Abstract
Intracellular signaling in insect olfactory receptor neurons remains unclear, with both metabotropic and ionotropic components being discussed. Here, we investigated the role of heterotrimeric Go and Gi proteins using a combined behavioral, in vivo and in vitro approach. Specifically, we show that inhibiting Go in sensory neurons by pertussis toxin leads to behavioral deficits. We heterologously expressed the olfactory receptor dOr22a in human embryonic kidney cells (HEK293T). Stimulation with an odor led to calcium influx, which was amplified via calcium release from intracellular stores. Subsequent experiments indicated that the signaling was mediated by the Gβγ subunits of the heterotrimeric Go/i proteins. Finally, using in vivo calcium imaging, we show that Go and Gi contribute to odor responses both for the fast (phasic) as for the slow (tonic) response component. We propose a transduction cascade model involving several parallel processes, in which the metabotropic component is activated by Go and Gi, and uses Gβγ.
Keywords: G proteins, insect odorant receptors, olfaction, signaling
Introduction
The sense of smell – olfaction – plays a major role for all animals and mediates behavioral and physiological responses. Odor molecules bind to the odorant receptors (ORs) present at the dendrites of the olfactory receptor neurons (ORNs) located at the peripheral olfactory organs, which send information to the central parts of the brain for further processing. Even though chemical senses are the most ancient in evolution, ORs have evolved creating several evolutionarily distinct and independent gene families, which differ in structure and in intracellular signaling. All OR families in vertebrates are G-protein-coupled receptors (GPCRs) (Buck & Axel, 1991; Mombaerts, 1999; Bargmann, 2006). They activate metabotropic G protein-dependent signaling cascades, but different OR families activate different cascades (Jones & Reed, 1989; Berghard & Buck, 1996; Berghard et al., 1996; Kaupp, 2010).
Insects have more than one family of receptors for olfaction. One family consists of ionotropic receptors (IRs) related to glutamate channels, which respond to odor binding by opening an ion channel (Benton et al., 2009). The other family consists of ORs with a predicted seven-transmembrane topology reminiscent of classical GPCRs, but with an inverted membrane topology and low sequence homology to all known GPCRs (Clyne et al., 1999; Gao & Chess, 1999; Vosshall et al., 1999; Benton et al., 2006; Lundin et al., 2007). Insect ORs form heteromeric complexes with a conserved ortholog protein called Orco (Larsson et al., 2004; Benton et al., 2006). It remains unclear whether and if so how insect ORs depend on G proteins for olfactory signaling. Two different hypotheses have been proposed for insect olfactory signal transduction: either insect ORs may act as ligand-gated ion channels (ionotropic signaling pathway) or they combine an ionotropic and a G protein-dependent pathway for olfactory signaling (Sato et al., 2008; Smart et al., 2008; Wicher et al., 2008). However, the involvement of different G proteins in insect olfactory signaling remains unclear.
Insect ORNs express several G proteins that could be involved in signal transduction, in particular the Go/i subgroup of G proteins (Miura et al., 2005; Rutzler et al., 2006; Boto et al., 2010; Kang et al., 2011). Therefore, in this study we tested whether Go/i are required for olfaction in behavior, for odor responses in the native tissue (antenna; in vivo) or when expressed in a heterologous cell-culture system (HEK293T cells: Human Embryonic Kidney 293T cells; in vitro). We found that in vivo disruption of Gαo/i subunits in the ORNs of Drosophila leads to olfactory behavioral deficits and reduced the amplitude of the odor responses regardless of odor identity and intensity. In vitro inhibition and over-expression of Gαo/i subunits indicated that the Gβγ heterodimer is the key player in the transduction mechanisms. Altogether, our results indicate a role of Go/i subgroup of G proteins in olfactory signaling in Drosophila.
Materials and methods
In vivo experiments
Flies
Flies were reared on standard corn meal medium containing yeast and were kept at 25 °C and a humidity of 50% on a 12/12-h light–dark cycle. We used 1–3-day-old flies for behavioral experiments and 7–14-day-old female flies of F1 progeny for in vivo calcium imaging experiments. The following lines were used: UAS-PTX (Katanaev et al., 2005), UAS-RNAi-Gαi (Kopein & Katanaev, 2009) [Vienna Drosophila RNAi Center (Dietzl et al., 2007)], UAS-GCaMP;Or22a-Gal4/Cyo [crossed from UAS-GCaMP;Cyo/Sp;+ flies provided by Jing Wang, University of California, San Diego, La Jolla, CA, USA (Nakai et al., 2001; Wang et al., 2003)], UAS-GCaMP;Or22a-Gal4/UAS-PTX [crossed from UAS-PTX (Katanaev et al., 2005) and UAS-GCaMP;Or22a-Gal4/Cyo] and UAS-GCaMP;Or22a-Gal4;UAS-RNAi-Gαi [crossed from UAS-RNAi-Gαi (Kopein & Katanaev, 2009) and UAS-GCaMP;Or22a-Gal4/Cyo].
Behavior
Approximately 150 young flies, with equal representation of males and females, were flipped into a large cylindrical bottle 8 cm in diameter and 14 cm in height, without anesthesia by CO2 or cold. Inside the bottles were two trap containers made of blue pipette tips, one with ca. 0.3 mL of mineral oil and one with an equal volume of kitchen apple vinegar. Flies were kept in bottles for 1 h at 25 °C, followed by counting the number of flies trapped in each container and those remaining in the bottle. Results are shown as mean ± standard error of mean (SEM), where n represents the number of experiments. The evaluation of the statistical significance of differences was tested with Student’s t-test.
In vivo preparation of flies
Flies were immobilized on ice for 15 min and then slipped with their neck into a horizontal slit in a plastic recording chamber. The head was fixed to the chamber using dental glue. Antennae were prevented from moving by an electron microscopy grid placed on top of the proximal part of the third antennal segment. The method of preparation leaves the animal surgically intact.
In vivo calcium imaging
Intact fly antennae were recorded as described before (Pelz et al., 2006). The calcium sensor GCaMP1.3 was expressed in the ORNs expressing the odorant receptor Or22a and the odor-evoked calcium changes were measured at the receptor neuron dendrites and somata through the intact antennal cuticle. The setup consists of an upright microscope (Olympus BX50WI, Tokyo, Japan) equipped with a 50× air objective (NA = 0.5) and a CCD/monochromator-based imaging system (Till Photonics, Gräfelfing, Germany). A monochromator (Polychrome II, Till Photonics) produced excitation light of 470 nm wavelength that was directed onto the antenna via a 500-nm low-pass filter and a 495-nm dichroic mirror; emission light was filtered through a 505-nm high-pass emission filter. Images were acquired with a TILL imago CCD camera with a binning of 8 × 8 on the chip. We varied the exposures time between 180 and 220 ms to adjust for different basal fluorescence values across preparations. Twenty-second films were recorded with an acquisition rate of 4 Hz.
