Abstract
Mycosis fungoides (MF) and Sezary syndrome (SS) are two major forms of cutaneous T-cell lymphoma (CTCL) characterized by resistance to apoptosis. A central pathway for T-cell apoptosis is activation-induced cell death (AICD) which is triggered through the T-cell receptor (TCR). This results in upregulation of FAS-ligand (FASL) and subsequent apoptosis through the FAS death receptor pathway. It has been known for more than a decade that TCR signaling is defective in CTCL; however, the underlying mechanism has not been apparent. In this report, we show that the E3 ubiquitin ligase, c-CBL, is over-expressed in CTCL and that its knockdown overcomes defective TCR signaling resulting in phosphorylation of PLCg1, calcium influx, ROS generation, up-regulation of FASL and extrinsic pathway apoptosis in CTCL cells expressing adequate FAS. In CTCL cells with suboptimal FAS expression, FAS can be upregulated epigenetically by derepression of the FAS promoter using methotrexate (MTX) which we showed previously has activity as a DNA methylation inhibitor. Using these combined strategies, FAS-low as well as FAS-high CTCL cells can be killed effectively.
INTRODUCTION
In this study, we use the term, CTCL, to refer specifically to MF/SS (Olsen et al., 2011). Based on a variety of phenotypic, genetic and functional investigations, there is ample evidence that CTCL is characterized by resistance to apoptosis (Meech et al., 2001; Braun et al., 2007; Contassot et al., 2008; Wu et al., 2009; Klemke et al., 2009; Wu and Wood., 2011; Stutz et al., 2012). CTCL cells often express only low levels of extrinsic apoptotic pathway death receptors such as FAS and are less responsive to apoptotic triggers than normal T cells. AICD is a key pathway for apoptosis among CD4+/CD45RO+ memory T cells, the subset from which both MF (effector memory differentiation) and SS (central memory differentiation) are derived. Following short term antigenic TCR stimulation of normal CD4+ T cells, FAS is up-regulated but FASL is not. The activated T cells are deleted by a passive form of intrinsic (mitochondrial pathway) apoptosis triggered by IL2 withdrawal following antigen clearance. In contrast, chronic TCR stimulation normally results in up-regulation of both FAS and FASL with subsequent AICD involving the extrinsic FAS death receptor pathway. For several years, it has been recognized that TCR signaling is defective in CTCL such that AICD is impeded (Fargnoli et al., 1997; Meech et al., 2001; Klemke et al., 2009). It has been observed that TCR-associated tyrosine kinases are not properly activated upon TCR engagement and that downstream signaling eventuating in FASL upregulation does not occur. Nevertheless, an underlying mechanism for these defects has not been elucidated.
One factor that regulates TCR signaling is c-CBL, a member of the Casitas B-lineage Lymphoma protein family and a ring-type E3 ubiquitin ligase that dampens TCR function by interacting with TCR-associated tyrosine kinases and promoting their degradation (Schmidt and Dikic, 2005; Swaminathan and Tsygankov, 2006; Loeser et al., 2007; Paolino and Penninger, 2010; Qiao et al., 2013). The TCR is expressed on the cell surface in association with CD3 proteins to form the TCR/CD3 complex. During AICD, engagement of TCR/CD3 normally leads to activation of proximate tyrosine kinases and a subsequent downstream cascade involving phosphorylation of phospholipase C gamma-1 (PLC-g1), calcium mobilization, generation of reactive oxygen species (ROS) and FASL up-regulation (Klemke et al., 2009).
In this report, we show that c-CBL is overexpressed in CTCL and that its knockdown restores signaling through PLC-g1 leading to upregulation of FASL and apoptosis in CTCL cells that express adequate FAS. In CTCL cells with low FAS expression (a common MF/SS phenotype), FAS can be up-regulated epigenetically by derepression of the FAS promoter using methotrexate (MTX). We showed previously that in addition to its role as an S-phase inhibitor that blocks purine synthesis, MTX has activity as a DNA methylation inhibitor by blocking synthesis of S-adenosylmethionine, the principal methyl donor for DNA methyltransferases (Wu and Wood, 2011). Using these combined strategies to modulate both FAS and FASL, FAS-low as well as FAS-high CTCL cells can be killed effectively.
RESULTS
c-CBL protein and transcript are over-expressed in CTCL
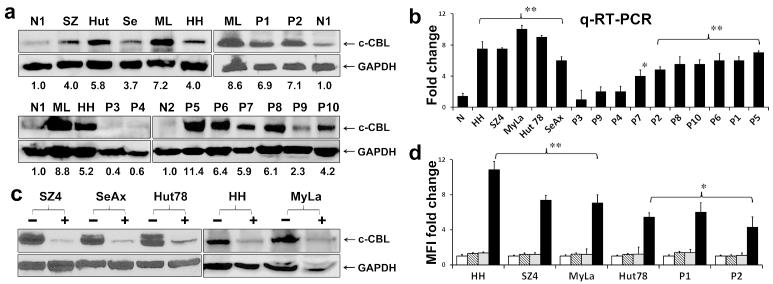
In order to assess cellular levels of c-CBL protein and mRNA, we analyzed CTCL cells using immunoblotting and quantitative RT/PCR, respectively. As shown in Figure 1a, 1b and Supplemental Figure 1a, compared to normal CD4+ T-cells in blood, baseline c-CBL protein and transcript were increased more than 3-fold in all five CTCL lines derived from patients with MF (MyLa, HH) or SS (SeAx, Hut78, SZ4). Among leukemic cells from SS patients, c-CBL protein and transcript were increased more than 3-fold in 10/14 and 7/10 cases, respectively, with good correlation between individual protein and transcript levels. Lesional skin biopsies (10 patch/plaque MF, 3 tumor MF, 2 SS) were assesses by quantitative multispectral image analysis of immunoperoxidase stained paraffin sections. Relative to reactive tonsil controls, c-CBL expression was 3-fold greater in the CTCL specimens regardless of lesion type (Supplemental Figures 1b and 1c).
