Summary
Background
Psoriasis is a chronic inflammatory skin disease characterized by hyperproliferation and aberrant keratinocyte differentiation. We have shown that treatment of reconstituted human skin with delphinidin, an anthocyanidin, present in pigmented fruits and vegetables, increased the expression and processing of caspase-14, which is involved in cornification. Delphinidin also increases the expression of epidermal differentiation marker proteins.
Objectives
To determine whether topical application of delphinidin can modulate pathological markers of psoriasiform lesions in flaky skin mice and if this is associated with increased epidermal differentiation and a reduction in proliferation and inflammation.
Methods
Five-week-old female homozygous flaky skin mice (fsn/fsn) were treated topically with delphinidin (0.5 mg per cm2 and 1 mg per cm2 skin areas, respectively), five times a week, up to 14 weeks of age.
Results
Treatment of flaky skin mice with delphinidin resulted in a reduction in (i) pathological markers of psoriasiform lesions; (ii) infiltration of inflammatory cells; and (iii) mRNA and protein expression of inflammatory cytokines. Delphinidin treatment also increased the expression and processing of caspase-14, and expression of filaggrin, loricrin, keratin-1 and keratin-10. Furthermore, there was a decrease in the expression of markers for cell proliferation (proliferating cell nuclear antigen and keratin-14) and modulation of tight junction proteins (occludin and claudin-1). In addition, delphinidin treatment increased the expression of activator protein-1 transcription factor proteins (JunB, JunD, Fra1 and Fra2).
Conclusions
Delphinidin could be a promising agent for treatment of psoriasis and other hyperproliferative skin disorders.
The epidermis produces a highly durable, flexible and self-repairing protective barrier between the internal body organs and the environment.1,2 The formation and maintenance of the skin barrier is regulated by cell proliferation and differentiation of epidermal keratinocytes.2,3 Impaired balance between keratinocyte differentiation and proliferation is observed in many skin disorders, such as psoriasis, atopic dermatitis and ichthyosis vulgaris.2,4 Psoriasis affects > 125 million people worldwide.5,6 In psoriatic lesions, the granular layer of the epidermis, where terminal differentiation occurs, is greatly reduced or even absent. In these lesions abnormal stratum corneum is formed (parakeratosis) along with thickening of the epidermis (acanthosis), formation of epidermal rete ridges (pappilomatosis), angiogenesis and increased infiltration of inflammatory cells into the dermis.7–9 As psoriasis is a chronic inflammatory skin disease, it is characterized by marked alteration in the expression and secretion of cytokines.10,11 Activator protein (AP)-1 transcription factor proteins play an important role in keratinocyte proliferation and differentiation.12,13 AP-1 is also known to regulate the production of inflammatory cytokines and thus has an important role in psoriasis pathogenesis.14,15
As there is no cure for psoriasis, there is a need to explore natural agents that possess the ability to abrogate the pathogenesis of psoriasis. Thus, identifying naturally occurring anti-inflammatory agents that possess the ability to induce terminal differentiation and inhibit hyperproliferation could be useful for the treatment of psoriasis. Delphinidin, an anthocyanidin abundantly present in pigmented fruits and vegetables, possesses both anti-inflammatory and antiproliferative properties.16–18 Recently, we have shown that treatment of normal human epidermal keratinocytes and reconstituted human skin with delphinidin induced epidermal differentiation.18
In the present study, we determined whether topical application of delphinidin can modulate pathological markers of psoriasiform lesions in the flaky skin mouse model. The flaky skin mutant mouse (fsn/fsn) carries a spontaneous autosomal recessive mutation in Ttc7 on chromosome 17. Mutation of Ttc7 in mice results in skin disorders resembling human psoriasis.19 Skin of the flaky skin mice exhibits the characteristics of psoriasis, including acanthosis, hyperkeratosis, parakeratosis and a mixed inflammatory infiltrate, including epidermal microabscesses, angiogenesis and blood vessel dilation. Consequently, these cutaneous lesions are termed psoriasiform or psoriasis-like skin disease. In addition, the Ttc7 mutation in mice results in multiorgan abnormalities characterized by an increase in immature B lymphocytes, pleiotropic abnormalities, anaemia, hyper-IgE, antidouble-stranded DNA autoantibodies, psoriasis dermatitis, forestomach epithelial hyperplasia and peripheral lymphadenopathy. This causes a reduction in lifespan. Mutations in the human homologue of Ttc7, TTC7A, causes combined immunodeficiency with intestinal atresias – a completely different disease from psoriasis.20 Even though the immunopathogenesis is not identical to human psoriasis, fsn/fsn mice are considered to be a useful model for studying hyperproliferative and inflammation-related skin disorders such as psoriasis.21–27 We observed a reduction in psoriasiform lesion pathogenesis in flaky skin mice after topical application of delphinidin. Delphinidin treatment also inhibited the proliferation of keratinocytes, induced keratinocyte differentiation and modulated the expression of tight junction proteins. Furthermore, delphinidin treatment inhibited proinflammatory cytokines, infiltration of macrophages and neutrophils, and increased the expression of AP-1 proteins in flaky skin mice.
Materials and methods
Materials
Delphinidin (> 99% pure) was purchased from Extrasynthese (Genay-Cedex, France).
Treatment of flaky skin mice
Flaky skin mice (CByJ.A-Ttc7fsn/J)+/+ (homozygous wild-type) and fsn/+ (heterozygous) were purchased from The Jackson Laboratory (Bar Harbor, ME, U.S.A.) and housed under pathogen-free conditions with a 12-h light/12-h dark schedule at the Animal Resource Facility, University of Alabama at Birmingham in accordance with the Institutional Animal Care and Use Committee guidelines. We bred these mice using fsn/+ × fsn/+ (heterozygous) breeding pairs from the Jackson Laboratory and identified fsn/fsn, fsn/+ and +/+ pups by genotyping with specific primers as described in Table S1 (see Supporting Information). Animals were fed a phytochemical-free diet (AIN-76 SEMI PD; Test Diet, Richmond, IN, U.S.A.) ad libitum. The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Five-week-old female homozygous flaky skin mice (fsn/fsn) were divided into three groups (eight mice in each group). Mice in groups 2 and 3 were treated topically with delphinidin 0.5 mg per cm2 and 1 mg per cm2 skin areas, respectively, five times a week, up to 14 weeks of age. Control mice in group 1 were treated with a similar volume of vehicle alone.
