Abstract
The generation of CD8+ T cells by vaccination represents an important goal for protective immunity to infectious pathogens. It is thus of utmost importance to understand the mechanisms involved in the generation of optimal CD8+ T cell responses. The forkhead box O (FoxO) family of transcription factors plays a crucial role in cellular responses to environmental change. Among them, FoxO3 is critically involved in the regulation of cellular proliferation, apoptosis, metabolism, and stress resistance to withdrawal of nutrients or cytokine growth factors. Since the role of FoxO3 has been poorly studied in the immune system, here we have evaluated its involvement in the CD8+ T cell response. We observe that CD8+ T cells deficient for FoxO3 undergo a significantly greater primary expansion than their wild-type counterparts in response to both infectious (vaccinia virus) or non-infectious (non replicating cellular vaccine) immunogens, resulting in a larger cohort of cells following contraction. These survivors, however, do not undergo a greater secondary response than wild type. Taken together, our data show that FoxO3 is a negative regulator of the CD8+ T cells response, specifically during the primary expansion.
Introduction
Understanding the mechanism(s), which promote effective CD8+ T cell responses, is essential to the design of new vaccines against infectious diseases and cancer. CD8+ T cells play an essential role in the clearance of either infected or abnormal cells through a variety of effector mechanisms1, 2, 3. This is preceded by a robust primary expansion in which rare precursors expand up to 10,000 fold4. After infection is brought under control, the majority of the cells will die5 (90–95%), with the remaining cells forming a long-lived memory pool, which can self-renew and rapidly produce new effector cells upon antigen re-encounter.
In the recent years the role of cellular metabolism in regulating CD8+ T cell function and memory has come to the forefront. Recent studies have shown that metabolism is important to regulate CD8+ T cell fate, survival and death6, 7, 8, 9. Several molecules have been implicated in T cell metabolism. The phosphatidyl-inositol-3-OH kinase (PI(3)K) pathway and subsequently Akt are activated after TCR triggering or cytokine stimuli such as IL-2 or IL-15. Akt activation is due to its phosphorylation status, and mTORC2 is involved in the phosphorylation of one of the Akt serine, whereas Akt activates mTORC1. Akt has been shown to negatively regulate FoxO molecules6, 10, 11, preventing their entry into the nucleus and their function as transcription factors.
The FoxO transcription factors are mammalian orthologs of the Caenorhabditis elegans longevity protein Daf-16 that are widely conserved through evolution and have been shown to play critical roles in cellular responses to environmental changes12, 13. Three of the four known FoxO orthologs (FoxO1, 3, and 4) have overlapping targets of transcriptional regulation and appear to be widely expressed and similarly regulated14. FoxO1 and FoxO3 are the main isoforms expressed in immune cells, but their expression levels differ between organs of the immune system and between lymphoid and myeloid cell types: FoxO1 expression is higher in spleen and lymph nodes as compared to FoxO3, which is the main transcript detected in the thymus and bone marrow15. FoxO3 plays a crucial role in regulating cellular proliferation, apoptosis, metabolism, and stress resistance to withdrawal of nutrients or cytokine growth factors (reviewed in10). Like FoxO1 and −4, the functions of FoxO3 are regulated post-transcriptionally, largely through phosphorylation16.
Although a role for FoxO1 in the CD8+ T cell memory formation has been established,17, 18, 19, 20 little is known about the function of FoxO3 in the CD8+ T cell response.
Information on the role of FoxO3 in immune functions has emerged from the study of genetically deficient (knockout) mice21. This study did not find evidence of immunological abnormalities in unmanipulated FoxO-deficient mice, either by histology or enumeration of T and B cells21. However, acute infection of FoxO3−/− mice with lymphocytic choriomeningitis virus (LCMV) or Vesicular Stomatitis Virus (VSV) revealed a more than 3 fold increase in the number of antigen-specific CD4+ and CD8+ T cells. The increased expansion of the primary responder lymphocytes coincided with dysregulated cytokine production by dendritic cells (DC)21, and highlights a key role for FoxO3 in the regulation of antigen presenting cell (APC) function, which was confirmed by subsequent studies22, 23, 24. More recently, a cell-intrinsic role for FoxO3 in regulating the CD8+ T cell response to infectious pathogens such as LCMV25, 26 or listeria27 was identified. This was based on the observation that CD8+ T cells lacking FoxO3, mounted proportionally larger responses, which was attributed to decreased apoptosis either during the primary expansion phase25, 26 or during the contraction phase27. Of critical relevance to memory function, however, none of these studies assessed whether secondary responses were influenced by a cell-intrinsic role of FoxO3. We have now examined whether FoxO3 regulates both primary and memory (recall) responses. We find that, in response to both inflammatory and non-inflammatory immunogens, FoxO3-deficient CD8+ T cells undergo a greater primary but comparable secondary response to wild-type (WT) controls. Therefore FoxO3 regulates primary but not memory CD8+ T cell responses.