Figure 1. c-CBL is over-expressed in CTCL and its inhibition induces FASL upregulation.
a. c-CBL protein. Grouped immunoblots show that relative to the highest expression among normal CD4+ blood T-cells (N1, N2), all five CTCL lines and 7/10 SS blood samples (P1,2,5-8,10) expressed more than 3-fold greater c-CBL protein levels as assessed by scanning densitometry. Numbers in the immunoblots refer to fold-differences in normalized c-CBL protein levels relative to control CD4+ T-cells. MyLa (ML), Hut-78 (Hut), HH (H), SZ4 (SZ), SeAx (Se). GAPDH is loading control.
b. c-CBL mRNA. Quantitative RT/PCR shows that relative to normal CD4+ blood T-cells pooled from three donors (N), all five CTCL lines and the same 7/10 SS blood samples (P1,2,5-8,10) expressed more than 3-fold greater c-CBL transcript levels. * p<0.05; ** p<0.01 in triplicate samples.
c. Grouped immunoblots show effective knockdown of c-CBL in CTCL lines using siRNA (+) relative to nonspecific siRNA (−). GAPDH is loading control.
d. Flow cytometry histogram of mean fluorescence intensities (MFI) shows marked upregulation of FASL expression (Y axis) induced by c-CBL knockdown in CTCL lines and SS leukemic blood samples (P1, P2). Bars represent isotype control (white), no treatment (striped), NS siRNA (gray), c-CBL siRNA (black). *p<0.05; **p < 0.01 for c-CBL siRNA relative to NS siRNA controls in triplicate samples.
c-CBL knockdown induces up-regulation of FASL
Given the frequently high expression of c-CBL in CTCL, we next explored the effects of reducing c-CBL on the expression of FASL. As shown in Figure 1c, we were able to significantly decrease c-CBL protein expression in CTCL lines using siRNA technology. As shown by flow cytometry in Figure 1d, this resulted in a significant increase in FASL expression by CTCL lines and leukemic cells from two SS patients (P1 and P2).
c-CBL knockdown induces apoptosis in CTCL cells that is enhanced by MTX
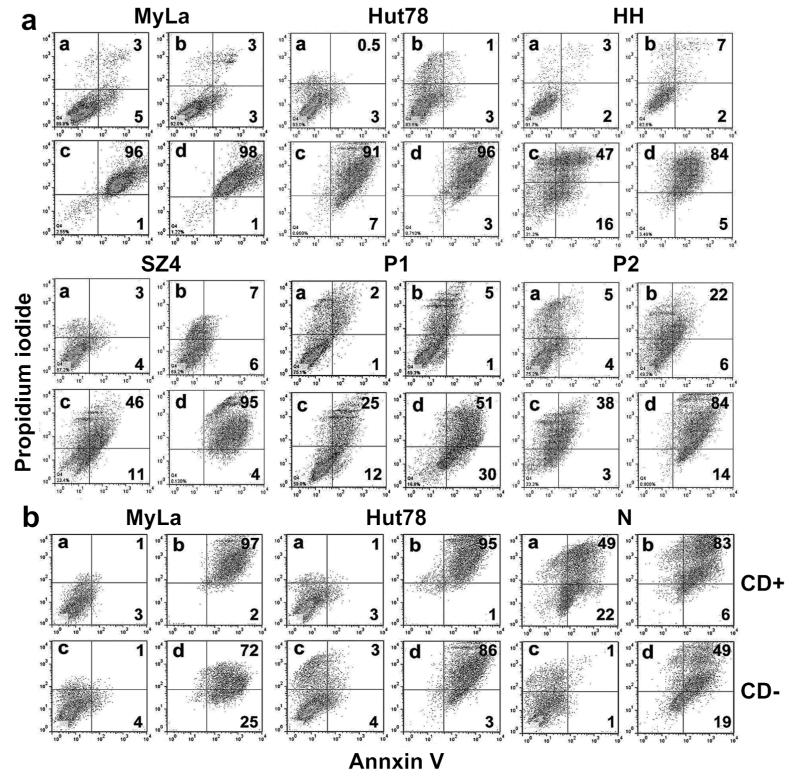
We determined the impact of c-CBL knockdown-induced FASL upregulation on apoptosis in CTCL lines and leukemic tumor cells from two SS patients using Annexin V/PI flow cytometry. Controls included isotype antibody, no treatment and nonsense (ns) siRNA. As shown in Figure 2a, Annexin V-positive apoptotic cells (the two right-hand quadrants in each plot) increased dramatically from the 3-9% range with ns siRNA to the 37-98%% range with c-CBL siRNA.
Figure 2. c-CBL inhibition induces apoptosis in CTCL and MTX enhances this effect.
a. Annexin V/propidium iodide flow cytometry dot plots show marked induction of apoptosis (right two quadrants of each panel) after c-CBL knockdown. In CTCL lines (MyLa, Hut-78) which express high baseline FAS death receptor, the effect is maximal with c-CBL knockdown alone. In the other samples, treatment with MTX (which upregulates low baseline FAS death receptor expression) enhances the effect of c-CBL knockdown in CTCL lines (HH, SZ4) and SS leukemic blood samples (P1, 2). a) NS siRNA, b) MTX, c) c-CBL siRNA, d) MTX plus c-CBL siRNA. Results representative of triplicate experiments.
b. MyLa and Hut-78 CTCL lines show marked apoptosis after c-CBL siRNA knockdown regardless of whether cells are treated with anti CD3/CD28 antibodies (CD+ vs CD−) but are insensitive to antibody treatment alone. In contrast, normal CD4+ blood T-cells (N) are sensitive to antibody treatment alone as well as c-CBL knockdown. a) and c) NS siRNA, b) and d) c-CBL siRNA. Results representative of duplicate experiments.