Preparation of skin lysates and Western blot analysis
For epidermal skin lysate preparation, epidermis from the whole skin was separated as described earlier.28 For Western blotting, 30–50 μg protein was resolved over 8–12% Tris–glycine gels and transferred onto a polyvinylidene fluoride membrane as described previously.17
Immunohistochemistry and immunofluorescence staining
For immunostaining, 5-μm sections were subjected to antigen retrieval and were incubated with primary antibodies followed by incubation with secondary antibody as described previously.17
RNA isolation, complementary DNA synthesis and mRNA expression analysis by quantitative real-time polymerase chain reaction
For mRNA expression analysis, total RNA was isolated from mouse skin using Tri reagent (Sigma-Aldrich, St Louis, MO, U.S.A.) and the concentration was determined by ultraviolet absorbance (Ultrospec™ 3100 pro; Amersham Biosciences, Biochrom Ltd, Cambridge, U.K.). First stand complementary DNA was transcribed from 1 μg RNA (iScript™ cDNA Synthesis Kit; Bio-Rad Laboratories, Hercules, CA, U.S.A.). Quantitative real-time polymerase chain reaction (PCR) was performed using Ssofast™ EvaGreen® Supermix (Bio-Rad Laboratories, Hercules, CA, U.S.A.) on a 7500 Fast Real-Time PCR System (Applied BioSystems, Carlsbad, CA, U.S.A.). Primer sequences are listed in Table S1 (see Supporting Information). The PCR was performed for 40 cycles of 3 s at 95 °C and 40 cycles of 30 s at 60 °C, followed by a melt curve stage. Relative gene expression was compared using the comparative Ct (2−ΔΔCt) method. Gene expression was determined and normalized using a value derived from the housekeeping gene GAPDH.
Statistical analysis
Data are shown as mean ± SEM. Levels of significance of differences among the groups were calculated using Student’s t-test. P < 0.05 was considered to be statistically significant.
Results
Delphinidin treatment reduces psoriasiform lesions and induces caspase-14 expression and processing in flaky skin mice
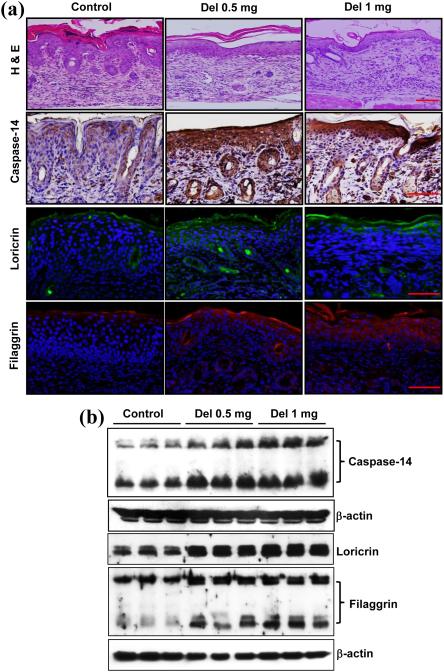
We first determined whether topical application of delphinidin reduces psoriasiform lesions in flaky skin mice. As shown in Figure 1 (a), haematoxylin and eosin staining revealed the presence of extensive psoriasiform lesions characterized by hyperkeratosis, parakeratosis, acanthosis and elongated rete ridges along with infiltration of inflammatory cells in the skin of mice in the control group. However, topical application of delphinidin to flaky skin mice (0.5 mg per cm2 and 1 mg per cm2 skin area) resulted in a reduction in thickening of the epidermis, aberrant cornification and inhibition of the formation of epidermal rete ridges. Caspase-14 plays an important role in terminal differentiation and cornification.29,30 Studies have shown that the expression of caspase-14 is downregulated in psoriatic skin compared with nonlesional and normal skin.31,32 Therefore, we determined the effect of delphinidin treatment on the expression and processing of caspase-14 in flaky skin mice. Immunostaining data clearly demonstrated that the protein expression of caspase-14 was increased in the epidermis of flaky skin mice compared with the control group. In addition, delphinidin treatment increased the expression and processing of caspase-14 (Fig. 1).
Fig. 1.
Effect of delphinidin (Del) treatment on pathological markers of psoriasiform lesions, caspase-14 expression and processing, and expression of filaggrin and loricrin proteins in flaky skin mice. (a) Haematoxylin and eosin (H&E) staining was performed on skin sections of flaky mice and were examined microscopically. Immunohistochemistry of caspase-14 and immunofluorescence staining for loricrin and filaggrin was performed on skin samples. Loricrin is shown in green, filaggrin in red and 4’,6-diamidino-2-phenylindole in blue. Representative pictures are shown. Scale bar = 20 μm. (b) Western blot analysis for protein expression of caspase-14, loricrin and filaggrin was performed in protein lysates. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are from a representative experiment repeated three times with similar results.