Results
FoxO3 regulates the expansion of primary CD8+ effector T cells
To evaluate the intrinsic role of FoxO3 in the CD8+ T cell response, we co-transferred a small number of WT and FoXO3−/− CD8+ T cells, both expressing a transgenic TCR (OT-I) specific for the Chicken Ovalbumin (OVA), into WT recipient mice. Hosts were then immunized with either a non-replicating cellular vaccine (Actm-OVA Kb−/− splenocytes)28 or replicating recombinant vaccinia virus containing OVA (vaccinia-OVA). Seven days after the immunization, at the peak of the primary response, we evaluated the expansion of both populations in lymphoid and non-lymphoid organs and observed that the CD8+ T cells lacking FoxO3 expanded significantly more than their WT counterparts (Figures 1A, B and C). However, the percentages of so called memory precursor effector cells defined by the expression of CD127 and lack of KLRG1 expression and short-lived effector cells (SLEC, CD127−KLRG1+)29 were comparable between the WT and FoxO3−/− CD8+ OT-I primary responder cells 7 days post-immunization (Figure 2A). This was confirmed by the fact that we did not see, any difference in the expression of T-bet (expressed by effector cells) or Eomes (expressed by memory cells) (Figure 2B). These data demonstrate that FoxO3 negatively regulates the overall expansion of primary CD8+ effector T cells.
Figure 1.
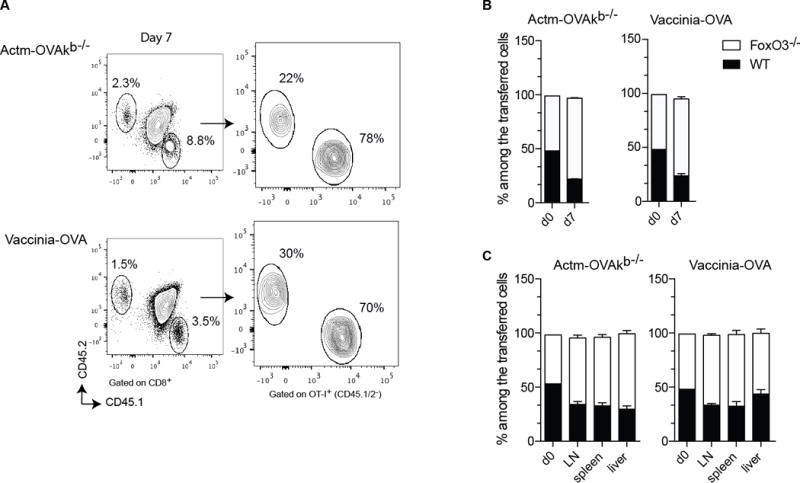
FoxO3 regulates the expansion of CD8+ T cells. A–C 500 OT-I CD45.1 FoxO3−/− and 500 OT-I CD45.2 cells were co-injected into WT (CD45.1/2) mice one day before immunization. The mice were infected with 1×106 vaccinia-OVA or with 5×106 Actm-OVAkb−/− splenocytes. In A and B the responses were measured in the blood at day 7. In C the ratio of OT-I was measured at day 7 in different organs. Data are representative of groups of 4 to 5 mice and represents the most representative result of 2 to 3 independent experiments.
Figure 2.