MTX upregulates FAS expression epigenetically by inhibiting promoter methylation and derepressing FAS expression (Wu and Wood, 2011). As shown in Figure 2a, when MTX was combined with c-CBL siRNA knockdown, apoptosis was increased to the 81- 99% range. The impact of c-CBL siRNA alone versus MTX plus c-CBL siRNA was less in MyLa (97% vs 99%) and Hut-78 (98% vs 99%) compared to HH (63% vs 89%) and SZ4 (57% vs 99%) because MyLa and Hut-78 have lower promoter methylation and higher baseline FAS expression than HH and SZ4 (Wu et al., 2009; Wu et al., 2011; Wu and Wood, 2011). In regard to potential clinical relevance, the impact of combination therapy was >2-fold in the SS leukemic cells where apoptosis increased from 37% to 81% (P1) and from 41% to 98% (P2).
We determined the effect of MTX on c-CBL expression by quantitative RT/PCR and could detect no significant difference in c-CBL transcript levels among five CTCL cell lines or three SS leukemic blood samples. These findings were confirmed by only minimal reductions in c-CBL protein levels detected by immunoblotting in three CTCL lines (Supplemental Figure 2). These data are consistent with our other evidence that MTX enhances apoptosis induced by c-CBL knockdown mainly by increasing FAS which is then available to interact with the increased FASL resulting from c-CBL knockdown.
Impact of antibody pretreatment on c-CBL knockdown in CTCL and normal T-cells
We determined whether treatment with anti-CD3 and anti-CD28 antibodies was required to induce apoptosis in FAS-high CTCL lines and normal CD4+ T cells subjected to c-CBL siRNA knockdown. Representative findings are shown in Figure 2b. CTCL cells were resistant to CD3/CD28 stimulation (3%-4% apoptosis with ns siRNA control) while normal T cells were much more sensitive (71% apoptosis). In contrast, CTCL cells were more sensitive to c-CBL siRNA knockdown in the absence of CD3/CD28 stimulation (97%-98%) than were normal T-cells (68%). This was also reflected in the pace of apoptosis. After 96 hours of knockdown, CTCL cells had more Annexin V/propidium iodide double-positive, late apoptotic cells in the right upper quadrant of each panel (72%-86%) than did normal T cells (50%).
Apoptosis induced by c-CBL knockdown is mediated by the FAS pathway
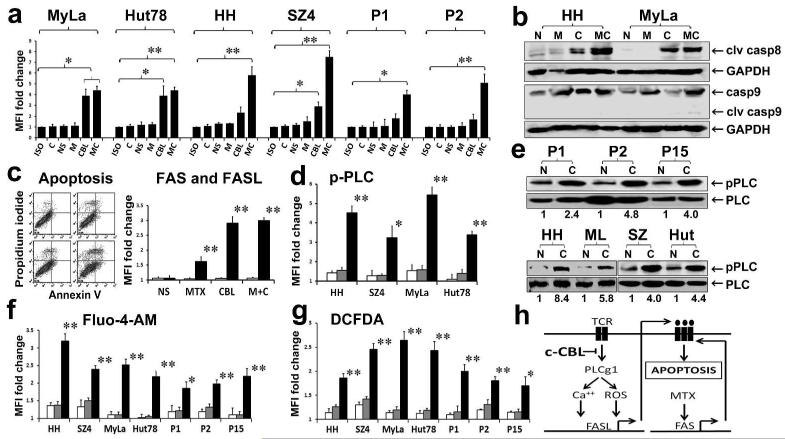
Given the fact that c-CBL knockdown significantly increased FASL expression (Figure 1d), we suspected that the associated increase in apoptosis (Figure 2) was due to extrinsic pathway apoptosis. As shown in Figure 3a, cleaved caspase 8 was significantly increased in multiple CTCL lines and SS leukemic cells as assessed by flow cytometry. Simultaneous measurement of cleaved caspase 9 using flow cytometry showed minimal or no increases (data not shown). These caspase 8 and 9 findings were confirmed by Western blotting in MyLa and HH (Figure 3b). This is consistent with activation of the extrinsic rather than intrinsic (mitochondrial) apoptotic pathway (Stutz et al., 2012). Several families of death receptors (e.g. FAS, TRAIL, TNF) can contribute to the generation of cleaved caspase 8 via the extrinsic apoptotic pathway. To determine the specific role of the FAS pathway in apoptosis induced by c-CBL knockdown and MTX, we tested the SeAx CTCL line. SeAx is FAS null because it lacks FAS genes (Wu et al., 2011), yet it does express TRAIL death receptors (Braun et al., 2007). As shown in Figure 3c, FAS expression remained undetectable and apoptosis was low (1-10%) under all conditions despite a marked increase in FASL expression. These findings indicate that c-CBL knockdown is not cytotoxic generally and that the marked extrinsic pathway apoptosis it typically induces in CTCL cells in association with FASL upregulation probably requires expression of the FAS death receptor. This is also supported by our prior studies showing that when SeAx cells are transfected with a FAS expression vector, they are readily killed by exogenous FASL (Wu et al., 2009).