Odorant preparation and application
Odorants [ethyl butyrate (EtBE), ethyl hexanoate (EtHE), 1-heptanol (HepL), 4-methoxybenzene (MeBM) and 1-butanol] were > 99.5% pure or of the highest purity available (Sigma-Aldrich, Taufkirchen, Germany). Pure odorants were diluted in 5 mL mineral oil (Sigma-Aldrich) in 20-mL headspace vials (Schmidlin, Neuheim, Switzerland) to their final concentration ranging from 10−7 to 10−2 dilution (v/v). The vials were filled with nitrogen to prevent the odors from oxidation and sealed and were positioned in a computer-controlled autosampler (CombiPAL, CTC analytics, Zwingen, Switzerland), which was used for odorant delivery to flies and was synchronized with the imaging setup via transistor-transistor-logic pulses. A constant air stream (1 mL/s) coming from a synthetic air bottle was guided through a teflon tubing (inner diameter: 1 mm), with the tubing exit placed approximately 5 mm away from the fly’s antennae. We used three different protocols for odor stimulation; in all cases during stimulation the constant air stream was interrupted with a computer-controlled solenoid valve and the autosampler injected up to 2.5 mL of headspace at 250 μL/s into the tube. However, the duration of time that the autosampler injected the headspace varied for different protocols: for the short single pulse protocol it was injected for only 1 s, for the double pulse protocol it was injected for 1 s twice with an interstimulus interval of 2 s and for the long single pulse protocol it was injected for 10 s.
Each stimulus protocol consisted of four blocks of 13 measurements each with an interstimulus interval of 2 min. Between the blocks the syringe of the autosampler was washed thoroughly (with pentane and afterwards heated to 44 °C) for 10 min. Each block started with three control measurements followed by nine odor presentations (the same odor was tested at three different concentrations from lower to higher; for each concentration first the short single pulse stimulation protocol was tested followed by the double pulse and long single pulse stimulation protocol) and ended with a control measurement (room air). After the end of four blocks three control measurements were tested again. The control measurements were: (1) a presentation of the diluent – mineral oil, (2) the reference odor 1-butanol at 10−2 dilution and (3) room air. The reference odor was used to monitor the fly’s responsive state. Four different odors were measured (one odor in each block) in a fly. An individual fly could show a consistent response up to 3 h.
Data analysis
Data analysis was performed with custom-made routines written in IDL software (Research Systems, Co, USA) and R (http://www.r-project.org/). Fluorescence values were converted to relative fluorescence changes (ΔF/F), taking the average of frames 5–22 for background fluorescence. Bleaching was corrected by fitting an exponential decay onto ΔF/F data (Silbering & Galizia, 2007). Measurements were chosen for further analysis if their flanking control block showed a stable response to the reference odor. For response calculation the area showing calcium responses to the first reference odor was chosen. For quantification of odor-evoked response magnitude for the phasic response and adapted response, the peak value (ΔF/F) between the defined time windows (within 3 s after odor stimulus onset) was taken, and for the tonic response the average response over the last 1 s of the stimulus was used. Results are given as mean ± SEM, where n represents the number of flies. The evaluation of statistical significance of differences was tested with two-way anova and multiple comparisons after anova were tested with Tukey’s honest significant differences (HSD) test. Statistical analysis and plots were done in R (http://www.r-project.org/).
In vitro experiments
Reagents
Probenecid, pluronic acid [20% solution in dimethyl sulfoxide (DMSO)], fluo-4 acetoxymethylesters (AM; 1 mm solution in DMSO), HEK293T cells, Dulbecco’s modified Eagle medium (DMEM), Opti-MEM reduced serum medium, penicillin/streptomycin, lipofectamine, 1 m HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and 1× Hank’s balanced salt solution (HBSS) were purchased from Invitrogen (www.invitrogen.com/GIBCO). Fetal calf serum (FCS), ionomycin (calcium ionophore) and pertussis toxin (PTX) were purchased from PAA (Velizy-Villacoublay, France), Sigma-Aldrich and Biotrend (Köln, Germany), respectively. Dantrolene sodium salt, DHBP (1,1-hiheptyl-4,4-bipyridinium) dibromide and ryanodine were purchased from Tocris Bioscience (Bristol, UK) and the stock solutions were made in DMSO. Live cell calcium imaging was performed in sterile μ-dishes (35 mm high, ibi treat surface) purchased from ibidi (Münich, Germany). Protease inhibitors (complete protease inhibitor cocktail), nitrocellulose membrane (Protran BA83), western bright ECL kit and X-ray films were purchased from Roche (IN, USA), Whatman (NJ, USA), Advansta (CA, USA) and Fujifilm super RX (Tokyo, Japan) respectively. Mouse monoclonal α-GFP primary antibody (catalog number: A-11120), rabbit polyclonal Gαi1/2 (catalog number: 371723) and Gαo/i (catalog number: 371726) primary antibodies and the secondary antibodies (mouse – catalog number: A00160 and rabbit – catalog number: A00098) were purchased from Molecular Probes (Eugene, OR, USA), Calbiochem (Billerica, MA, USA) and Genscript (NJ, USA), respectively.
EtBE was purchased from Sigma-Aldrich (> 99.5% purity). Odorant solutions were prepared freshly for every experiment in the assay buffer of stock concentration of 100 mm. The desired odorant concentration was prepared by serial dilution of stock odorant solution in assay buffer. Assay buffer was prepared by adding 1 part of 1 m HEPES to 49 parts of 1× HBSS. The pH of the buffer was adjusted to 7.3 with sodium hydroxide.
Expression vector
The odorant receptors of Drosophila melanogaster (dORs) used in this study are pCDNA3-dOr22a-GFP and pCDNA3-dOr83b-GFP (Orco) (Neuhaus et al., 2005). Human wild-type Gα subunits, GαoA, Gαi1 and Gαi2, were purchased from Missouri S&T cDNA Resource Center (sequence information of the proteins is available from http://www.cdna.org/Alpha-Subunits-c70.html).
Cell culture and transfection
HEK293T cells were maintained as an adherent culture in DMEM supplemented with 10% FCS and penicillin (100 U/mL final concentration)/streptomycin (100 μg/mL) at 37 °C with 5% CO2. For transfection, HEK293T cells were cultured at a density of ˜1 × 106 cells per well of a six-well plate and transiently transfected with 1 μg of pcDNA3-dOr22a-GFP and 1 μg of pcDNA3-dOr83b (Orco)-GFP (dORs) using 7 μL of lipofectamine in 500 μL of serum-free medium (Opti-MEM). For over-expression studies, 1 μg of pcDNA3.1-Gαo or pcDNA3.1-Gαi1 or pcDNA3.1-Gαi2 was also transiently transfected together with 1 μg of each dOR. Eight to 12 h post-transfection cells were split (1:5) into μ-dishes for calcium imaging or into 12-well plates with 12 mm poly-l-lysine-coated cover slips for transfection quantification.
Pharmacology
HEK293T cells were incubated with 500 ng of PTX per mL of DMEM for 2–3 h at 48 h post-transfection. For experiments with calcium-free buffer, the medium was replaced with 900 μL of calcium-free buffer [standard assay buffer minus calcium chloride with 1 mm ethylene glycol tetraacetic acid (EGTA)] just before imaging. HEK293T cells were treated with dantrolene sodium (10, 20 and 40 μm) or DHBP dibromide (5, 10 and 20 μm) or ryanodine (10, 20 and 40 μm) for 20–30 min prior to imaging.