Delphinidin treatment increases filaggrin and loricrin protein expression in flaky skin mice
Filaggrin, a major structural protein, synthesized as profilaggrin in the granular layer of the skin, is involved in the terminal differentiation and formation of the cornified envelope of stratum corneum.33,34 Importantly, approximately 80% of patients with psoriasis have a deficiency of filaggrin in their skin.35,36 Therefore, we examined the effect of delphinidin treatment on protein expression of filaggrin in flaky skin mice and found that filaggrin expression in delphinidin-treated mice was increased compared with the skin of control mice (Fig. 1a). Caspase-14 is involved in the cleavage of profilaggrin into filaggrin units,37,38 and, as a consequence of enhanced expression and processing of caspase-14 in delphinidin-treated flaky skin mice, an increase in filaggrin protein expression was also observed in the delphinidin-treated mice compared with mice in the control group (Fig. 1b). Loricrin, an insoluble cornified precursor protein, is expressed in the granular layer and comprises 70–80% of the total protein mass of the cornified layer.2,39 In patients with psoriasis, expression of loricrin in lesional and nonlesional skin is reduced when compared with normal skin.10 Therefore, we also examined the effect of delphinidin treatment on loricrin expression in flaky skin mice. We found that delphinidin treatment increased the protein expression of loricrin compared with mice of the control group (Fig. 1). In addition, we observed that topical application of delphindin to wild-type mice increased the expression of differentiation marker proteins compared with mice not treated with delphinidin (Fig. S1; see Supporting Information).
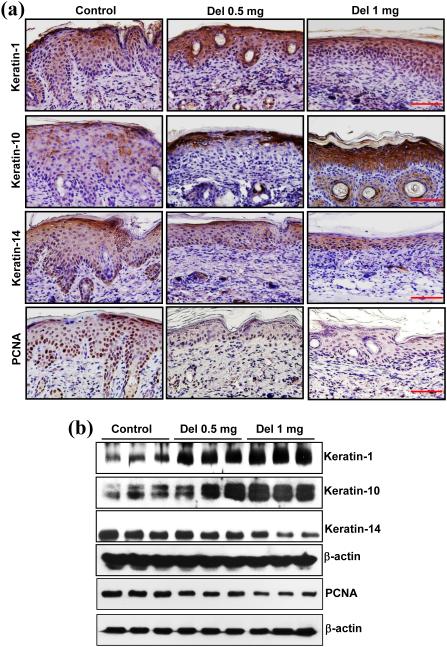
Delphinidin treatment modulates expression of keratins involved in proliferation and differentiation in flaky skin mice
Keratins belong to a heterogeneous family of water-insoluble proteins and are the most abundant structural proteins found in the cytoplasm of epithelial cells.40,41 In normal epidermis keratin-1 and keratin-10 are synthesized at suprabasal layers, whereas keratin-5 and keratin-14 are expressed in the proliferating basal layer of the epidermis. Owing to aberrant differentiation in psoriasis, expression of keratin-1 and keratin-10, which is involved in differentiation of epithelial cells, is downregulated. Therefore, we analysed the protein expression of keratin-1 and keratin-10 in delphinidin-treated flaky skin mice. Topical application of delphinidin restored the differentiation properties as observed by increased staining and enhanced protein expression of keratin-1 and keratin-10 compared with the control mice (Fig. 2). As psoriasis is characterized by hyperproliferation of keratinocytes, we investigated the effect of delphinidin treatment on keratinocyte proliferation in flaky skin mice. Treatment with delphinidin significantly reduced the expression of proliferating cell nuclear antigen in flaky skin mice compared with control mice (Fig. 2). In addition, psoriatic hyperproliferation of keratinocytes leads to increased expression of keratin-14; therefore, we analysed keratin-14 expression in flaky skin mice. As shown in Figure 2, protein expression of keratin-14 was significantly reduced in delphinidin-treated mice compared with control mice. These results suggest that delphinidin treatment of flaky skin mice reduced the expression of keratin-14 related to keratinocyte proliferation, with a concomitant increase in differentiation-related proteins keratin-1 and keratin-10.
Fig. 2.
Effect of delphinidin (Del) treatment on protein expression of proliferating cell nuclear antigen (PCNA) and keratin-1, keratin-10 and keratin-14 in flaky skin mice. (a) Immunohistochemistry of PCNA, keratin-1, keratin-10 and keratin-14 on skin sections of flaky mice was performed. Representative pictures are shown. Scale bar = 20 μm. (b) Western blot analysis for protein expression of PCNA, keratin-1, keratin-10 and keratin-14 was performed in protein lysates. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are from a representative experiment repeated three times with similar results.
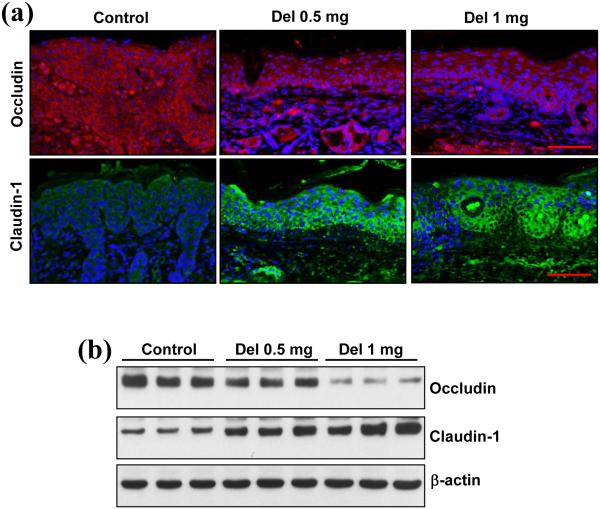
Delphinidin treatment modulates the expression of the tight junction proteins occludin and claudin-1 in flaky skin mice
Tight junction proteins play an important role in the establishment and maintenance of functional biological barriers.42,43 Altered expression of the tight junction proteins occludin and claudin-1 has been observed in psoriasis and in many other human inflammatory diseases.44 Accumulating evidence suggests that downregulation of claudin-1 expression in psoriasis results in hyperproliferation and impaired differentiation of keratinocytes.43,45 Therefore, we evaluated the protein expression of claudin-1 and found that delphinidin treatment markedly increased the protein expression of claudin-1 in flaky skin mice compared with control mice (Fig. 3). In addition, expression of occludin is increased in psoriasis and other inflammatory diseases.46,47 We found that the expression of occludin in the flaky skin mice treated with delphinidin was reduced compared with the control group (Fig. 3).
Fig. 3.