Figure 3. c-CBL inhibition in CTCL induces FAS pathway apoptosis and downstream TCR signaling.
a. Flow cytometry histogram of mean fluorescence intensities (MFI) shows marked upregulation of cleaved caspase 8 expression (Y axis) induced by c-CBL knockdown in CTCL lines and SS leukemic blood samples (P1, 2). Bars represent isotype control (Iso), no treatment (C), NS siRNA (NS), MTX (M), c-CBL siRNA (CBL) and MTX plus c-CBL siRNA (MC). Analogous to the induction of apoptosis (Figure 3), samples with suboptimal baseline FAS death receptor expression (HH, SZ4, P1, P2) showed enhanced cleavage of caspase 8 when MTX was combined with c-CBL knockdown. *p<0.05; **p < 0.01 relative to NS siRNA controls in triplicate samples.
b. Grouped immunoblots show marked increase in cleaved caspase 8 but minimal change in cleaved caspase 9 under similar conditions to those described in A. NS siRNA (N), MTX (M), c-CBL siRNA (C), MTX plus c-CBL siRNA (MC).
c. (Left). Annexin V/propidium iodide flow cytometry dot plots show no induction of apoptosis (right two quadrants of each panel) after c-CBL knockdown in the SeAx CTCL line which lacks the gene for the FAS death receptor. a) NS siRNA, b) MTX, c) c-CBL siRNA, d) MTX plus c-CBL siRNA. Results representative of triplicate experiments. (Right). Flow cytometry histogram of mean fluorescence intensities (MFI) shows marked upregulation of FASL (black bars) but not FAS (white bars) induced by c-CBL knockdown in CTCL line, SeAx. Histogram shows fold change compared to the “no treatment” control which is set as 1. FAS levels were always null, so the white bars were at the X axis but are shown slightly larger to be visible. NS siRNA (NS), MTX (MTX), c-CBL siRNA (CBL), MTX plus c-CBL siRNA (M+C). **p<0.01 relative to NS siRNA controls in triplicate samples.
d. Flow cytometry histogram of mean fluorescence intensities (MFI) shows marked upregulation of phospho-PLC-g1 induced by c-CBL knockdown. Fold change is shown with the isotype control value set as 1. Bars show no treatment (striped), NS siRNA (gray) and c-CBL siRNA (black). *p<0.05; **p < 0.01 relative to NS siRNA controls in triplicate samples.
e. Grouped immunoblots show 2-8 fold increased phospho-PLC-g1/total PLCg1 ratios (numbers) after c-CBL siRNA knockdown (C) relative to NS siRNA control (N) set as 1. For Figs. 3E, 3F and 3G, samples include four CTCL lines (HH, MyLa, SZ4, Hut-78) and primary tumor cells from 3 SS patients (P1, P2, P15).
f. Flow cytometry histogram of fluo-4-AM mean fluorescence intensities (MFI) show that calcium flux is enhanced by c-CBL knockdown. Fold change is shown with the isotype control value set as 1. Bars show no treatment (striped), NS siRNA (gray) and c-CBL siRNA (black). **p<0.01 relative to NS siRNA controls in triplicate samples.
g. Flow cytometry histogram of DCFDA mean fluorescence intensities (MFI) show that ROS generation is enhanced by c-CBL knockdown. Fold change is shown with the isotype control value set as 1. Bars show no treatment (striped), NS siRNA (gray) and c-CBL siRNA (black).
**p<0.01 relative to NS siRNA controls in triplicate samples.
h. In CTCL, the TCR signaling cascade that normally leads to FASL upregulation is blocked by high c-CBL levels. When c-CBL is inhibited, signaling is restored and FASL increases. This leads to apoptosis if there is adequate FAS death receptor expression. If FAS is low, then MTX can be used to derepress the FAS promoter leading to FAS upregulation and subsequent apoptosis. With this approach, both FAS-high and FAS-low CTCL cells can be killed.
c-CBL knockdown enhances signaling downstream of the TCR that results in FASL upregulation
There are several known steps in the signaling cascade that links TCR engagement to FASL upregulation. These include phosphorylation of PLC-g1 at Tyr 783, calcium ion influx and generation of ROS. We used immunoblotting and flow cytometry to determine the effects of c-CBL knockdown on these parameters. As shown in Figure 3d, siRNA knockdown of c-CBL increased PLC-g1 phosphorylation in all four CTCL lines tested. This increase was confirmed by Western blotting in these four cell lines and primary tumor blood samples from three SS patients (Figure 3e). The phospho-PLCg1/total PLCg1ratio increased 2-8 fold (median 4 fold). Similarly, induction of calcium ion influx (Figure 3f) and ROS (Figure 3g) also occurred in all four CTCL lines and tumor cells from all three SS cases tested, as assessed by Fluo-4-AM flow cytometry and DCFDA flow cytometry, respectively.
DISCUSSION
FAS expression in T-cells is inducible by a wide variety of stimuli including MTX (Wu and Wood, 2011); however, expression of FASL is tightly controlled (Meech et al., 2001) and as shown in this report and elsewhere, CTCL cells generally express only very low levels of it (Stutz et al., 2012; Wu et al., 2009). Therefore, our current findings provide an approach for the upregulation of this missing trigger for the FAS apoptotic pathway. In this study, we showed that the E3 ubiquitin-ligase, c-CBL, is often overexpressed in CTCL at both the protein and mRNA levels. This suggests a transcriptional regulatory defect although the specific etiology of c-CBL dysregulation remains to be determined. Known mutations of CBL proteins are believed to reduce rather than enhance the ubiquitin-mediated down-regulation of activated protein tyrosine kinases. These kinases are then available to drive cell proliferation and promote neoplastic transformation (Saito et al. 2012). The distribution of germline and somatic mutations are similar and cluster in the linker or ring finger domains. Germline and/or somatic mutations have been reported in chronic myelomonocytic, juvenile myelomonocytic and acute lymphoblastic leukemias as well as in lung cancers (Lo et al. 2011). CBL mutations can activate the RAS pathway. Patients with germline CBL mutations have been grouped together with those diagnosed as Noonan syndrome and related disorders under the umbrella of so-called “RASopathies”.