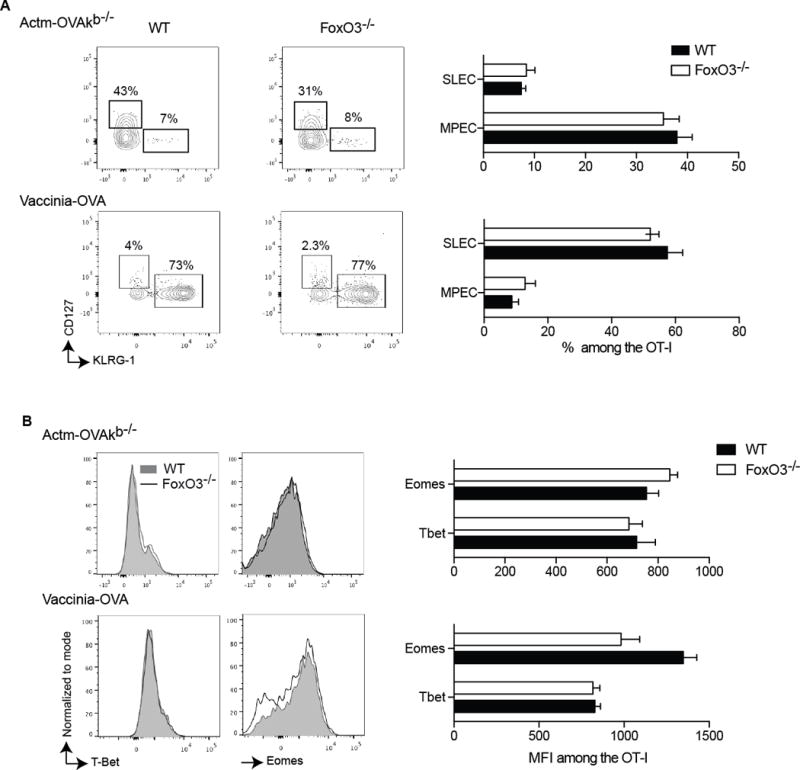
FoxO3 does not regulate the number of memory precursor effector cells (MPEC). A−B 500 OT-I CD45.1 FoxO3−/− and 500 OT-I CD45.2 were co- injected into WT (CD45.1/2) mice one day before immunization. The mice were infected with 1×106 vaccinia-OVA or with 5×106 Actm-OVAkb−/− A represents the FACS plots and the percentage of OT-I expressing CD127+KLRG1− (MPEC) or CD127−KLRG1+ (SLEC) at day 7 post immunization. C represents the histograms and the MFI for the transcription factors Tbet and Eomes at day 7- post immunization within the OT-I. Data are representative of groups of 4 to 5 mice and represents the most representative result of 2 to 3 independent experiments.
FoxO3 does not influence the functional differentiation of primary CD8+ effector T cells
To investigate if in addition to the quantity, FoxO3 also negatively regulates the quality of the primary response, cytokine production was measured 7days post immunization. There was no difference noted however, in the proportion of cells able to produce IFNγ or IFNγ and TNF-α, between the WT and the FoxO3−/− CD8 OT-I T cells (Figure 3A). Moreover there was no difference in the amount of IL-2 production (Figure 3B), indicating that FoxO3 does not control the cytokine production of primary CD8+ effector T cells.
Figure 3.