Effect of delphinidin (Del) treatment on expression of occludin and claudin-1 in flaky skin mice. (a) Immunofluorescence staining for occludin and claudin-1 on skin sections of flaky mice was performed. Occludin is shown in red, claudin-1 in green and 4’,6-diamidino-2-phenylindole in blue. Representative pictures are shown. Scale bar = 20 μm. (b) Western blot analysis for protein expression of occludin and claudin-1 was performed in protein lysates. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are from a representative experiment repeated three times with similar results.
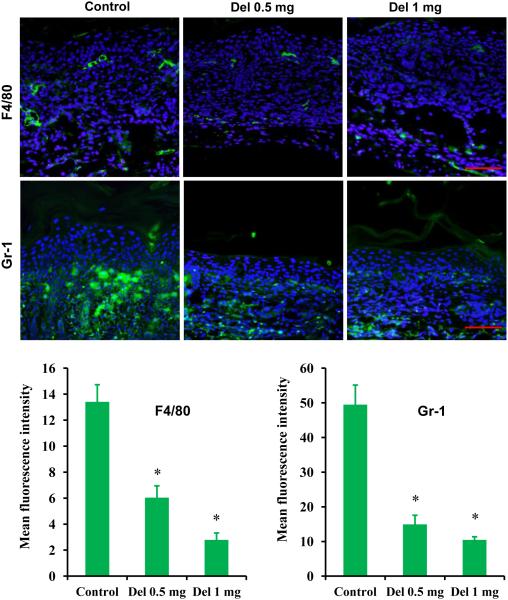
Delphinidin treatment inhibits infiltration of inflammatory cells in flaky skin mice
Psoriasis is characterized by increased dermal infiltration of inflammatory cells, neutrophils, macrophages, dendritic cells and natural killer cells.48,49 Moreover, infiltration of macrophages, especially around the epidermal–dermal junction, increases in psoriatic skin.48,50 Therefore, infiltration of macrophages in the flaky skin mice was examined, and we found that the number of infiltrating macrophages was decreased in the skin of delphinidin-treated mice, as shown by F4/80 staining, compared with the skin of control mice. In addition, delphinidin treatment also reduced Gr-1-positive infiltratory neutrophils in the skin (Fig. 4).
Fig. 4.
Effect of delphinidin (Del) treatment on infiltration of F4/80 and Gr-1 in flaky skin mice. Immunofluorescence staining for F4/80 and Gr-1 on skin sections of flaky mice was performed. Sections were incubated with primary antibody against F4/80 and Gr-1 overnight at 4 °C followed by incubation with specific Alexa Flour 488-labelled secondary antibodies for 1 h at room temperature in the dark. After washing, the sections were incubated for 10 min in the dark with VECTASHIELD® Mounting Media (Vector Labs, Burlingame, CA, U.S.A.) containing 4’,6-diamidino-2-phenylindole (DAPI) and immediately analysed microscopically. F4/80 and Gr-1 staining is shown in green and DAPI in blue. Representative pictures are shown. Scale bar = 20 μm. For quantification, each colour image was separated into its red–green–blue channel component using ImageJ software (National Institutes of Health, Bethesda, MD, U.S.A.), and green channel was used to analyse mean fluorescent intensity. Data shown here are mean fluorescence intensity ± SEM. Significant differences were determined as *P < 0.05 vs. control.
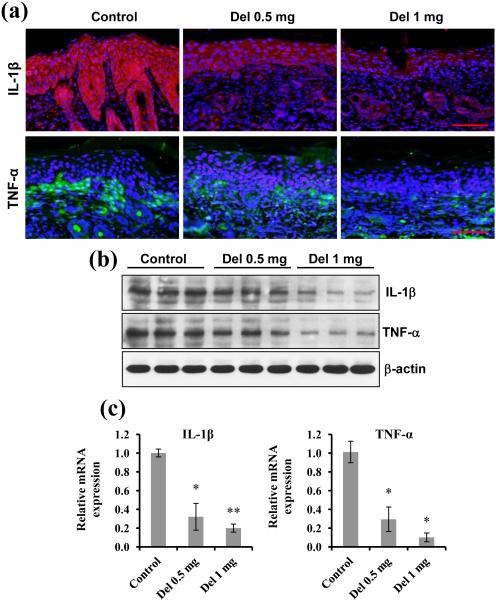
Delphinidin treatment inhibits protein and mRNA expression of inflammatory cytokines in flaky skin mice
Inflammatory cytokines, such as tumour necrosis factor (TNF)-α and interleukin (IL)-1β, produced by macrophages and neutrophils are the predominant mediators of skin immunopathology in psoriasis.5,10,11 TNF-α is involved in the early stages of inflammation and maintains the inflammatory state through secretion of other inflammatory cytokines.51,52 We found that delphinidin treatment significantly reduced the protein production and mRNA expression of TNF-α in flaky skin mice (Fig. 5). IL-1β regulates expression of cytokines and promotes infiltration of inflammatory cells.53,54 We determined the effect of delphinidin treatment on the protein and mRNA expression of IL-1β in flaky skin mice and found that delphinidin treatment reduced the expression of IL-1β both at the protein and the mRNA level (Fig. 5). A growing body of evidence points to a critical contribution of IL-17/IL-23 and associated cytokines in the pathogenesis of psoriasis in mice and humans.55,56 Therefore, the expression of IL-17/IL-23 in the skin of delphinidin-treated flaky skin mice was determined. As shown in Figure 6 (a), mRNA expression of IL-17 and IL-23 was significantly downregulated in the delphinidin-treated mice compared with control mice. TNF-α induces the expression of IL-6, which stimulates the proliferation of keratinocytes and the production of IL-8, along with other cytokines and growth factors.57,58 We found that delphinidin treatment significantly reduced the mRNA expression of the cytokines IL-6 and IL-8 in flaky skin mice compared with control mice (Fig. 6a). Collectively, these data suggest that delphinidin treatment in flaky skin mice reduces infiltration of inflammatory cells and production of inflammatory mediators.
Fig. 5.