Consistent with the known function of c-CBL in the degradation of receptor-associated tyrosine kinases (Loeser et al., 2007; Paolino and Penninger, 2010; Qiao et al., 2013), we considered the possibility that some neoplasms might exhibit enhanced rather than diminished c-CBL function. Specifically, we postulated that c-CBL hyperactivity might help explain the long-recognized defective TCR signaling characteristic of CTCL cells (Fargnoli et al., 1997; Meech et al., 2001; Klemke et al., 2009). Furthermore, given the importance of intact TCR signal transduction to the induction of FASL during AICD, we also postulated that c-CBL abnormalities might play a central role in the well-known resistance of CTCL cells to apoptosis.
Our data support these hypotheses as depicted in Figure 3h. Knockdown of c-CBL using siRNA technology resulted in major increases in FASL expression among all CTCL cells tested. Several hallmarks of TCR-mediated signal transduction that lead to FASL upregulation were also enhanced. These included PLC-g1 phosphorylation, calcium ion influx and generation of ROS. This was associated functionally with the restoration of apoptosis in CTCL cells with adequate FAS expression. Among CTCL cells with suboptimal FAS expression, MTX augmented apoptosis. We demonstrated previously that MTX enhances FAS expression in CTCL cell lines and leukemic cells by inhibiting methylation of the FAS promoter and thereby derepressing gene expression (Wu and Wood, 2011). This mechanism of action for MTX appears to result from inhibition of the synthesis of S-adenosylmethionine, the main methyl donor used by DNA methyltransferases. Therefore, either alone or in combination with MTX, c-CBL knockdown was able to overcome apoptotic resistance in all CTCL cells tested regardless of their baseline FAS expression level.
Our experiments showed that treatment with anti-CD3 and anti-CD28 antibodies is not required for c-CBL knockdown to induce apoptosis in CTCL cells. Furthermore, normal T cells were less sensitive to the pro-apoptotic effects of c-CBL knockdown than CTCL cells, suggesting relative tumor selectivity of this approach. At present, there are no well characterized small molecule inhibitors specific for c-CBL. It is possible that agents affecting other E3 ubiquitin ligases or the proteasome pathway more broadly might affect the function of c-CBL. Existing inhibitors such as thalidomide, lenalidomide and bortezomib have already shown some efficacy in CTCL (Querfeld et al., 2011; Jain et al., 2012) and additional agents are being developed (Lydeard and Harper, 2010). Our current findings might help explain the mechanism of action of these drugs in CTCL. Histone deacetylase inhibitors like tenovin-1 can also indirectly affect the function of some E3 ubiquitin ligases (Chen et al., 2007). Our data suggest that an appropriate small molecule inhibitor of c-CBL, either alone or in combination with MTX, might provide an effective approach to the treatment of CTCL. Because MTX’s ability to inhibit DNA methylation is shared by other folate antagonists, currently approved drugs such as pralatrexate and pemetrexed might also be of value in this therapeutic context. Our prior work also showed that interferon-alpha increased FAS expression in CTCL cells by a STAT-1 mediated mechanism distinct from the demethylating action of MTX (Wu et al., 2011) and that their combined effects on FAS-mediated apoptotic sensitivity were synergistic (Wu and Wood, 2011). Therefore, addition of this agent to a multidrug regimen might also prove beneficial in some cases.
In a larger context, our c-CBL knockdown data provide proof-of-principle that, whether by targeting c-CBL or other mechanisms, upregulation of FASL is an effective strategy for killing CTCL tumor cells driven by various proliferative signals such as the well documented constitutive activation of NF-kB (Sors et al., 2008). The physiologic balance between apoptosis and proliferation in normal T-cells is disrupted in CTCL by constitutive over-activity of both c-CBL and proliferative stimulation. This leads to blocked AICD and unchecked proliferation. However, when c-CBL is blocked, FASL is upregulated. AICD can then occur despite constitutively active proliferative drivers.
Aside from c-CBL, there are several alternative approaches that might be exploited to upregulate FASL. It has been reported that IL-2 plus PKC activators (PMA or bryostatin) that act downstream of the TCR can induce leukemic CTCL cells to express FASL and undergo apoptosis ex-vivo (Meech et al., 2001). Similarly, UV bherapy and photodynamic therapy might also be able to overcome defective TCR signaling by inducing ROS and upregulating FASL through a JNK-Jun pathway. This mechanism for FASL upregulation has been reported in squamous cell carcinomas (Ahmad et al., 2000; Ali et al., 2002; Furre et al., 2006). However, AP1 transcription factors containing c-Jun repress FAS expression. Therefore, concomitant therapy with MTX or other DNA methylation inhibitors might be important for ensuring the presence of sufficient FAS to interact with FASL and trigger apoptosis. In fact, our recent studies show that PDT induces FAS-ligand in CTCL and that MTX+PDT induces both FAS and FAS-ligand resulting in significantly enhanced apoptosis relative to PDT alone (Salva et al., 2014). Lastly, it has been shown that nonsteroidal anti-inflammatory agents such as diclofenac can promote extrinsic pathway apoptosis by decreasing the inhibitory factor, c-FLIP (Braun et al., 2012).