Western blot
HEK293T cells were harvested 2 days after transfection (dOr22a + Orco, dOr22a + Orco + Gαo, dOr22a + Orco + Gαi1 and dOr22a + Orco + Gαi2) with ice cold homogenization buffer (50 mm HEPES and 0.2 mm EGTA) with protease inhibitors and homogenized using a dounce homogenizer. Cell debris and nuclei were removed by centrifugation (2000 g, 5 min at 4 °C), supernatant was further centrifuged (> 18 000 g, 1 h) and the resultant membrane pellet was solubilized in resuspension buffer (50 mm HEPES, 0.2 mm EGTA, 5 mm MgCl2 and 100 mm NaCl). Samples were loaded on 10% SDS-PAGE gels, and transferred to a nitrocellulose membrane. The nitrocellulose membranes were blocked with PBS (137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4.2H2O and 2 mm KH2PO4, pH 7.4) containing 5% non-fat dry milk and incubated with mouse monoclonal α-GFP antibody 1:1000 in 5% milk for 1 h. After washing in PBS, membranes were incubated with mouse secondary antibodies coupled to HRP 1:10 000 in 5% milk for 1 h. Detection was performed with ECL on X-ray films. After the detection of GFP bands the nitrocellulose membranes were stripped, blocked and incubated with rabbit Gαi1/2 antibody 1:1000 in 5% milk for 1 h. After washing in PBS, membranes were incubated with goat-α-rabbit secondary antibodies coupled to HRP 1:10 000 in 5% milk for 1 h. Gαi1/2 subunits were detected by the same method described above and after detection nitrocellulose membranes were stripped again, blocked and incubated with rabbit Gαo/i antibody 1:1000 in 5% milk for 1 h. After this step the membranes were treated as for Gαi1/2 antibody staining. Western blots were quantified using the Gel Analysis tools in ImageJ (http://rsbweb.nih.gov/ij/). For loading control we used antibodies against the membrane-bound protein reggie1/flotillin2 (BD Biosciences – catalog no. 610383).
Quantification of vector expression
At 48 h, post-transfected cells were washed in PBS (3×), then fixed in 4% paraformaldehyde solution in PBS for 20 min, then again washed with PBS (3×). Nuclei were stained by adding 300 μL of DAPI (300 nm in PBS) for 5 min. Then, cells were rinsed with PBS several times. Cells were mounted on glass slides using mounting solution. Cells were imaged using a laser scanning microscope (LSM 510 Meta; Carl Zeiss, Oberkochen, Germany) equipped with an oil immersion objective (40× objective, NA = 1.30; Carl Zeiss), and the percentage of GFP-positive cells was quantified (expression efficiency).
In vitro calcium imaging
Forty-eight-hour post-transfected HEK293T cells in μ-dishes were washed twice with assay buffer. One milliliter of assay buffer containing 2 μm fluo-4 AM, 0.01% pluronic acid and 2.5 mm probenecid was added to each dish and incubated at 37 °C for 45 min. The fluo-4 solution was removed and washed twice with the assay buffer and replaced with 900 μL of assay buffer. The dishes were then incubated for a further 30 min at 37 °C prior to calcium imaging. Fluorescence images were acquired through the bottom of the dish using an inverted laser scanning confocal microscope (LSM 510 Meta; Carl Zeiss) equipped with air objective (20× objective, NA = 0.5; Carl Zeiss). Excitation wavelength was 488 nm, and the detection filter was a 505-nm long pass filter. For every measurement the detector gain was adjusted in such a way that PMT detectors were not saturated. We imaged with an acquisition rate of 0.2 Hz for 250 s for all experiments except for calcium-free buffer experiments (0.1 Hz for 300 s). One hundred microliters of the odorant (e.g. 100 mm EtBE was added so that the final concentration of the odorant was 10 mm) or the solvent (assay buffer; control) was added to the cells in 900 μL of buffer between the 10th and 11th frames. To determine the maximal fluorescence of the cells, ionomycin (final concentration 2 μm) was added between the 40th and 41st frames.
Data analysis
Data analysis was done using custom made routines written in KNIME (Konstanz Information miner; http://www.knime.org/) and R (http://www.r-project.org/). The background fluorescence of the images (area of the image excluding the area of the cells) was subtracted from the mean fluorescence intensity of each frame. For each cell, the average fluorescence intensity of first ten frames before any application was defined as Bo (baseline odor). The fluorescence intensity before ionomycin addition (39th frame) was defined as Bi (baseline ionomycin). Similarly, for each cell, maximum fluorescence intensity after odor or assay buffer addition (11–39th frames) and after ionomycin addition (40–50th frames) were defined as Ro and Ri, respectively (response odor, and response ionomycin). Stimulus responses were calculated as Ro/Bo (odor response) and Ri/Bi (ionomycin response), respectively. Cells with Bo < 3000 a.u. (putatively without GFP expression and/or no fluo-4 loading) and Ri/Bi < 1.5 (no ionomycin response indicating dead cells) were excluded during analysis.
Data were log-transformed to reduce the right-skew of the distribution. Results were given as median with 25 and 75% quartiles (log transformed), and n represents the number of cells from 10–50 different experiments of 4–20 independent transfections. Differences were tested statistically with Mann–Whitney U test (comparison between two groups) and Kruskal–Wallis rank sum test (comparison between more than two groups). Multiple comparisons were performed after the Kruskal–Wallis rank sum test using a post-hoc multiple comparisons test.
Results
Go inactivation leads to behavioral deficits in Drosophila
We performed a behavioral screen to test whether the Gαo subgroup of G proteins is involved in olfactory responses. We expressed PTX [a specific inhibitor of Go in Drosophila (Katanaev & Tomlinson, 2006)] in all olfactory receptor neurons that express the olfactory co-receptor Orco, using the GAL4-UAS system (Duffy, 2002). Flies were kept in a chamber and could choose between remaining in the chamber or entering one of two vials: one with an attractive odor (apple vinegar) and one with neutral mineral oil. The percentage of flies choosing the vinegar trap was higher in the control group than in the PTX group, while more PTX flies remained in the chamber (Fig.1; Student’s t-test, P = 0.02, n = 5 for the PTX group and n = 28 for the control group). These data suggest that flies may be less sensitive towards odorants when levels of active Go are reduced in all the ORNs expressing Orco.
Figure 1.
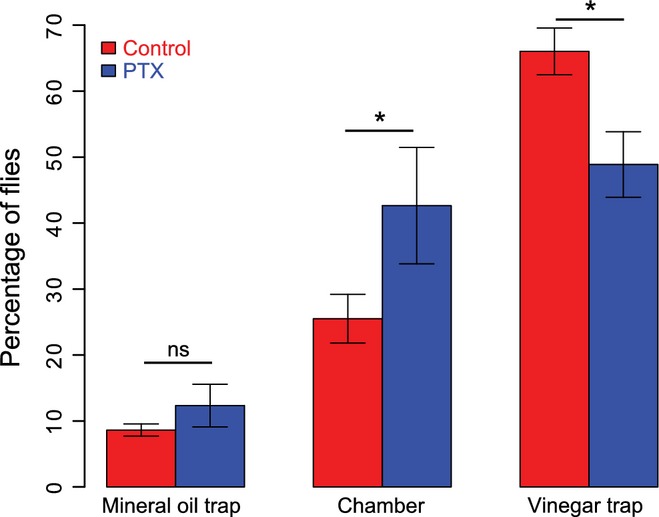
Flies with reduced Gαo activity showed olfactory behavioral deficits. Bar plot (mean ± SEM) showing the percentage of flies that entered the non-odor (mineral oil; left) or the odor (vinegar; right) trap or that did not enter any trap (chamber; middle). Asterisks indicate statistical significance between the control (red) and PTX (blue) group for each condition, Student’s t-test, P = 0.02, n = 5 and 28 experiments for the PTX and control group, respectively.