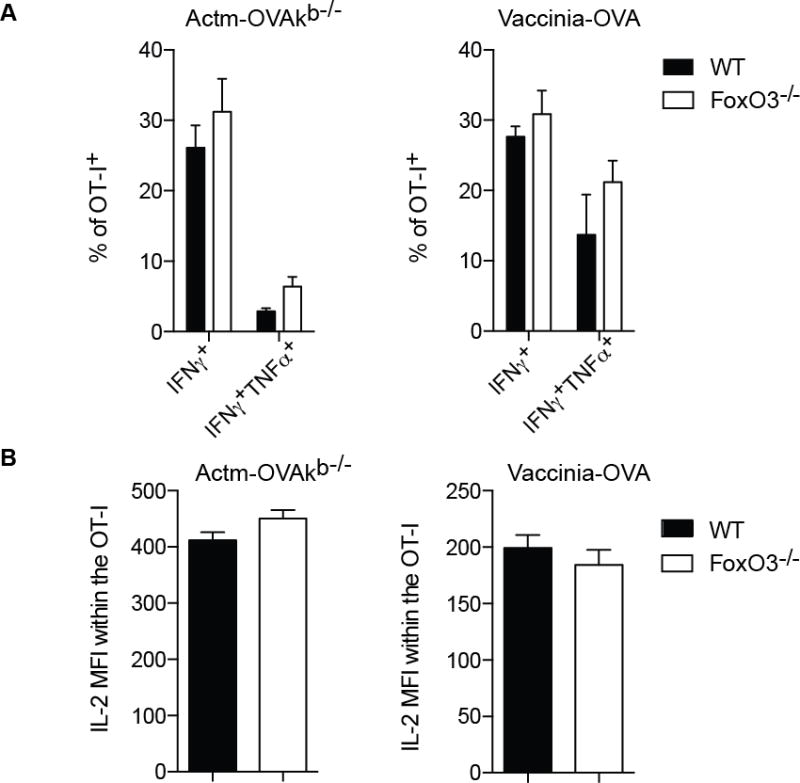
FoxO3 does not influence cytokine production. A–B 500 OT-I CD45.1 FoxO3−/− and 500 OT-I CD45. 2 were coinjected into WT (CD45.1/2) mice one day before immunization. The mice were infected with 1×106 vaccinia-OVA or with 5×106 Actm-OVAkb−/−. The cytokine response was measured in the spleen at day 7. A represents the percentage of OT-I producing IFNγ or IFNγTNF. B represents the MFI of IL-2 within the OT-I. Data are representative of groups of 4 to 5 mice and represents the most representative result of 2 to 3 independent experiments.
FoxO3 does not control the initial contraction or expansion phase of secondary responder CD8+ T cells
To investigate if FoxO3 controls the magnitude of the memory response through enhanced cell death, we analyzed the contraction phase that follows the primary expansion. In contrast to the enhanced expansion of the FoxO3−/− primary responder OT-I CD8+ T cells, we did not observe any significant change in the proportion between the WT and FoxO3 responder cells during the contraction phase, indicating that FoxO3 does not promote cell death or counteract survival of the primary responder cells (Figure 4A). To investigate if, similar to the primary expansion, FoxO3 also negatively regulates the magnitude of the secondary response; recipient mice were rechallenged with Listeria-OVA 43 days after the initial priming and analyzed 5 days later (Figures 4A and B). WT and FoxO3−/− OT-I responder cells expanded at an equal rate during the secondary response, indicating that in contrast to the primary response, FoxO3 does not control the magnitude of the secondary expansion.
Figure 4.
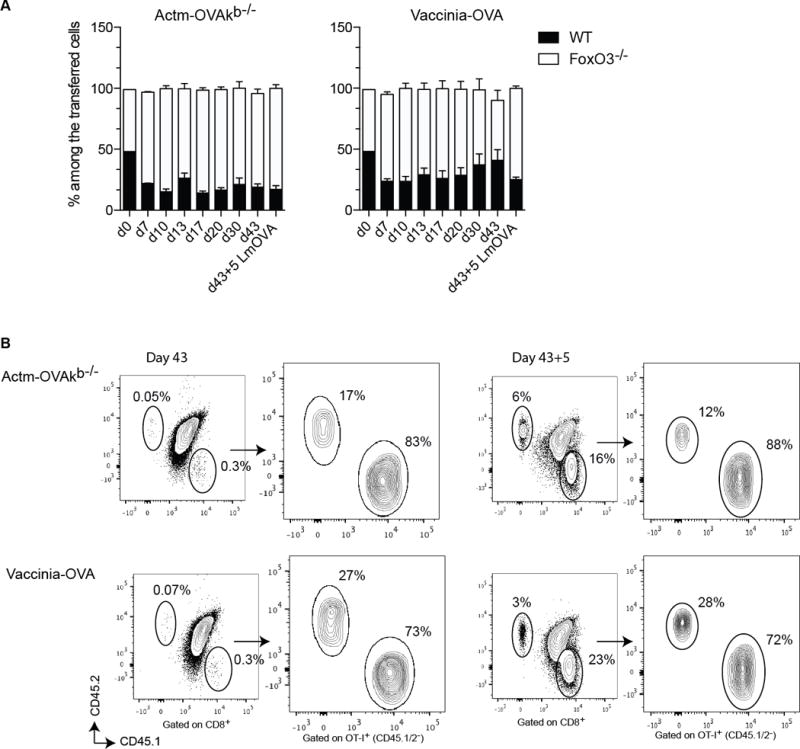
FoxO3 does not regulate the contraction and secondary expansion. A–B 500 OT-I CD45.1 FoxO3−/− and 500 OT-I CD45.2 were co-injected into WT (CD45.1/2) mice one day before immunization. The mice were infected with 1×106 vaccinia-OVA or with 5×106 Actm-OVAkb−/−. In A the response was measured in the blood at different time points during priming, contraction, and memory. The mice were then rechallenged at day 43 with 5000 Lm-OVA and the response was measured in the blood 5 days later. B represents the FACS plot during memory and secondary response. Data are representative of groups of 4 to 5 mice and represents the most representative result of 2 to 3 independent experiments.