MATERIALS AND METHODS
CTCL cell lines, SS blood specimens and MF/SS skin lesions
Human CTCL lines derived from patients with MF (MyLa, HH) or SS (SZ4, Hut-78, SeAx) were studied. All have been described previously (Wu et al., 2009; Wu and Wood, 2011). Cells were cultured under standard conditions and harvested by centrifugation. All clinical samples were obtained with institutional review board approval. All patients gave written informed consent and all protocols adhered to the Declaration of Helsinki principles. Blood mononuclear cells were obtained from 15 SS patients (P1-P15). This included 6 men and 9 women: 2 stage IIIB, 12 stage IVA, 1 stage IVB. In all cases, the majority of cells were tumor cells as assessed by V-beta TCR antibodies, CD4+26− phenotype and/or Sezary preparations (11 in the 86-99% range; 4 in the 61-71% range). Lesional skin biopsies were obtained from 8 men and 7 women: 10 patch/plaque MF (2 stage IA, 5 stage IB, 3 stage IIB), 3 tumor MF (all stage IIB), 2 SS (stages IVA and IVB). Samples were obtained prior to, or one month off therapy. Multispectral image analysis using the Nuance computerized microscope system was employed for quantitative measurement of in-situ c-CBL protein expression in these biopsies as described previously by us (Wu et al., 2014).
In some experiments, cells were stimulated with a combination of monoclonal antibodies directed against CD3 (OKT3; eBioscience; San Diego, CA and CD28 (CD28.2; BD Biosciences; San Jose, CA) prior to other interventions. Cell culture plates were pre-coated with anti-CD3 antibody (30ug/ml) for 24 hours at 4°C. Cells then were stimulated with plate-bound anti-CD3, soluble anti-CD28 (3ug/ml) and goat anti-mouse (2.5ug/ml) antibodies (BD Biosciences; San Jose, CA) for 24 hours. In some experiments, cells were treated with MTX which was obtained from MP Biomedicals (Santa Ana, California), dissolved in DMSO and diluted to 10 μM in culture medium.
Flow cytometry
Expression of FAS, FASL, phospho PLC-g1 and cleaved caspase 8 was assessed by flow cytometry. Cells were stained with FITC conjugated antibody against FAS/CD95 (DX2), biotin conjugated (NOK-1) or unconjugated (G247-4) antibody against FAS-ligand/CD178 (BD Biosciences,San Jose, CA), anti-phospho-PLC-g1 (Tyr783) and anti-cleaved caspase-8 (18C8) (Cell Signaling; Beverly, MA). Isotype-matched mAbs of irrelevant specificity were used as negative controls. Briefly, 2 × 105 lymphoid cells were washed twice with phosphate-buffered saline (PBS), and blocked with 1:10 normal goat serum in phosphate-buffered saline for 20 minutes. For phospho-PLC-g1 and cleaved caspase 8 detection, cells were fixed by Cytofix Buffer and permeablized by Perm Buffer III (BD Biosciences, San Jose, CA). Cells were then immunostained for 30 minutes at room temperature and washed twice with PBS. If needed, secondary staining with FITC-conjugated avidin or goat anti-mouse IgG (BD Biosciences, San Jose, CA) was then performed followed by further washing. Cells were then resuspended in FACS buffer (2% BSA in PBS), and analyzed with a FACSCalibur bench top flow cytometer (BD Biosciences, San Jose, CA). Data analysis was performed using FlowJo software (Treestar, Ashland, OR).
For Annexin V/PI apoptosis analysis, control and treated cells were collected, washed with PBS and then resuspended in 100 μl binding buffer (0.1 M Hepes, pH 7.4; 1.4 M NaCl; 25 mM CaCl2) and stained with FITC conjugated Annexin V and propidium iodide (BD Biosciences, CA) as per vendor’s guidelines. After staining, flow cytometric analysis was performed as described above. Annexin V single-positive cells were interpreted as early apoptotic cells (right lower quadrant of dot plots). Annexin V/PI double-positive cells were interpreted as late apoptotic cells (right upper quadrant of dot plots).
Calcium mobilization and reactive oxygen species (ROS) assays
To assess calcium ion flux, cells were stained with Fluo-4-AM dye, and to assess generation of reactive oxygen species (ROS), cells were stained with DCFDA and subjected to flow cytometric analysis as described above. For Fluo-4-AM staining, cells were washed and resuspended in PBS, and Fluo-4-AM was added to a final concentration of 1uM. Cells then were incubated in room temperature for one hour. For DCFDA staining, DCFDA was directly added into 2 ml of cell culture to the final concentration of 5uM and incubated in 37°C for 30 minutes. Stained cells were washed and analyzed by flow cytometry.
Immunoblotting
Following treatments, cells were washed with ice-cold PBS and lysed with RIPA buffer. For immunoblot analysis, 30 μg of each protein sample was subjected to SDS-PAGE and transferred onto a nitrocellulose membrane. Blots were exposed to anti-c-CBL (D4E10), cleaved caspase 8 (18C8), caspase 9 (C9), PLC-g1 (D9H10) and phospho-PLC-g1 (Tyr 783) primary antibodies (Cell Signaling; Beverly, MA) and HRP conjugated appropriate secondary antibodies (Cell Signaling; Danvers, MA), followed by enhanced chemiluminescent detection (Thermo Fisher Scientific, Inc., Rockford, IL) using the Fotodyne digital imaging system (Fotodyne, Hartland, WI). Loading control antibody was anti-GAPDH (D16H11; Cell Signaling; Beverly, MA). Numbers in immunoblot lanes represent scanning densitometry relative to controls.