Heterologous expression of Drosophila ORs leads to odor-induced calcium influx
To study the role of Go proteins in detail we used a heterologous cell system by transiently expressing dORs in HEK293T cells. We used a particular odorant receptor, dOr22a, which is highly responsive to EtBE in vivo (Hallem et al., 2004; Pelz et al., 2006), together with the olfactory co-receptor dOrco (C-terminal GFP fusion OR constructs, see Materials and methods). We quantified transfection efficiency by counting GFP-positive cells. Transfection efficiency was 52 ± 3.3% (mean ± SEM, n = 6 transfections, > 500 cells in total; see Fig.2). Most of the GFP fluorescence was observed in cytoplasm (Fig.2). To confirm that the receptors were also correctly localized in the plasma membrane, we isolated membranes from cells expressing dORs. We obtained GFP-positive bands on Western blots corresponding to the ˜70-kDa proteins (Lane 2; Fig.2D), which is close to the calculated molecular weight of dORs fused to GFP (dOr22a-GFP ˜74 kDa and Orco-GFP ˜81 kDa). Cell membranes of non-transfected HEK293T cells show no bands (Lane 1; Fig.2D). These results indicate that dORs are expressed in HEK293T cells and can be transported to the plasma membrane.
Figure 2.
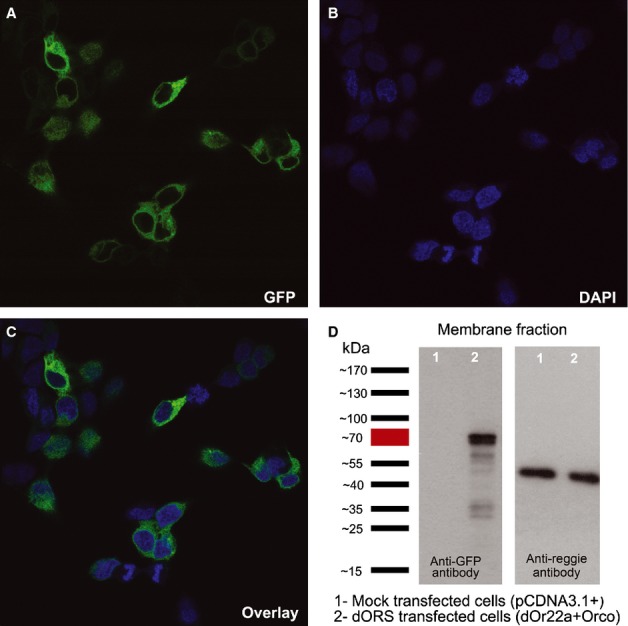
Expression of dORs in HEK393T cells. (A–C) Confocal images of HEK293T cells transfected with dOr22a and Orco (48 h post transfection): GFP expression (A), DAPI staining (nuclei, B) and the overlay (C). (D) Western blots of receptor-transfected and mock transfected HEK293T cell membranes showing the expression of dORs (GFP staining; left blot); the membrane-bound protein reggie1/flotillin2 was used as the loading control (right blot). Representative blot from eight individual transfections.
We incubated the cells with the calcium-sensitive dye fluo-4 and recorded odor-evoked calcium transients (Fig.3A). Adding the odorant EtBE (10−2m) to control (mock-transfected) cells elicited a negligible calcium response. In contrast, upon cell transfection with dORs, 32% of the cells showed a robust calcium response to EtBE (Fig.3B; Kruskal–Wallis test, P< 2.2e-16, 348 ≤ n≤6663 cells). As mock-transfected cells do not increase their calcium concentration upon odor addition, and our expression efficiency is 52% (see above), this number of 32% responding cells corresponds to 62% of transfected cells. Addition of solvent alone to transfected or mock-transfected cells produced a negligible response (Fig.3B). Odor responses were concentration dependent, with stronger responses to higher odor concentrations (10−7 to 10−2 m; data not shown). We used 10−2m EtBE for the subsequent experiments. In a Ca2+-free buffer (1 mm EGTA), odor-induced calcium responses were abolished (Fig.3C; Mann–Whitney U test, P< 2.2e-16, 536 ≤ n ≤ 1407 cells), and hence the Ca2+ current necessitates a membrane-bound calcium channel, which could be either the olfactory receptor itself or another channel activated by a second messenger cascade. We tested the viability of the cells using the calcium ionophore ionomycin, which releases calcium mostly from intracellular stores and not from extracellular space (Yoshida & Plant, 1992; Mason & Grinstein, 1993; Morgan & Jacob, 1994; Cavarra et al., 2003). Addition of ionomycin elicited a strong calcium response under all conditions. Responses elicited by ionomycin in Ca2+-free buffer were not significantly different from the ionomycin response in calcium buffer (Supporting Information Fig. S1A; Mann–Whitney U test, P = 0.38, 536 ≤ n ≤ 1407 cells), confirming the localization of ionomycin to intracellular Ca2+-store membranes.
Figure 3.
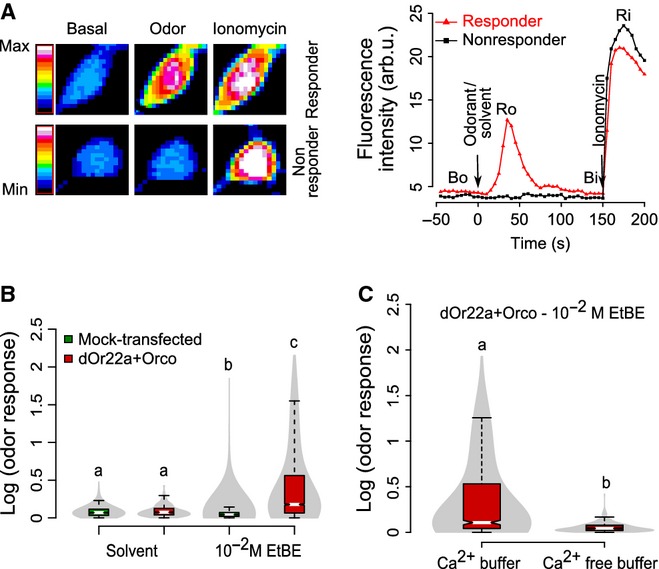
dORs expressed in HEK293T cells are functional. (A) Transfected HEK293T cells respond to odor stimulus. Mean fluorescence intensity change of a responder and a non-responder cell transfected with dORs to EtBE 10−2 m dilution is shown as a false color-coded picture (left) and as the time course (right) for each of the three stages of our calcium assay. Time points for Bo, Ro, Bi and Ri are shown. (B) Violin plot (combination of boxplot and kernel density distribution plot; boxplots were colored based on the group; the gray color indicates the probability density of the data) of the calcium response [log (odor response) = log (Ro/Bo)] of mock and dORs transfected cells to solvent (assay buffer, 348 ≤ n≤560 cells from ten independent experiments) or to odorant (EtBE 10−2 m, 1161 ≤ n≤6663 cells from 12 to 50 independent experiments). Medians with different letters differ significantly (Kruskal–Wallis test, P<2.2e-16, 348 ≤ n≤6663 cells). (C) Calcium-free buffer abolishes odor responses. Violin plot of odor-induced calcium response of transfected cells with (n = 537 cells from ten independent experiments) or without (n = 1411 cells from 20 independent experiments) the presence of calcium in extracellular buffer. Medians with different letters differ significantly (Mann–Whitney U test, P<2.2e-16, 537 ≤ n≤1411 cells).