Discussion
In this study we have investigated the impact of FoxO3 during the CD8+ T cell response. Using co-transfer of antigen-specific WT and FoxO3−/− CD8+ T cells we were able to show that FoxO3 plays a cell intrinsic role in the primary expansion of the effector cells that follows the first encounter with their specific antigen. On the contrary, we did not find any involvement of FoxO3 in the functional differentiation of the primary effector cells nor did we find an effect of FoxO3 during the contraction phase or memory formation.
Firstly we found that the FoxO3 deficient CD8 primary responder cells expanded significantly more than the cells expressing FoxO3, indicating a negative regulatory function of FoxO3 in the primary expansion of the CD8+ T cell. These results together with previous findings showing that FoxO3 induces the expression of the pro-apoptotic molecules Bim and Puma in CD8+ T cells (27), suggest that FoxO3 promotes cell death during the initial primary response. We did however, not observe any difference in the quality of the effector response and both WT and FoxO3−/− CD8+ T cells were able to produce IL-2 and IFNγ and TNF-α to the same extent, implying that FoxO3 does not affect the quality of the effector CD8+ T cells generated following infectious or non-infectious immunization.
Surprisingly and in contradiction to two previously published studies25, 26, 27, we did not find any effect of FoxO3 during the contraction phase or the generation of the secondary response. The differences could be due first to the fact that we are using a FoxO3 deficient strain of different origin compared to the other studies, where in the Sullivan et al. and the Tzelepis et al., studies a FoxO3a-trap was used whereas we used the FoxO3Kca 21(FoxO3−/−), but since in both cases the FoxO3 protein is absent, it should not explain the differences in our results. In addition, different immunization strategies were used in the published studies compared to our approach here, which might contribute to the discrepancy. To control for this, however, we included 2 types of immunization strategies, a cellular vaccine and an infectious pathogen. Since both approaches rendered the same results, we concluded that the different immunization strategies are likely not the cause of the different outcome of the studies. Another possibility is the difference in the number of cells that was transferred which was much larger in the published studies as compared to our study here. It is well established that the precursor frequency has an impact on the efficiency and the nature of the memory generation28, 30. In fact most facets of the CD8+ T cell response, including kinetics, proliferation, surface molecule expression, effector function and the efficiency of memory generation are substantially altered when the initial number of TCR transgenic T cells is sufficiently high to inhibit the endogenous CD8+ T cell response to the same Ag. Those data suggest that the use of TCR transgenic T cells to model the endogenous CD8+ T cell response may only be reliable under conditions where these cells represent only a fraction of the endogenous repertoire. In our case using a low precursor frequency, we noted no difference in the ratio of WT compared to FoxO3 deficient cells during the contraction or upon a secondary challenge, implying that FoxO3 did not affect those phases. Altogether, our results indicate that FoxO3 is not essential for the generation of CD8+ memory T cells. This is in contrast to FoxO1, which was shown to promote CD8+ central memory formation17, 18, 19, 20 by repressing T-bet and the effector differentiation. Our results are also in line with the notion that there is no compensation by FoxO3 when FoxO1 is absent or conversely. Thus it seems that FoxO transcription factors have differential roles in the CD8+ T cell response where FoxO3 regulates the expansion during priming whereas FoxO1 repress the effector function and participates in the central memory formation.
Material and methods
Mice
C57BL/6, were purchased from The Jackson Laboratory (Bar Harbor, Maine). OT-I CD45.1+ and Act-mOVA/Kb−/− mice on a C57BL/6J background have been previously described31. The OT-I FoxO3−/− CD45.1+ strain was generated by intercross between FoxO3Kca and OT-I CD45.1+ mice. Mice were maintained by in-house breeding at the La Jolla Institute for Allergy and Immunology under specific pathogen-free conditions in accordance with guidelines by the Association for Assessment and Accreditation of Laboratory Animal Care International.