Quantitative RT/PCR
RNA was isolated with Trizol reagent (Invitrogen, CA), treated with DNase (Promega, WI) and first strand cDNA created with M-MLV reverse transcriptase (Promega, WI) according to vendor’s protocol. Quantitative RT/PCR was performed with SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA). Relative c-CBL mRNA expression was calculated using the ΔΔCT method with GAPDH as an endogenous control. The primer sequences and conditions for quantitative RT-PCR are shown below:
| Sequence (5′ → 3′) | Length | Tm | Location | |
|---|---|---|---|---|
| Fw c-CBL Primer | GTGATCCCTGGACAGGAAGA | 20 | 60.0 | 1959-1979 |
| Rev c-CBL Primer | CATTGGCAGATGAGGAAGGT | 20 | 62.4 | 2156-2176 |
| Fw GAPDH Primer | TGTGGGCATCAATGGATTTGG | 21 | 60.9 | 231-251 |
| Rev GAPDH Primer | ACACCATGTATTCCGGGTCAAT | 22 | 61.4 | 346-325 |
c-CBL knockdown
Knockdown of c-CBL was performed using siRNA technology. Four pooled siRNA oligos targeting c-CBL (#1027416) or non-specific siRNAs (#1027280) were obtained from Qiagen (Valencia, CA), and were transfected into cells by electroporation using the Nucleofector device and the L kit (Lonza, Switzerland). Briefly, cells were cultured under normal conditions to the log growth stage, 3×106 cells were centrifuged and resuspended in 100 μl of transfection solution L, siRNA was added to a final concentration of 1uM and transfected using program X 001. The c-CBL siRNA sequences were: 5′CCCGCCGAACTCTCTCAGATA3′, 5′CCGTACTATCTTGTCAAGATA3′, 5′CCCATACTTCGTATTCTTAAA3′, 5′CTGGCGCTAAAGAATAGCCCA3′. Cells were cultured for 4 days prior to detection or further processing. Controls included isotype antibody, no treatment and nonsense (NS) siRNA.
Statistics
Statistical analysis was performed by Student’s t-test. A p-value < 0.05 was considered statistically significant. Error bars in histograms show standard deviations of triplicate measurements.
Supplementary Material
Acknowledgements
Supported in part by Merit Review funding from the Department of Veterans Affairs, NCI grant P30CA014520 to the UW Carbone Comprehensive Cancer Center. NIH CTSA UL1TR000427 to the UW Institute for Clinical and Translational Research and NIH T32AR055893 grant (K.Salva).
Abbreviations
- MF
Mycosis fungoides
- SS
Sezary syndrome
- CTCL
cutaneous T-cell lymphoma
- AICD
activation-induced cell death
- TCR
T-cell receptor
- FASL
FAS-ligand
- MTX
methotrexate
- PLC-g1
phospholipase C gamma-1
- ROS
reactive oxygen species
- siRNA
short interfering RNA
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
REFERENCES
- Ahmad N, Gupta S, Feyes DK, et al. Involvement of Fas (APO-1/CD95) during photodynamic therapy-mediated apoptosis in human epidermoid carcinoma A431 cells. J Invest Dermatol. 2000;115:1041–1046. doi: 10.1046/j.1523-1747.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Ali SM, Chee SK, Yuen GY, et al. Photodynamic therapy induced Fas-mediated apoptosis in human carcinoma cells. Int J Mol Med. 2002;9:257–270. [PubMed] [Google Scholar]
- Braun FK, Fecker LF, Schwarz C, et al. Blockade of death receptor-mediated pathways coincides with distinct apoptosis resistance in cutaneous T-cell lymphoma cells. J Invest Dermatol. 2007;127:2425–2437. doi: 10.1038/sj.jid.5700868. [DOI] [PubMed] [Google Scholar]
- Braun FK, Al-Yacoub N, Plotz M, et al. Nonsteroidal anti-inflammatory drugs induce apoptosis in cutaneous T-cell lymphoma cells and enhance their sensitivity for TNF-related apoptosis-inducing ligand. J Invest Dermatol. 2012;132:429–439. doi: 10.1038/jid.2011.316. [DOI] [PubMed] [Google Scholar]
- Chen WY, Weng JH, Huang CC, et al. Histone deacetylase inhibitors reduce steroidogenesis through SCF-mediated ubiquitination and degradation of steroidogenic factor 1 (NR5A1) Mol Cell Biol. 2007;27:7284–90. doi: 10.1128/MCB.00476-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contassot E, Kerl K, Roques S, et al. Resistance to FasL and TRAIL-mediated apoptosis in Sezary syndrome associated with impaired death receptor and FLIP expression. Blood. 2008;111:4780–4787. doi: 10.1182/blood-2007-08-109074. [DOI] [PubMed] [Google Scholar]
- Fargnoli M-C, Edelson RL, Berger CL, et al. Diminished TCR signaling in cutaneous T-cell lymphoma is associated with decreased activities of Zap70, Syk and membrane-associated Csk. Leukemia. 1997;11:1338–1346. doi: 10.1038/sj.leu.2400745. [DOI] [PubMed] [Google Scholar]
- Furre IE, Moller MT, Shahzidi S, et al. Involvement of both caspase-dependent and - independent pathways in apoptotic induction by hexaminolevulinate-mediated photodynamic therapy in human lymphoma cells. Apoptosis. 2006;11:2031–2042. doi: 10.1007/s10495-006-0190-x. [DOI] [PubMed] [Google Scholar]
