repair. Biomaterials. 1990;11:97–107. doi: 10.1016/0142-9612(90)90123-8. [DOI] [PubMed] [Google Scholar]
- Woerly S, Marchand R, Lavallée C. Interactions of copolymeric poly(glyceryl methacrylate)-collagen hydrogels with neural tissue: effects of structure and polar groups. Biomaterials. 1991;12:197–203. doi: 10.1016/0142-9612(91)90200-t. [DOI] [PubMed] [Google Scholar]
- Woerly S, Pinet E, de Robertis L, Van Diep D, Bousmina M. Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeuroGel). Biomaterials. 2001;22:1095–1111. doi: 10.1016/s0142-9612(00)00354-9. [DOI] [PubMed] [Google Scholar]
- Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood-brain barrier: an engineering perspective. Front. Neuroengineering. 2013;6 doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray LS, Rnjak-Kovacina J, Mandal BB, Schmidt DF, Gil ES, Kaplan DL. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials. 2012;33:9214–9224. doi: 10.1016/j.biomaterials.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Molnar P, Gregory C, Das M, Boland T, Hickman JJ. Electrophysiological characterization of embryonic hippocampal neurons cultured in a 3D collagen hydrogel. Biomaterials. 2009;30:4377–4383. doi: 10.1016/j.biomaterials.2009.04.047. [DOI] [PubMed] [Google Scholar]
- Xue Q, Liu Y, Qi H, Ma Q, Xu L, Chen W, Chen G, Xu X. A novel brain neurovascular unit model with neurons, astrocytes and microvascular endothelial cells of rat. Int. J. Biol. Sci. 2013;9:174–189. doi: 10.7150/ijbs.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell. Mol. Life Sci. CMLS. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Lai KWC, Xi N, Yang J. Development of automated patch clamp system for electrophysiology. 2013 IEEE International Conference on Robotics and Biomimetics (ROBIO) 2013:2185–2190. [Google Scholar]
- Zaman MH. The role of engineering approaches in analysing cancer invasion and metastasis. Nat. Rev. Cancer. 2013;13:596–603. doi: 10.1038/nrc3564. [DOI] [PubMed] [Google Scholar]
- Zamecnik J. The extracellular space and matrix of gliomas. Acta Neuropathol. (Berl.) 2005;110:435–442. doi: 10.1007/s00401-005-1078-5. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao Y, Yan S, Yang Y, Zhao H, Li M, Lu S, Kaplan DL. Preparation of uniaxial multichannel silk fibroin scaffolds for guiding primary neurons. Acta Biomater. 2012;8:2628–2638. doi: 10.1016/j.actbio.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Zheng YP, Mak AFT. Extraction of effective Young's modulus of skin and subcutaneous tissues from manual indentation data. Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 1997;5:2246–2249. 1997. [Google Scholar]
- Zhong Y, Bellamkonda RV. Dexamethasone-coated neural probes elicit attenuated inflammatory response and neuronal loss compared to uncoated neural probes. Brain Res. 2007;1148:15–27. doi: 10.1016/j.brainres.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Blewitt M, Hobgood A, Willits RK. Comparison of neurite growth in three dimensional natural and synthetic hydrogels. J. Biomater. Sci. Polym. Ed. 2013;24:301–314. doi: 10.1080/09205063.2012.690277. [DOI] [PubMed] [Google Scholar]
- Zschüntzsch J, Schütze S, Hülsmann S, Dibaj P, Neusch C. Heterologous expression of a glial Kir channel (KCNJ10) in a neuroblastoma spinal cord (NSC-34) cell line. Physiol. Res. Acad. Sci. Bohemoslov. 2013;62:95–105. doi: 10.33549/physiolres.932264. [DOI] [PubMed] [Google Scholar]
- Zustiak SP, Pubill S, Ribeiro A, Leach JB. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds as a cell delivery vehicle: characterization of PC12 cell response. Biotechnol. Prog. 2013;29:1255–1264. doi: 10.1002/btpr.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]