T cell preparations
CD8+ T cell transfer
OT-I CD45.2+ and OT-I FoxO3−/− CD45.1+, were harvested from blood and the number of OT-I cells was determined by counting and by FACS staining for V2+V5+. WT mice CD45.1/2 were co-transferred with 500 OT-I CD45.2+ and 500 OT-I FoxO3−/− CD45.1+cells by retro-orbital (RO) injection.
In vivo experiments
OT-I cells were transferred respectively into C57BL/6J CD45.1/2 mice one day prior to immunization. The mice were immunized with either 5×106 Act-mOVA/Kb−/− splenocytes by RO injection (prime) and re-challenged by RO injection with 5000 cfu Listeria monocytogenes-OVA, or were inoculated ip with 1×106 pfu vaccinia-OVA (priming), and boosted by RO injection with 5000 cfu Listeria monocytogenes-OVA.
Ex-vivo restimulation and antibody staining
Cytokine production in CD8+ T cells was assessed as described: splenocytes from immunized mice were resuspended in IMDM medium (Invitrogen) supplemented with 8% FCS (Omega Scientific), 1% L-glutamine (Invitrogen), 100 μg/ml streptomycin, 100 U/ml penicillin, and 50 μM 2-ME (Sigma-Aldrich). Cells (1–2 × 106) were plated in 96-well round bottom plates in case of Act-mOVA/Kb−/− and Vaccinia-OVA stimulation in 200 μl medium plus OVA257–264 peptide (SIINFEKL) at 1 μg/ml in presence of GolgiPlug (BD Biosciences) for 5 h at 37°C. Cells were stained with anti-CD8 (5H10, PE-TR), CD44 (IM7, Alexa-Fluor 700), CD45.1 (A20, Pacific Blue) and CD45.2 (104, Percp-Cy5.5) followed by fixation with Cytofix-Cytoperm (BD Biosciences) for 20 min at 4°C. Fixed cells were subjected to intracellular cytokine staining in Perm/Wash buffer (BD Biosciences) for 30 min at 4°C. Anti-TNF (MP6-XT22, Pe-cy7), IFN- (XMG1.2, APC) and IL-2 (JES6-5H4, PE). The antibodies were purchased from BD Pharmingen, eBiosciences or biolegend. The samples were acquired on a LSRII flow cytometer (Becton Dickinson) and analyzed using FlowJo software.
Transcription factor and Flow cytometry analysis
Cells were stained with CD8 (5H10, PE-TR), CD44 (IM7, Alexa-Fluor 700), CD45.1 (A20, Pacific Blue), CD45.2 (104, Percp-Cy5.5 or FITC), CD62L (MEL-14, APC eFluor780), KLRG-1 (2F1, PeCy7), CD127 (A7R34, APC). In the case of transcription factor study the staining was followed by fixation with Cytofix-Cytoperm (BD Biosciences) for 20 min at 4°C. Fixed cells were subjected to intracellular staining in Perm/Wash buffer (BD Biosciences) for 30 min at 4°C with Eomes (Dan11mag, PercP-eFluor710) and Tbet (eBio4B10, APC). The antibodies were purchased from BD Pharmingen, eBiosciences or Biolegend. The samples were acquired on an LSRII flow cytometer (Becton Dickinson) and analyzed using FlowJo software.
Statistical analysis
Data were analyzed using PRISM software (GraphPad, San Diego, CA). Differences between groups were examined for statistical significance using an unpaired two-tailed Student’s t test. Unless otherwise indicated, data represent the mean ± SEM, with * = p<0.05 considered statistically significant.
Acknowledgments
We thank Cheryl Kim and Kurt Van Gunst for assistance with flow cytometry. We thank Dr. Stephen M. Hedrick for providing the FoxO3Kca mice. This work was supported by National Institutes of Health grants AI070010 [Schoenberger]. This is manuscript number 1713 from La Jolla Institute for Allergy and Immunology, La Jolla, CA.