Effect of delphinidin (Del) treatment on protein and mRNA expression of interleukin (IL)-1β and tumour necrosis factor (TNF)-α in flaky skin mice. (a, b) Western blot and immunofluorescence analyses for protein expression of IL-1β and TNF-α in the flaky mice skin sections treated with and without delphinidin were performed. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are from a representative experiment repeated three times with similar results. Scale bar = 20 μm. (c) Quantitative real-time polymerase chain reaction (PCR) was performed using Ssofast™ EvaGreen® Supermix (Bio-Rad Laboratories, Hercules, CA, U.S.A.) on a 7500 Fast Real-Time PCR System (Applied BioSystems, Carlsbad, CA, U.S.A.). Gene expression was determined and normalized using a value derived from the housekeeping gene GAPDH. Data shown here are relative mRNA expression ± SEM. Significant differences were determined as *P < 0.05 and **P < 0.01 vs. control.
Fig. 6.
Effect of delphinidin (Del) treatment on mRNA expression of inflammatory cytokines and activator protein (AP)-1 factor proteins in flaky skin mice. (a) Quantitative real-time polymerase chain reaction (PCR) was performed using Ssofast™ EvaGreen® Supermix (Bio-Rad Laboratories, Hercules, CA, U.S.A.) on a 7500 Fast Real-Time PCR System (Applied BioSystems, Carlsbad, CA, U.S.A.) to determine the effect of delphinidin on inflammatory cytokines. Gene expression was determined and normalized using a value derived from the housekeeping gene GAPDH. Data shown here are relative mRNA expression ± SEM. Significant differences were determined as *P < 0.05 and **P < 0.01 vs. control. (b) Western blot analysis for JunB, JunD, Fra-1 and Fra-2 protein expression was performed. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for lamin. The immunoblots shown here are from a representative experiment repeated three times with similar results. IL, interleukin; TGF-β, transforming growth factor-β.
Delphinidin treatment upregulates activator protein-1 transcription factor proteins in flaky skin mice
Accumulating evidence has shown that AP-1 regulates the expression of caspase-14, loricrin, profilaggrin and cytokeratins involved in keratinocyte differentiation.12,59–62 Studies have shown that c-Jun and Jun B, and, to a lesser extent, other members of AP-1 (Jun D, Fra 1 and Fra 2) bind to the caspase-14 AP-1 site and play an important role in transcriptional control of caspase-14.62 Abrogation of JunB/AP-1 in keratinocytes triggers chemokine and cytokine expression, which recruits inflammatory cells to the epidermis, thereby contributing to psoriasis.13,14 As topical application of delphinidin significantly reduced psoriasiform lesions, infiltration of inflammatory cells and production of inflammatory mediators, we next determined the effect of delphinidin on AP-1 transcription factor proteins. We found that delphinidin treatment significantly enhanced the expression of Jun proteins (c-Jun, JunB, JunD) and Fos proteins (Fra-1 and Fra-2) in flaky skin mice compared with control mice (Fig. 6b).
Discussion
Psoriasis is a common chronic skin condition characterized by epidermal hyperproliferation, aberrant differentiation of keratinocytes, impaired barrier function and infiltration of inflammatory cells into the dermis and epidermis. Despite recent advances in understanding of the pathogenesis and underlying mechanism(s) involved in psoriasis, current treatment approaches produce varying and often subtle results. Moreover, the high cost of newer synthetic agents makes their use unfeasible for many patients. Therefore, the quest for agents that have antipsoriatic activity with a low profile of adverse effects and manageable cost continues. In this regard, bioactive natural ingredients hold promise because of their low cost and minimal adverse effects.
Delphinidin is known to possess antioxidant, anti-inflammatory, antitumorigenic and antiangiogenic properties.16–18,63 We have recently shown that delphinidin induces differentiation in human epidermal keratinocytes and reconstituted human skin.18 Recently, Hoss et al. confirmed our findings,64 thus reinforcing the use of delphinidin for psoriasis therapy. In the present study, application of delphinidin to flaky skin mice abrogated the histological characteristics of psoriasiform lesions and greatly reduced infiltration of inflammatory cells such as neutrophils and macrophages (Figs 1–4). In addition, in terms of overall gross appearance, the flaky skin mice that received delphinidin looked healthier compared with nontreated flaky skin mice.
A dysregulated immune profile involving elevated myeloid lineage leucocytes appears to be a major factor in the immunopathogenesis of psoriasis.65 Accumulating evidence has shown that drugs causing a reduction in infiltration of neutrophils in flaky skin mice and patients with psoriasis lead to a reduction in epidermal thickness, the absence of microabscesses and rapid clinical improvement of psoriasis.66,67 Furthermore, inflammatory cytokines regulate the infiltration of neutrophils and macrophages, and induce keratinocyte proliferation.68–71 Reduction in the production of these inflammatory cytokines was also seen in the flaky skin mice after delphinidin treatment (Figs 5 and 6). In summary, these hallmarks of psoriasis – infiltration of inflammatory cells and production of inflammatory mediators – are significantly reduced by delphinidin treatment.
In order to maintain the normal architecture of skin, the epidermis is continuously renewed through the coupled process of cell proliferation and differentiation. Studies have shown that caspase-14 is involved in the cleavage of profilaggrin into filaggrin, which is a major structural protein involved in the terminal differentiation and formation of cornified envelops in the stratum corneum.33,34,72 In addition, loricrin has an important role in epidermal skin barrier formation and is considered to be a marker of epidermal differentiation. In contrast to envelope proteins, keratins are differentially expressed at various developmental and differentiation stages of the skin. Apart from their structural functions, keratin intermediate filaments are known to regulate epithelial cell proliferation, migration and differentiation, thus maintaining the overall structural integrity of the epidermis.1,2 Tight junction proteins also regulate cell proliferation and differentiation. Their altered expression is observed in psoriasis and other inflammatory diseases.42,43,72 We found that treatment with delphinidin restored markers of differentiation in flaky skin mice, as shown by increased expression and processing of caspase-14 and expression of filaggrin, loricrin, keratin-1 and keratin-10 (Figs 1 and 2). In addition, there was a decrease in the expression of cell proliferation markers and modulation of tight junction proteins (Figs 2 and 3).