We noted that ionomycin responses were smaller in cells that had previously responded to an odor (‘responders’) than in the other cells (‘non-responders’, Fig. S1B, Kruskal–Wallis test, P < 2.2e-16, 1161 ≤ n ≤ 4574 cells). Responders and non-responders were classified based on the odor response, with a threshold at Ro/Bo = 1.5 (Fig. S2). This suggests that a preceding odor response leads to a reduction of available Ca2+ in a subsequent ionomycin response, possibly due to depletion of intracellular calcium stores. Therefore, we tested for a contribution of intracellular calcium stores to the odorant-evoked responses. HEK293 cells express ryanodine receptors (Querfurth et al., 1998). The substance ryanodine activates ryanodine receptors in the nanomolar range, and blocks them in the micromolar range (Meissner, 1994; Sutko et al., 1997). We blocked calcium-induced calcium release (CICR) channels using 10–40 μm ryanodine (959 ≤ n ≤ 1331 cells), and saw a significant and dose-dependent reduction in odorant-evoked Ca2+ responses (Fig.4A; Kruskal–Wallis test, P < 2.2e-16, 959 ≤ n ≤ 6663 cells). Other blockers of CICR also led to a reduced response [dantrolene (1404 ≤ n ≤ 2071 cells) and DHBP dibromide (594 ≤ n ≤ 1952 cells), Fig.4B and C, Kruskal–Wallis test, P <2.2e-16, 594 ≤ n ≤ 6663 cells)]. In total about 50% of odor-induced calcium release was from the intracellular calcium sources via CICR channels. These experiments show that an odorant-induced calcium response is amplified by CICR from intracellular stores.
Figure 4.
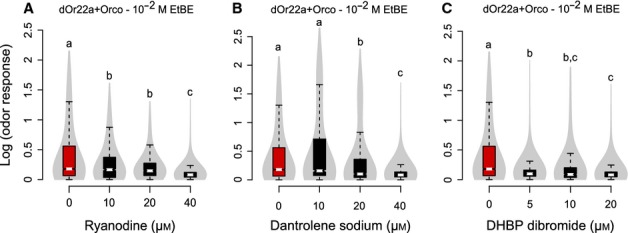
CICR inhibitors reduced odor-induced calcium responses. Violin plots of odor-induced calcium responses [log (odor response) = log (Ro/Bo)] of transfected cells with (black boxplots, 594 ≤ n≤2071 cells from 10 to 20 independent experiments) or without (red boxplot, n = 6663 cells from 50 independent experiments) pretreatment of the CICR inhibitors ryanodine (A), dantrolene sodium (B) and DHBP dibromide (C). Medians with different letters are statistically significant (Kruskal–Wallis test, P<2.2e-16, 594 ≤ n≤6663 cells).
Go and Gi contribute to OR response of dOr22a in vitro
Having shown that dOr22a is functional when heterologously expressed in HEK cells, we sought to investigate whether Go is involved in this response, as suggested by the behavioral effects seen in the living fly. We applied PTX to the transfected cells (dORs; n = 1380 cells), and found that odorant responses decreased significantly (Fig.5A, Mann–Whitney U test, P <2.2e-16, 1380 ≤ n≤6663 cells). The fact that responses were not abolished completely suggests that either the effect of PTX was not complete or the PTX-sensitive cascade represents only part of the odorant transduction cascade. We show here that insect ORs are able to link to a mammalian G-protein signaling cascade. However, in mammalian cells PTX does not inhibit only Go (as in insects), but also inhibits Gi and Gt (Gilman, 1987). We did not consider Gt as a relevant signaling molecule because it is a G protein specific to the visual system and is not expressed in HEK cells (Reeves et al., 1996). While insect genomes encode only one type of Gi, mammals encode three Gαi subunits: Gαi1, Gαi2 and Gαi3; all three are expressed in HEK cells according to the RT-PCR data while Gαo mRNA is present at lower levels (Atwood et al., 2011). We detected strong Gαo/i signals in these cells by Western blots using antibodies separately recognizing Gαi1/2 and Gαo/i3 subunits (Fig. S3A). Therefore, the PTX effect on HEK293T cell odor responses could be due to the inhibition of Go, Gi1–3, or any combination thereof.
Figure 5.
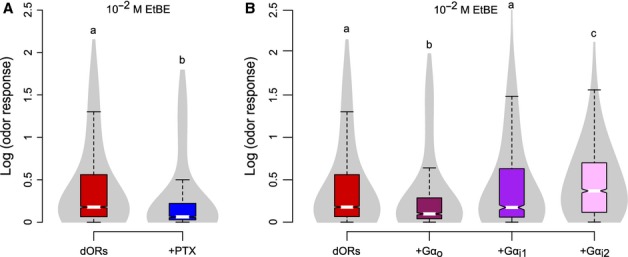
Odors induce calcium increase in HEK cells that heterologously express dORs via a Go/i-mediated pathway. (A) Treatment with PTX reduces odor responses. Violin plot of transfected cells with (blue boxplot, n = 1380 cells from 23 independent experiments) or without (red boxplot, n = 6663 cells from 50 independent experiments) treatment of the Gαo/i inhibitor PTX. Medians with different letters differ significantly (Mann–Whitney U test, P<2.2e-16, 1380 ≤ n≤6663 cells). (B) Over-expression of Gαo reduces odor responses, while over-expression of Gαi2 increases odor responses. Violin plot of transfected cells with (912 ≤ n≤1531 cells from 10 to 20 independent experiments) or without (n = 6663 cells from 50 independent experiments) over-expression of human Gαo/i subunits. Medians with different letters differ significantly (Kruskal–Wallis test, P<2.2e-16, 912 ≤ n≤6663 cells).