- Jain S, Zain J, O’Conner O. Novel therapeutic agents for cutaneous T-cell lymphoma. J Hematol Oncol. 2012;5:24. doi: 10.1186/1756-8722-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke CD, Brenner D, Weiss EM, et al. Lack of T-cell receptor-induced signaling is crucial for CD95 ligand up-regulation and protects cutaneous T-cell lymphoma cells from activation-induced cell death. Cancer Res. 2009;69:4175–83. doi: 10.1158/0008-5472.CAN-08-4631. [DOI] [PubMed] [Google Scholar]
- Lo F-Y, Tan Y-HC, Cheng H-C, et al. An E3 ubiquitin ligase c-Cbl: a new therapeutic target of lung cancer in cell and animal models. Cancer. 2011;117:5344–5350. doi: 10.1002/cncr.26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser S, Penninger JM. The ubiquitin E3 ligase Cbl-b in T-cell tolerance and tumor immunity. Cell Cycle. 2007;6:2478–2485. doi: 10.4161/cc.6.20.4797. [DOI] [PubMed] [Google Scholar]
- Lydeard JR, Harper JW. Inhibitors for E3 ubiquitin ligases. Nature Biotech. 2010;28:682–684. doi: 10.1038/nbt0710-682. [DOI] [PubMed] [Google Scholar]
- Meech SJ, Edelson R, Walsh P, et al. Reversible Resistance to Apoptosis in Cutaneous T-cell Lymphoma. Ann NY Acad Sci. 2001;941:46–58. doi: 10.1111/j.1749-6632.2001.tb03710.x. [DOI] [PubMed] [Google Scholar]
- Olsen EA, Whittaker S, Kim Y, et al. Clinical endpoints and response criteria in mycosis fungoides and Sezary syndrome. J Clin Oncol. 2011;29:2598–607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino M, Penninger JM. Cbl-b in T-cell activation. Semin Immunopathol. 2010;32:137–48. doi: 10.1007/s00281-010-0197-9. [DOI] [PubMed] [Google Scholar]
- Qiao G, Zhao Y, Li Z, et al. T-cell activation threshold regulated by E3 ubiquitin ligase Cbl-b determines fate of inducible regulatory T-cells. J Immunol. 2013;191:632–639. doi: 10.4049/jimmunol.1202068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfeld C, Rosen ST, Guitart J, et al. Results of an open-label multicenter phase 2 trial of lenalidomide monotherapy in refractory mycosis fungoides and Sézary syndrome. Blood. 2014;123:1159–66. doi: 10.1182/blood-2013-09-525915. [DOI] [PubMed] [Google Scholar]
- Saito Y, Aoki Y, Muramatsu H, et al. Casitas B-cell lymphoma mutation in childhood T-cell acute lymphoblastic leukemia. Leuk Res. 2012;36:1009–1015. doi: 10.1016/j.leukres.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salva KA, Nihal M, Wu J, et al. Epigenetically enhanced photodynamic therapy (ePDT) is superior to conventional PDT for inducing apoptosis in cutaneous T-cell lymphoma (CTCL) J Invest Dermatol. 2014;134:S117. abstr. [Google Scholar]
- Schmidt MHH, Dikic I. The CBL interactome and its functions. Nature Rev- Mol Cell Biol. 2005;6:907–918. doi: 10.1038/nrm1762. [DOI] [PubMed] [Google Scholar]
- Sors A, Jean-Louis F, Begue E, et al. Inhibition of IKK in CTCL down-regulates NF-kB activation, induces cell death, and potentiates response to chemotherapeutic agents. Clin Cancer Res. 2008;14:901–911. doi: 10.1158/1078-0432.CCR-07-1419. [DOI] [PubMed] [Google Scholar]
- Stutz N, Johnson RD, Wood GS. The Fas apoptotic pathway in cutaneous T-cell lymphomas: Frequent expression of phenotypes associated with resistance to apoptosis. J Am Acad Dermatol. 2012;67:1327–34. doi: 10.1016/j.jaad.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Swaminathan G, Tsygankov AY. The Cbl Family Proteins: Ring Leaders in Regulation of Cell Signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- Wu J, Nihal M, Siddiqui J, et al. Low FAS/CD95 expression in CTCL correlates with reduced sensitivity to apoptosis that can be restored by FAS up-regulation. J Invest Dermatol. 2009;129:1165–1173. doi: 10.1038/jid.2008.309. [DOI] [PubMed] [Google Scholar]
- Wu J, Salva K, Stutz N, et al. Quantitative Gene Analysis of Methylation and Expression (Q-GAME) in Fresh or Fixed Cells and Tissues. Exp Dermatol. 2014;23:304–9. doi: 10.1111/exd.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Siddiqui J, Nihal M, et al. Structural alterations capable of affecting FAS/CD95 gene function in cutaneous T-cell lymphoma (CTCL) Arch Biochem Biophys. 2011;508:185–91. doi: 10.1016/j.abb.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wood GS. Reduction of FAS/CD95 promoter methylation upregulates FAS protein and enhances sensitivity to apoptosis in cutaneous T-cell lymphoma. Arch Dermatol. 2011;147:443–9. doi: 10.1001/archdermatol.2010.376. [DOI] [PubMed] [Google Scholar]
- Wu J, Wood GS. c-CBL ubiquitin E3 ligase is over-expressed in Sezary syndrome and its inhibition restores T-cell receptor signaling, upregulates FAS-ligand expression and promotes activation-induced cell death. J Invest Dermatol. 2014;134:S32. abstr. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.