The AP-1 transcription factor consists of homo- or heterodimers of members of the Fos and Jun protein families.73 Reduced AP-1 binding activity and decreased transcription of c-Fos and c-Jun has been reported in psoriasis.74,75 Specifically, the expression of JunB and caspase-14 has been shown to be greatly reduced in psoriatic skin.13,31,32 More importantly, the inducible epidermis-specific deletion of JunB in combination with c-Jun in the epidermis of adult mice lead to development of strongly thickened epidermis with prominent rete ridges, thickened keratinized upper layers with parakeratosis and increased subepidermal vascularization. We found that delphinidin treatment increased the expression of AP-1 proteins in flaky skin mice (Fig. 6).
Our study shows that topical application of delphinidin to flaky skin mice reduces pathological markers of psoriasiform lesions. It induces epidermal differentiation and reduces proliferation and inflammation. In addition, delphinidin treatment modulates the expression of tight junction proteins. Furthermore, delphinidin treatment increased the protein expression of JunB. These promising biological effects coupled with the relatively low cost and toxicity of natural agents makes delphinidin a promising agent for the treatment of psoriasis and other hyperproliferative skin disorders.
Supplementary Material
Fig S1. Five-week-old female homozygous wild-type [(CByJ.A-Ttc7fsn/J); +/+] mice were divided into two groups (six mice in each group). Mice in group 1 were treated topically with vehicle. Mice in group 2 were treated topically with delphinidin 1 mg per cm2 skin areas, five times a week, up to 14 weeks of age. At the end of the treatment mice were euthanized and skin lysates were analysed for protein expression of caspase-14, filaggrin, loricrin, keratin-1 and keratin-10 by Western blot analysis. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are from a representative experiment repeated three times with similar results.
What’s already known about this topic?
Psoriasis is a chronic inflammatory skin disease characterized by increased epidermal hyperproliferation, aberrant keratinocyte differentiation, impaired barrier function and infiltration of inflammatory cells.
What does this study add?
Topical application of delphinidin, an anthocyanidin present in pigmented fruits and vegetables, reduced psoriasiform lesions that were associated with induction of epidermal differentiation, a reduction in keratinocyte proliferation, and modulation in the expression of tight junction proteins.
• Delphinidin treatment inhibited proinflammatory cytokines, infiltration of inflammatory macrophages and neutrophils, and increased the expression of activator protein-1 proteins.
Acknowledgments
Funding sources
This work was supported by National Institutes of Health Grants R21 AT004966 and RO1 AR059742, and University of Alabama Skin Disease Research Center Pilot and Feasibility Grant (P30AR050948).
Footnotes
Conflicts of interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
References
- 1.Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- 2.Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–8. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 4.Hoffjan S, Stemmler S. On the role of the epidermal differentiation complex in ichthyosis vulgaris, atopic dermatitis and psoriasis. Br J Dermatol. 2007;157:441–9. doi: 10.1111/j.1365-2133.2007.07999.x. [DOI] [PubMed] [Google Scholar]
- 5.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 6.Stenderup K, Rosada C, Dam TN, et al. Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. J Invest Dermatol. 2011;131:2033–9. doi: 10.1038/jid.2011.161. [DOI] [PubMed] [Google Scholar]
- 7.Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 8.Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- 9.Chua RA, Arbiser JL. The role of angiogenesis in the pathogenesis of psoriasis. Autoimmunity. 2009;42:574–9. doi: 10.1080/08916930903002461. [DOI] [PubMed] [Google Scholar]
- 10.Nickoloff BJ. The cytokine network in psoriasis. Arch Dermatol. 1991;127:871–84. [PubMed] [Google Scholar]
- 11.Rivas-Bejarano JJ, Valdecantos WC. Psoriasis as autoinflammatory disease. Dermatol Clin. 2013;31:445–60. doi: 10.1016/j.det.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Eckert RL, Adhikary G, Young CA, et al. AP1 transcription factors in epidermal differentiation and skin cancer. J Skin Cancer. 2013;2013:537028. doi: 10.1155/2013/537028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenz R, Eferl R, Kenner L, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–75. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 14.Zenz R, Eferl R, Scheinecker C, et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10:201–10. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmets CA. An animal model of psoriasis in mice deficient in epidermal Jun proteins. Arch Dermatol. 2006;142:1499–500. doi: 10.1001/archderm.142.11.1499. [DOI] [PubMed] [Google Scholar]
- 16.Seong AR, Yoo JY, Choi K, et al. Delphinidin, a specific inhibitor of histone acetyltransferase, suppresses inflammatory signaling via prevention of NF-κB acetylation in fibroblast-like synoviocyte MH7A cells. Biochem Biophys Res Commun. 2011;410:581–6. doi: 10.1016/j.bbrc.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Pal HC, Sharma S, Strickland LR, et al. Delphinidin reduces cell proliferation and induces apoptosis of non-small-cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLOS One. 2013;8:e77270. doi: 10.1371/journal.pone.0077270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamcheu JC, Afaq F, Syed DN, et al. Delphinidin, a dietary antioxidant, induces human epidermal keratinocyte differentiation but not apoptosis: studies in submerged and three-dimensional epidermal equivalent models. Exp Dermatol. 2013;22:342–8. doi: 10.1111/exd.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundberg JG, Boggess D, Sundberg BA, et al. Epidermal dendritic cell populations in the flaky skin mouse mutant. Immunol Invest. 1993;22:389–401. doi: 10.3109/08820139309063417. [DOI] [PubMed] [Google Scholar]