Abbreviations
- APC
Antigen Presenting Cell
- DC
Dendritic Cells
- Eomes
Eomesodermin
- FoxO
Forkhead box O
- −/−
knockout
- Listeria
Listeria monocytogenes
- LCMV
lymphocytic choriomeningitis virus
- MPEC
memory precursor effector cells
- OVA
chicken Ovalbumine
- PI(3)K
phosphatidyl-inositol-3-OH kinase
- SLEC
short-lived effector cells
- Tbet
T-box expressed in T cells
- VSV
Vesicular Stomatitis Virus
- WT
wild-type
Footnotes
Authorship: S.T., A.L. and S.F. designed and performed the experiments and analyzed the data. S.P.S. provided the funds and resources to pursue these study. S.F. wrote the manuscript. S.T., A.L. and S.P.S. provided significant assistance in writing and editing the manuscript.
Disclosures: The authors declare no conflicts of interest.
References
- 1.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290(5495):1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 2.Berke G. The CTL’s kiss of death. Cell. 1995;81(1):9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 3.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 4.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3(7):619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 6.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. 2011;11(2):109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5(11):844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 8.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs SR, Rathmell JC. Lymphocyte selection by starvation: glucose metabolism and cell death. Trends Immunol. 2006;27(1):4–7. doi: 10.1016/j.it.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 11.Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34(2):224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenyon C. A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann N Y Acad Sci. 2010;1204:156–162. doi: 10.1111/j.1749-6632.2010.05640.x. [DOI] [PubMed] [Google Scholar]
- 13.Lam EW, Francis RE, Petkovic M. FOXO transcription factors: key regulators of cell fate. Biochem Soc Trans. 2006;34(Pt 5):722–726. doi: 10.1042/BST0340722. [DOI] [PubMed] [Google Scholar]
- 14.Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27(16):2258–2262. doi: 10.1038/onc.2008.29. [DOI] [PubMed] [Google Scholar]
- 15.Dejean AS, Hedrick SM, Kerdiles YM. Highly specialized role of Forkhead box O transcription factors in the immune system. Antioxid Redox Signal. 2011;14(4):663–674. doi: 10.1089/ars.2010.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boccitto M, Kalb RG. Regulation of Foxo-dependent transcription by post-translational modifications. Curr Drug Targets. 2011;12(9):1303–1310. doi: 10.2174/138945011796150316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. Immunity. 2012;36(3):374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MV, Ouyang W, Liao W, Zhang MQ, Li MO. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity. 2013;39(2):286–297. doi: 10.1016/j.immuni.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess Michelini R, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med. 2013;210(6):1189–1200. doi: 10.1084/jem.20130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tejera MM, Kim EH, Sullivan JA, Plisch EH, Suresh M. FoxO1 controls effector-to-memory transition and maintenance of functional CD8 T cell memory. Journal of immunology. 2013;191(1):187–199. doi: 10.4049/jimmunol.1300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, et al. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10(5):504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JC, Espeli M, Anderson CA, Linterman MA, Pocock JM, Williams NJ, et al. Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell. 2013;155(1):57–69. doi: 10.1016/j.cell.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, et al. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121(4):1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Bronte V. Tolerogenic pDCs: spotlight on Foxo3. J Clin Invest. 2011;121(4):1247–1250. doi: 10.1172/JCI57190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan JA, Kim EH, Plisch EH, Peng SL, Suresh M. FOXO3 regulates CD8 T cell memory by T cell-intrinsic mechanisms. PLoS Pathog. 2012;8(2):e1002533. doi: 10.1371/journal.ppat.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan JA, Kim EH, Plisch EH, Suresh M. FOXO3 regulates the CD8 T cell response to a chronic viral infection. Journal of virology. 2012;86(17):9025–9034. doi: 10.1128/JVI.00942-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzelepis F, Joseph J, Haddad EK, Maclean S, Dudani R, Agenes F, et al. Intrinsic role of FoxO3a in the development of CD8+ T cell memory. Journal of immunology. 2013;190(3):1066–1075. doi: 10.4049/jimmunol.1200639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat Immunol. 2011;12(9):908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104(28):11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26(6):827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T-cells via CD27–CD70 interactions. Nat Commun. 2012;3:948. doi: 10.1038/ncomms1948. [DOI] [PMC free article] [PubMed] [Google Scholar]