We next over-expressed Gαo, Gαi1 or Gαi2 in HEK293T cells together with dOr22a and dOrco. This treatment did not modify the expression levels of dORs (Fig. S3). Then, we measured odorant-evoked calcium responses in these cells, using calcium imaging. We found that over-expression of Gαi2 (n = 1531 cells) increased the response, over-expression of Gαi1 (n = 1007 cells) did not affect the response and over-expression of Gαo (n = 912 cells) decreased the response (Fig.5B, Kruskal–Wallis test, P<2.2e-16, 912 ≤ n≤6663 cells). These results indicate that Gαi2 contributes to dOr22a-Orco activity in HEK293T cells. G proteins are heterotrimeric with three subunits: α, β and γ. When a G protein is activated, it leads to dissociation of the heterotrimer into Gα-GTP and Gβγ. Gα-GTP and Gβγ may independently activate signaling pathways in the cell. Thus, the decrease in calcium response of cells over-expressing Gαo could be due to sequestration of Gβγ in the cells by the over-expressed Gαo subunit, leading towards low levels of Gβγ available for Gαi2 activity, as shown in other systems (Katanayeva et al., 2010).
Go and Gi amplify the physiological response of Or22a in vivo
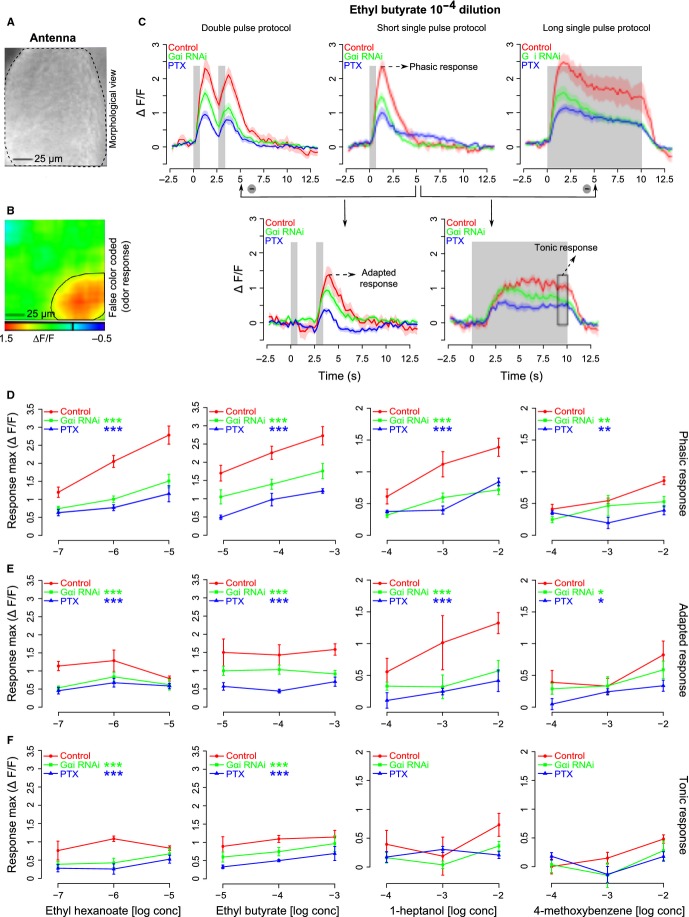
With this information from heterologous expression in hand, we went back to the intact animal. G proteins are involved in neural signaling. In particular, in Drosophila GABAB receptors that use Go for signaling are expressed on receptor cell axon terminals (Olsen & Wilson, 2008; Root et al., 2008). We therefore quantified odorant-evoked calcium responses in the dendritic segment and the soma, excluding axonal terminals. We performed in vivo calcium imaging from Or22a-expressing neurons in intact antennae of Drosophila (Fig.6A) with or without inhibition of the function of Gαo/i subunits in all the ORNs expressing the odorant receptor 22a (Or22a-GAL4). We used the genetically encoded calcium-dependent fluorescent sensor G-CaMP1.3 in Or22a ORNs. Calcium responses were quantified from the fluorescence emitted through the intact cuticle from an area (as shown in Fig.6B) corresponding to the area of expression of Or22a (de Bruyne et al., 2001; Dobritsa et al., 2003). Responses increased with increasing odor concentration, and for EtBE were in the range 1.5–3% ΔF/F for an odorant concentration from 10−5 to 10−3 (Fig.6D; two-way anova, F2,44 = 15, P<1.0e-05, n = 5–7 flies).
Figure 6.
Odor-mediated calcium changes in the antenna of female flies are affected by the levels of Go/i subgroup of G proteins. (A) Morphological view of an antenna of a female Drosophila melanogaster; black dotted lines mark the margin of the antenna. Image was taken from a CCD camera. (B) False color-coded picture of the response to ethyl butyrate 10−5 m dilution measured on the antenna; the black circle indicates the area from which responses were calculated. Orientation as in A. (C) Mean traces of response to ethyl butyrate 10−4 m dilution for different stimulation protocols and genotypes tested (shading indicates SEM, n = 5–7 flies for every genotype). Gray bars in the plot indicate the time and duration of odor delivery. Red, green and blue colors indicate control, Gαi RNAi (downregulation of Gαi) and PTX (reduction of Gαo) groups of flies, respectively, and the color coding is maintained throughout the figure. Response magnitudes of the phasic response, adapted response and tonic response were calculated from the traces labeled with the same names (for more details see Materials and methods). (D–F) Dose–response curves (mean ± SEM) for the phasic response, adapted response and tonic response, respectively, for ethyl hexanoate (left most), ethyl butyrate (middle left), 1-heptanol (middle right) and 4-methoxy benzene (right most) to the genotypes tested. Asterisks indicate statistical significance compared with the control group for all the concentrations tested, two-way anova (treatment and concentration are used as factors; ***P<0.001, **P<0.01, *P = 0.05, n = 5–7 flies for every genotype).
We inhibited Go by co-expressing PTX, and reduced the levels of Gi by driving an RNA-interference construct. The efficiency of both transgenic lines in affecting Go and Gi has been tested before (Katanaev et al., 2005; Katanaev & Tomlinson, 2006; Dietzl et al., 2007; Kopein & Katanaev, 2009; Bredendiek et al., 2011). We needed a separate treatment for Gi because, unlike in mammals, PTX does not inhibit Gi signaling in insects (Katanaev & Tomlinson, 2006). Both treatments led to a significant reduction in calcium responses (Fig.6C–F), irrespective of whether we used a very potent ligand (EtHE or EtBE), an intermediate ligand (HepL) or a weak ligand (MeBM). This indicates that both Gi and Go are involved in sensory signaling in vivo. The effect of PTX treatment was stronger than the Gαi-RNAi treatment for the odorant EtBE (Fig.6D and E), which may indicate that Go has a stronger role than Gi in these cases.
