Abstract
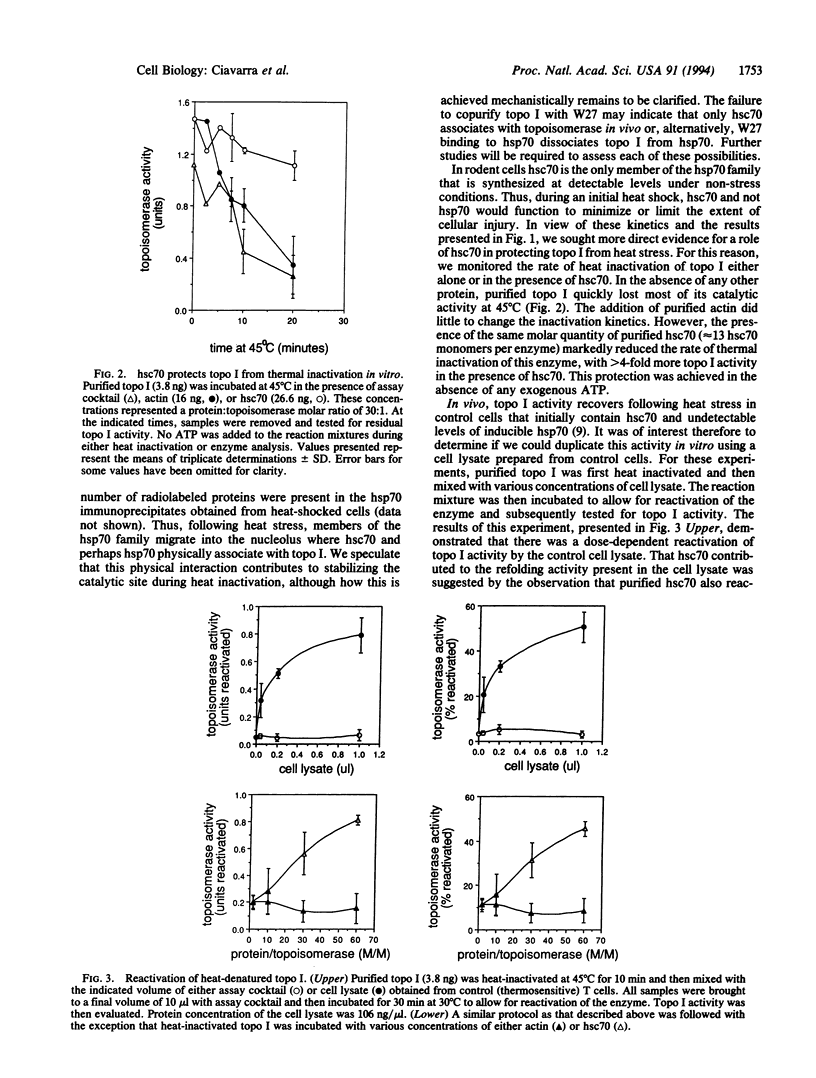
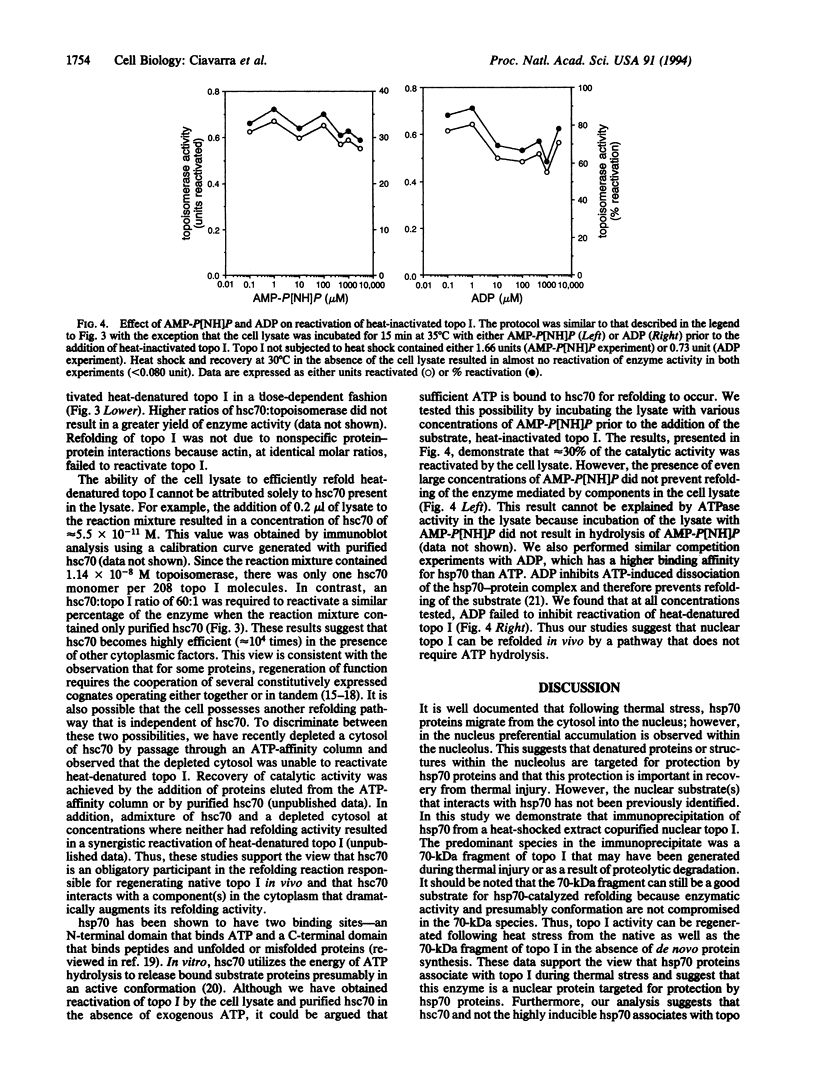
We previously demonstrated that in murine T cells thermotolerance correlated with heat shock protein 70 (hsp70) synthesis and protection of nuclear type I topoisomerase (topo I). Topo I activity returned to normal levels following heat stress even in cells not rendered thermotolerant by a prior heat shock. Recovery of topo I activity was not dependent on de novo protein synthesis, suggesting that the cell possesses a pathway(s) for refolding this nuclear protein. In this report we demonstrate that topo I and hsc70, the constitutively produced member of the hsp70 family, associated in vivo during heat stress. That this association may play a physiologically important role in protecting topo I activity from heat stress was suggested by the observation that hsc70 protected topo I from heat inactivation in vitro. hsc70 but not actin also reactivated previously heat-denatured topo I in a dose-dependent fashion. However, refolding of heat-denatured topo I by purified hsc70 was inefficient relative to a hsc70-containing cell lysate. Protection from heat inactivation as well as reactivation by hsc70 did not require exogenous ATP. Similarly, reactivation by the cell lysate was not inhibited by ADP or a nonhydrolyzable analogue of ATP. Thus, our studies suggest that nuclear topo I complexes with hsc70 during heat stress, which may explain, at least in part, why hsp70 proteins accumulate in the nucleus, particularly the nucleolus. This interaction may limit heat-induced protein damage and/or accelerate restoration of protein function in an ATP-independent reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Buchner J., Schmidt M., Fuchs M., Jaenicke R., Rudolph R., Schmid F. X., Kiefhaber T. GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry. 1991 Feb 12;30(6):1586–1591. doi: 10.1021/bi00220a020. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Chen B. F., Castora F. J. Mechanism of ATP inhibition of mammalian type I DNA topoisomerase: DNA binding, cleavage, and rejoining are insensitive to ATP. Biochemistry. 1988 Jun 14;27(12):4386–4391. doi: 10.1021/bi00412a027. [DOI] [PubMed] [Google Scholar]
- Cheng M. Y., Hartl F. U., Martin J., Pollock R. A., Kalousek F., Neupert W., Hallberg E. M., Hallberg R. L., Horwich A. L. Mitochondrial heat-shock protein hsp60 is essential for assembly of proteins imported into yeast mitochondria. Nature. 1989 Feb 16;337(6208):620–625. doi: 10.1038/337620a0. [DOI] [PubMed] [Google Scholar]