- 20.Helms C, Pelsue S, Cao L, et al. The Tetratricopeptide repeat domain 7 gene is mutated in flaky skin mice: a model for psoriasis, autoimmunity, and anemia. Exp Biol Med (Maywood) 2005;230:659–67. doi: 10.1177/153537020523000908. [DOI] [PubMed] [Google Scholar]
- 21.Sundberg JP, France M, Boggess D, et al. Development and progression of psoriasiform dermatitis and systemic lesions in the flaky skin (fsn) mouse mutation. Pathobiology. 1997;65:261–8. doi: 10.1159/000164138. [DOI] [PubMed] [Google Scholar]
- 22.Schon M, Behmenburg C, Denzer D, et al. Pathogenic function of IL-1 beta in psoriasiform skin lesions of flaky skin (fsn/fsn) mice. Clin Exp Immunol. 2001;123:505–10. doi: 10.1046/j.1365-2249.2001.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atochina O, Harn D. Prevention of psoriasis-like lesions development in fsn/fsn mice by helminth glycans. Exp Dermatol. 2006;15:461–8. doi: 10.1111/j.1600-0625.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 24.Sundberg JP, Dunstan RW, Roop DR, et al. Full-thickness skin grafts from flaky skin mice to nude mice: maintenance of the psoriasiform phenotype. J Invest Dermatol. 1994;102:781–8. doi: 10.1111/1523-1747.ep12377741. [DOI] [PubMed] [Google Scholar]
- 25.Morita K, Hogan ME, Nanney LB, et al. Cutaneous ultrastructural features of the flaky skin (fsn) mouse mutation. J Dermatol. 1995;22:385–95. doi: 10.1111/j.1346-8138.1995.tb03412.x. [DOI] [PubMed] [Google Scholar]
- 26.Nanney LB, Sundberg JP, King LE. Increased epidermal growth factor receptor in fsn/fsn mice. J Invest Dermatol. 1996;106:1169–74. doi: 10.1111/1523-1747.ep12347791. [DOI] [PubMed] [Google Scholar]
- 27.Hsu S, Dickinson D, Borke J, et al. Green tea polyphenol induces caspase 14 in epidermal keratinocytes via MAPK pathways and reduces psoriasiform lesions in the flaky skin mouse model. Exp Dermatol. 2007;16:678–84. doi: 10.1111/j.1600-0625.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 28.Afaq F, Saleem M, Krueger CG, et al. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–33. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 29.Eckhart L, Declercq W, Ban J, et al. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol. 2000;115:1148–51. doi: 10.1046/j.1523-1747.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 30.Denecker G, Ovaere P, Vandenabeele P, et al. Caspase-14 reveals its secrets. J Cell Biol. 2008;180:451–8. doi: 10.1083/jcb.200709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippens S, Kockx M, Denecker G, et al. Vitamin D3 induces caspase-14 expression in psoriatic lesions and enhances caspase-14 processing in organotypic skin cultures. Am J Pathol. 2004;165:833–41. doi: 10.1016/S0002-9440(10)63346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh DS, Borke JL, Singh BB, et al. Psoriasis is characterized by altered epidermal expression of caspase 14, a novel regulator of keratinocyte terminal differentiation and barrier formation. J Dermatol Sci. 2005;37:61–3. doi: 10.1016/j.jdermsci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Sandilands A, Sutherland C, Irvine AD, et al. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009;122:1285–94. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012;132:751–62. doi: 10.1038/jid.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim BE, Howell MD, Guttman-Yassky E, et al. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier. J Invest Dermatol. 2011;131:1272–9. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huffmeier U, Traupe H, Oji V, et al. Loss-of-function variants of the filaggrin gene are not major susceptibility factors for psoriasis vulgaris or psoriatic arthritis in German patients. J Invest Dermatol. 2007;127:1367–70. doi: 10.1038/sj.jid.5700720. [DOI] [PubMed] [Google Scholar]
- 37.Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233–41. doi: 10.1038/jid.2011.153. [DOI] [PubMed] [Google Scholar]
- 38.Hoste E, Denecker G, Gilbert B, et al. Caspase-14-deficient mice are more prone to the development of parakeratosis. J Invest Dermatol. 2013;133:742–50. doi: 10.1038/jid.2012.350. [DOI] [PubMed] [Google Scholar]
- 39.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–19. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–97. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- 42.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 43.Kirschner N, Poetzl C, Vonden-Driesch P, et al. Alteration of tight junction proteins is an early event in psoriasis: putative involvement of proinflammatory cytokines. Am J Pathol. 2009;175:1095–106. doi: 10.2353/ajpath.2009.080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kucharzik T, Walsh SV, Chen J, et al. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–9. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson REB, Poddar R, Walker JM, et al. Altered claudin expression is a feature of chronic plaque psoriasis. J Pathol. 2007;212:450–8. doi: 10.1002/path.2200. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida Y, Morita K, Mizoguchi A, et al. Altered expression of occluding and tight junction formation in psoriasis. Arch Dermatol Res. 2001;293:239–44. doi: 10.1007/s004030100221. [DOI] [PubMed] [Google Scholar]
- 47.Peltonen S, Riehokainen J, Pummi K, et al. Tight junction components occludin, ZO-1, and claudin-1, -4 and -5 in active and healing psoriasis. Br J Dermatol. 2007;156:466–72. doi: 10.1111/j.1365-2133.2006.07642.x. [DOI] [PubMed] [Google Scholar]