Go and Gi are involved in both early and late response phases
Calcium responses in the dendrites of sensory cells do not only reflect signal transduction cascades, but also events linked to sensory adaptation (Leinders-Zufall et al., 1998). As these are also linked to second messenger cascades, we specifically addressed whether Gi or Go are involved in sensory adaptation by choosing appropriate odorant pulse protocols. Single-pulse stimulation was used to test the early response phase (phasic response, Fig.6C). Double pulses were used to probe for adaptation or sensitization of ORNs: responses to the second stimulus are always lower than those to the first stimulus. If Gi or Go were involved in adaptation or sensitization, we would expect a modified response to the second odor pulse in the respective mutants. The response to the second pulse was quantified by subtracting the single-pulse response from the double-pulse response (Fig.6C). The tonic response component was measured using a 10-s long odor pulse protocol, and was isolated by subtracting the response of a 1-s short odor pulse prior to quantification (Fig.6C). We found that dOr22a-cells responded both to the first pulse and to the second pulse, and that they responded to 10-s long pulses for the entire length of the 10 s (Fig.6C). Interestingly, responses to the second pulse (adapted response) and the late component of the response to the 10-s pulse (tonic response) had an inverse odorant-concentration response: for best ligands high concentrations led to weaker responses, for intermediate ligands the response was concentration independent (two-way anova, F2,35 = 3, P = 0.6 for EtHE, F2,42 = 0.19, P = 0.8 for EtBE, n = 5–7 flies), and for weak ligands the response increased with increasing concentration (two-way anova, F2,35 = 4.2, P = 0.02 for HepL, F2,24 = 4.8, P = 0.02 for MeBM, n = 5–7 flies), indicating stronger adaptation to better ligands (Fig.6E and F). Overall, however, with increasing phasic response, the adapted and the tonic responses increased only slightly, as seen by the significant but shallow regression slope in Fig. S4. Reducing the effective concentration of Go or of Gi in olfactory receptor neurons reduced but did not abolish calcium responses for all aspects of the odor response: the phasic response (Fig.6D, two-way anova, F2,43 = 45, P<2.7e-11 for EtHE, F2,44 = 45, P<1.9e-11 for EtBE, F2,41 = 24.7, P<8.9e-08 for HepL, F2,27 = 8.5, P = 0.001 for MeBM, n = 5–7 flies), the adapted response (Fig.6E, two-way anova, F2,35 = 11.7, P<1.2e-4 for EtHE, F2,42 = 23.6, P<1.3e-07 for EtBE, F2,35 = 13.4, P<4.6e-05 for HepL, F2,24 = 4.4, P = 0.02 for MeBM, n = 5–7 flies) and the tonic response (Fig.6F, two-way anova, F2,37 = 9.3, P<5.0e-4 for EtHE, F2,37 = 8.4, P<9.3e-04 for EtBE, F2,33 = 1.4, P = 0.2 for HepL, F2,23 = 1.1, P = 0.34 for MeBM, n = 5–7 flies; note that the tonic response of weak ligands was not a statistically significant response), arguing in favor of a role of these G proteins that is directly related to the receptor protein itself and its signal transduction mechanism, rather than to an associated second messenger cascade.
Discussion
Go/i subgroup of G proteins is important for olfactory signaling
Insect ORs are seven-transmembrane proteins, but their relationship with GPCRs remains unclear. Most importantly, their topology is inverted with respect to canonical GPCRs, in that the C terminus is extracellular (Benton et al., 2006; Lundin et al., 2007; Smart et al., 2008; Tsitoura et al., 2010). Whether these receptors are linked to G proteins remains controversial: some studies show that the OR-Orco heteromer acts as an ionic channel (Sato et al., 2008; Smart et al., 2008; Yao & Carlson, 2010; Nakagawa et al., 2012), while others suggest a combined metabotropic and ionotropic action of the complex (Kain et al., 2008; Wicher et al., 2008; Chatterjee et al., 2009; Deng et al., 2011; Sargsyan et al., 2011; Getahun et al., 2013). In the latter case, the metabotropic action may be most relevant for the late odor responses or for the modulation of odor responses (Wicher et al., 2008; Deng et al., 2011; Getahun et al., 2013). In this study, we show that G proteins are indeed relevant for olfactory transduction in insects. In behavioral experiments, inhibition of Go leads to reduced odor responses (Fig.1). As this effect could derive from the role of G proteins in the periphery, or in the neural network of the antennal lobe (Olsen & Wilson, 2008; Root et al., 2008), we analysed olfactory transduction in vitro. In heterologous expression systems, a contribution by Go/i becomes apparent, and in particular its Gβγ component likely (Fig.5). Because G proteins differ in mammals and in insects, we went back to Drosophila. Using in vivo calcium imaging, we show that this effect is localized to the dendrites, and affects the entire temporal span of an odor response, including the very first odor response (Fig.6).
Multiple cascades are involved in olfaction
In our experiments, manipulation of G protein cascades (Go/i) never completely abolished odor responses, suggesting that a strongly reduced titer of G proteins is sufficient for the response, or that the G proteins studied here are simply a component of the transduction cascade. In particular, there is strong evidence for a parallel ionotropic current (Sato et al., 2008; Smart et al., 2008; Wicher et al., 2008; Yao & Carlson, 2010), and other G proteins (e.g. Gs) may also be involved (Wicher et al., 2008; Deng et al., 2011). Furthermore, with our data we cannot exclude that calcium influx may also be caused by a membrane-bound calcium channel that is controlled by an intracellular second messenger cascade. In HEK cells, the odorant-induced calcium response is amplified by CICR from intracellular stores (Fig.4A–C). Whether this also occurs in vivo remains to be investigated. Together, we obtain a picture that involves multiple cascades, all initiated by the odorant binding to a receptor. Whether these multiple cascades are part of a redundant signaling system, adding stability and reliability to olfactory transduction, or whether these cascades are used to modulate olfactory responses (e.g. by circadian rhythms, attention, arousal states) remains to be investigated.
Different ORs may rely on different heterotrimeric G proteins
The results shown here are obtained with the olfactory receptor dOr22a, a general odorant receptor with a broad odor–response profile, but exquisitely sensitive to a few odorants (EtHE and EtBE) (Hallem et al., 2004; Pelz et al., 2006). Even though all Drosophila ORs belong to the same molecular family, the role that G proteins play in odorant olfaction need not be the same. This would explain why some studies support the involvement of G proteins in olfactory signaling (Kain et al., 2008; Wicher et al., 2008; Chatterjee et al., 2009; Deng et al., 2011; Sargsyan et al., 2011; Getahun et al., 2013), but others appear to suggest differences. For example, inhibition of Go by expressing PTX in all ORNs reduced the odor response measured by electroantennograms and single sensillum spike rates of SSR (Chatterjee et al., 2009), confirming our results. On the other hand, no effect for Go reduction was found in two other studies (Yao & Carlson, 2010; Deng et al., 2011), but in those studies Orco-GAL4 was used and not Or22a, and other odors and concentrations were used. Thus, it may be that different receptors use Go and Gi to a varying degree; other G proteins may also be used by other ORs. Parallel usage of different G proteins by the same receptor has been reported in several systems, including the resulting variety of second messenger cascades (Hermans, 2003). Note that not all insect OR cells express OR receptor proteins. Some receptor cells express receptors from another molecular family (IRs) (Benton et al., 2009), which were not affected by our manipulations in the behavioral experiments and were not studied here.
Potential role of the βγ heterodimer in olfactory transduction
Odorant-induced calcium responses of dORs in HEK293T cells were reduced when inhibiting endogenous Go/i by PTX, and when over-expressing Gαo, while over-expression of the mammalian Gαi2 subunit enhanced the odor response. These results suggest that the Gβγ heterodimer might take a role in the transduction cascade, as shown for other systems: Gαs mediates the expansion of Drosophila wings after hatching, and when Gαo is over-expressed, Gαo antagonizes the effect of Gαs by competing with the Gβγ heterodimer, thus reducing Gβγ effective concentration (Katanayeva et al., 2010). We propose a similar mechanism for our results: over-expression of Gαo could antagonize the function of Gαi2 by sequestering Gβγ heterodimers and thus reducing its effective concentration.