- Ciavarra R. P., Duvall W., Castora F. J. Induction of thermotolerance in T cells protects nuclear DNA topoisomerase I from heat stress. Biochem Biophys Res Commun. 1992 Jul 15;186(1):166–172. doi: 10.1016/s0006-291x(05)80789-2. [DOI] [PubMed] [Google Scholar]
- Ciavarra R. P., Simeone A. T lymphocyte stress response. I. Induction of heat shock protein synthesis at febrile temperatures is correlated with enhanced resistance to hyperthermic stress but not to heavy metal toxicity or dexamethasone-induced immunosuppression. Cell Immunol. 1990 Sep;129(2):363–376. doi: 10.1016/0008-8749(90)90212-a. [DOI] [PubMed] [Google Scholar]
- Ciavarra R. P., Simeone A. T lymphocyte stress response. II. Protection of translation and DNA replication against some forms of stress by prior hyperthermic stress. Cell Immunol. 1990 Nov;131(1):11–26. doi: 10.1016/0008-8749(90)90231-f. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Johnston R. N., Kucey B. L. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science. 1988 Dec 16;242(4885):1551–1554. doi: 10.1126/science.3201244. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Lazarus G. M., Henrich J. P., Kelly W. G., Schmitz S. A., Castora F. J. Purification and characterization of a type I DNA topoisomerase from calf thymus mitochondria. Biochemistry. 1987 Sep 22;26(19):6195–6203. doi: 10.1021/bi00393a036. [DOI] [PubMed] [Google Scholar]
- Li G. C., Li L., Liu R. Y., Rehman M., Lee W. M. Heat shock protein hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2036–2040. doi: 10.1073/pnas.89.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Pfund W. P., Mehta V. B., Trask D. K. Eukaryotic type I topoisomerase is enriched in the nucleolus and catalytically active on ribosomal DNA. EMBO J. 1985 May;4(5):1237–1243. doi: 10.1002/j.1460-2075.1985.tb03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleros D. R., Welch W. J., Fink A. L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Schmitt B., Buhre U., Vosberg H. P. Characterisation of size variants of type I DNA topoisomerase isolated from calf thymus. Eur J Biochem. 1984 Oct 1;144(1):127–134. doi: 10.1111/j.1432-1033.1984.tb08440.x. [DOI] [PubMed] [Google Scholar]
- Skowyra D., Georgopoulos C., Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990 Sep 7;62(5):939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- Subjeck J. R., Sciandra J. J., Johnson R. J. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol. 1982 Aug;55(656):579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Mizzen L. A. Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes. J Cell Biol. 1988 Apr;106(4):1117–1130. doi: 10.1083/jcb.106.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]