- 48.Clark RA, Kupper TS. Misbehaving macrophages in the pathogenesis of psoriasis. J Clin Invest. 2006;116:2084–7. doi: 10.1172/JCI29441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stratis A, Pasparakis M, Rupec RA, et al. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. J Clin Invest. 2006;116:2094–104. doi: 10.1172/JCI27179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shukuya R, Hasegawa T, Niwa Y, et al. Granulocyte and monocyte adsorption apheresis for generalized pustular psoriasis. J Dermatol. 2011;38:1130–4. doi: 10.1111/j.1346-8138.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb AB, Chamian F, Masud S, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–9. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Peters T, Kess D, et al. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J Clin Invest. 2006;116:2105–14. doi: 10.1172/JCI27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groves RW, Rauschmayr T, Nakamura K, et al. Inflammatory and hyperproliferative skin disease in mice that express elevated levels of the IL-1 receptor (type I) on epidermal keratinocytes. Evidence that IL-1-inducible secondary cytokines produced by keratinocytes in vivo can cause skin disease. J Clin Invest. 1996;98:336–44. doi: 10.1172/JCI118797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renne J, Schafer V, Werfel T, et al. Interleukin-1 from epithelial cells fosters T cell-dependent skin inflammation. Br J Dermatol. 2010;162:1198–205. doi: 10.1111/j.1365-2133.2010.09662.x. [DOI] [PubMed] [Google Scholar]
- 55.DiCesare A, DiMeglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–50. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 56.Rizzo HL, Kagami S, Phillips KG, et al. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186:1495–502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- 57.Teunissen MB, Koomen CW, deWaal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–59. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 58.Gudjonsson JE, Johnston A, Sigmundsdottir H, et al. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Welter JF, Eckert RL. Differential expression of the fos and jun family members c-fos, fosB, Fra-1, Fra-2, c-jun, junB and junD during human epidermal keratinocyte differentiation. Oncogene. 1995;11:2681–87. [PubMed] [Google Scholar]
- 60.Jang SI, Steinert PM, Markova NG. Activator protein 1 activity is involved in the regulation of the cell type-specific expression from the proximal promoter of the human profilaggrin gene. J Biol Chem. 1996;271:24105–14. doi: 10.1074/jbc.271.39.24105. [DOI] [PubMed] [Google Scholar]
- 61.Mehic D, Bakiri L, Ghannadan M, et al. Fos and jun proteins are specifically expressed during differentiation of human keratinocytes. J Invest Dermatol. 2005;124:212–20. doi: 10.1111/j.0022-202X.2004.23558.x. [DOI] [PubMed] [Google Scholar]
- 62.Ballaun C, Karner S, Mrass P, et al. Transcription of the caspase-14 gene in human epidermal keratinocytes requires AP-1 and NFkappaB. Biochem Biophys Res Commun. 2008;371:261–6. doi: 10.1016/j.bbrc.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 63.Afaq F, Syed DN, Malik A, et al. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007;127:222–32. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 64.Hoss E, Austin HR, Batie SF, et al. Control of late cornified envelope genes relevant to psoriasis risk: upregulation by 1,25-dihydroxyvitamin D3 and plant-derived delphinidin. Arch Dermatol Res. 2013;305:867–78. doi: 10.1007/s00403-013-1390-1. [DOI] [PubMed] [Google Scholar]
- 65.Michalak-Stoma A, Pietrzak A, Szepietowski JC, et al. Cytokine network in psoriasis revisited. Eur Cytokine Netw. 2011;22:160–8. doi: 10.1684/ecn.2011.0294. [DOI] [PubMed] [Google Scholar]
- 66.Schon M, Denzer D, Kubitza RC, et al. Critical role of neutrophils for the generation of psoriasiform skin lesions in flaky skin mice. J Invest Dermatol. 2000;114:976–83. doi: 10.1046/j.1523-1747.2000.00953.x. [DOI] [PubMed] [Google Scholar]
- 67.Ikeda S, Takahashi H, Suga Y, et al. Therapeutic depletion of myeloid lineage leukocytes in patients with generalized pustular psoriasis indicates a major role for neutrophils in the immunopathogenesis of psoriasis. J Am Acad Dermatol. 2013;68:609–17. doi: 10.1016/j.jaad.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 68.Terui T, Ozawa M, Tagami H. Role of neutrophils in induction of acute inflammation in T-cell-mediated immune dermatosis, psoriasis: a neutrophil-associated inflammation-boosting loop. Exp Dermatol. 2000;9:1–10. doi: 10.1034/j.1600-0625.2000.009001001.x. [DOI] [PubMed] [Google Scholar]
- 69.Vander-Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–45. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 70.Komine M, Karakawa M, Takekoshi T, et al. Early inflammatory changes in the “perilesional skin” of psoriatic plaques: is there interaction between dendritic cells and keratinocytes? J Invest Dermatol. 2007;127:1915–22. doi: 10.1038/sj.jid.5700799. [DOI] [PubMed] [Google Scholar]
- 71.Buerger C, Richter B, Woth K, et al. Interleukin-1β interferes with epidermal homeostasis through induction of insulin resistance: implications for psoriasis pathogenesis. J Invest Dermatol. 2012;132:2206–14. doi: 10.1038/jid.2012.123. [DOI] [PubMed] [Google Scholar]
- 72.Hvid M, Johansen C, Deleuran B, et al. Regulation of caspase 14 expression in keratinocytes by inflammatory cytokines-a possible link between reduced skin barrier function and inflammation? Exp Dermatol. 2011;20:633–36. doi: 10.1111/j.1600-0625.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 73.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 74.Basset-Seguin N, Escot C, Moles JP, et al. C-fos and c-jun proto-oncogene expression is decreased in psoriasis: An in situ quantitative analysis. J Invest Dermatol. 1991;97:672–8. doi: 10.1111/1523-1747.ep12483807. [DOI] [PubMed] [Google Scholar]
- 75.Johansen C, Kragballe K, Rasmussen M, et al. Activator protein 1 DNA binding activity is decreased in lesional psoriatic skin compared with nonlesional psoriatic skin. Br J Dermatol. 2004;151:600–707. doi: 10.1111/j.1365-2133.2004.06088.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Five-week-old female homozygous wild-type [(CByJ.A-Ttc7fsn/J); +/+] mice were divided into two groups (six mice in each group). Mice in group 1 were treated topically with vehicle. Mice in group 2 were treated topically with delphinidin 1 mg per cm2 skin areas, five times a week, up to 14 weeks of age. At the end of the treatment mice were euthanized and skin lysates were analysed for protein expression of caspase-14, filaggrin, loricrin, keratin-1 and keratin-10 by Western blot analysis. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are from a representative experiment repeated three times with similar results.