There is a certain difference in terms of involvement of Gαo/i subunits in Or22a-mediated signaling as judged by our in vitro vs. in vivo experiments, as Drosophila Gαo and Gαi are involved in vivo, while in HEK293T cells the mammalian Gαo appears to prevent the signaling mediated by Gi2. We wish to stress, however, that in this reconstituted system, the identity of the exact mammalian Gα-subunit coupling to the Drosophila ORs could not be predicted beforehand. Sequence similarity within the Gαo/i subfamily of G proteins is very high, with the sequence identity of Drosophila Gαo to mammalian members of this family being 82% (to human Gαo), 69% (human Gαi1), 68% (human Gαi2) and 69% (human Gαi3). Drosophila Gαi has 65, 78, 76 and 77% identity to the human proteins, respectively (percentage identities mentioned here are obtained from sequence alignment using Clustal W). We propose that the ability of Or22a to couple to mammalian Gαi2 can be used as evidence that this dOR signals through Go/i proteins, as corroborated by our in vivo experiments. Further, this reconstituted system allowed us to predict the important role of the Gβγ subunits and internal Ca2+ stores in Or22a-mediated responses.
Certain care should be taken when interpreting the data we obtained in this reconstituted system. It is formally possible that in the HEK293 cells we use, the Gα and the Gβγ subunits released upon the action of dORs open channels which are not necessarily present in the insect olfactory neurons, or that these human G proteins act on the human channels in a slightly different way from insect cells. Given this, the primary conclusion we draw from the analysis in the reconstituted system is that the dORs under study can activate heterotrimeric G proteins – a conclusion of an unquestionable importance, given the inverted topology of insect olfactory receptors as compared with normal GPCRs. However, we would like to go further and, given the high degree of conservation between mammalian and insect proteins and signaling systems, propose that also in the Drosophila olfactory neurons, the G protein subunits released upon the action of dORs can open calcium channels in a way similar to that observed in HEK293 cells.
Transduction cascade might be similar to vomeronasal receptors
It is not unusual that the Go/i group of heterotrimeric G proteins is involved in olfactory signal transduction. In the vomeronasal system of vertebrates, two groups of olfactory receptors are described: V1Rs (vomeronasal receptor type-1) and V2Rs (vomeronasal receptor type-2) and are shown to signal via the Go/i subgroup of G proteins. V1Rs activate Gi2 and induce Gβγ-mediated calcium signaling upon ligand binding, while V2Rs activate Go and also induce calcium influx (Berghard & Buck, 1996; Berghard et al., 1996; Lucas et al., 2003; Touhara & Vosshall, 2009; Kaupp, 2010). Here, we show that also insect ORs activate the Go/i subgroup of G proteins and signal via them, in a way reminiscent of V1Rs and V2Rs. Note, however, that insect ORs and V1Rs and V2Rs have evolved entirely independently: V1Rs and V2Rs are genuine GPCRs, while insect ORs have an inverted membrane topology. The similarity in transduction cascades is, to our knowledge, entirely convergent.
Transduction and signaling with multithreading
Taken together, we propose that Drosophila ORs activate (at least) two parallel pathways upon odor detection, both leading to the depolarization of ORNs. One pathway is via a ligand-gated ion channel, i.e. an ionotropic pathway, as proposed elsewhere (Sato et al., 2008; Smart et al., 2008). Both the ligand selective OR and the co-receptor (Orco) were shown to contribute to the cation channel activity (Nichols et al., 2011; Pask et al., 2011; Nakagawa et al., 2012). This ionotropic mechanism was not studied here, and indeed may even use an ion channel that is detached from the OR/Orco heteromer. The second pathway acts via Go/i (metabotropic pathway) using also the Gβγ heterodimer. Specifically, upon activation of the receptor, Gαo/i and the Gβγ heterodimer are released and Gβγ activates the signaling cascade. Gβγ could activate the influx of calcium ions through Orco or the OR-Orco complex, or perhaps via other channels. Calcium responses from either of the two pathways may then be amplified by CICR from intracellular stores. These results add an intriguing component to the still open full picture of insect olfactory transduction.
Acknowledgments
This work was funded by the Graduate School of Chemical Biology, University of Konstanz, Germany, by the University of Konstanz, and by the Swiss National Science Foundation (grant No. 31003A_138350 to V.L.K.). We thank Alexey Koval for contributing to the experiments. We thank Professor Eva M. Neuhaus, Charite – Universitatsmedizin Berlin, Germany, for the kind gift of dOR receptor constructs (pcDNA3-Or22a-GFP and pcDNA3-Or83b-GFP) used in in vitro experiments, and Martin Horn, University of Konstanz, for helping with the image analysis in KNIME (http://knime.org/). Images were recorded for in vitro experiments from the Bioimaging Center (BIC) of the University of Konstanz, Germany. The authors have no financial interests or conflict of interest to disclose.
Glossary
- CICR
calcium-induced calcium release
- DHBP dibromide
1,1-diheptyl-4,4-bipyridinium dibromide
- DMEM
Dulbecco’s modified Eagle medium
- DMSO
dimethyl sulfoxide
- dORs
Drosophila odorant receptors
- EGTA
ethylene glycol tetraacetic acid
- EtBE
ethyl butyrate
- EtHE
ethyl hexanoate
- FCS
fetal calf serum
- GPCRs
G-protein-coupled receptors
- HBSS
Hank’s balanced salt solution
- HEK293T cells
human embryonic kidney 293T cells
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HePL
1-heptanol
- IRs
ionotropic receptors
- KNIME
Konstanz Information Miner
- MeBM
4-methoxybenzene
- ORNs
olfactory receptor neurons
- ORs
odorant receptors
- PTX
pertussis toxin
- V1Rs
vomeronasal receptor type-1
- V2Rs
vomeronasal receptor type-2
Supporting Information
Fig. S1. Calcium from intracellular stores contributes to the odor-mediated calcium response in HEK293T cells.
Fig. S2. Classification of responders and non-responders.
Fig. S3. Expression levels of dORs were unaltered by over-expression of Gαo/i.
Fig. S4. Adapted and tonic responses are less variable than phasic responses.
References
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. doi: 10.1186/1471-2164-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghard A, Buck LB. Sensory transduction in vomeronasal neurons: evidence for G alpha o, G alpha i2, and adenylyl cyclase II as major components of a pheromone signaling cascade. J. Neurosci. 1996;16:909–918. doi: 10.1523/JNEUROSCI.16-03-00909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghard A, Buck LB, Liman ER. Evidence for distinct signaling mechanisms in two mammalian olfactory sense organs. Proc. Natl. Acad. Sci. USA. 1996;93:2365–2369. doi: 10.1073/pnas.93.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boto T, Gomez-Diaz C, Alcorta E. Expression analysis of the 3 G-protein subunits, Gα, Gβ, and Gγ, in the olfactory receptor organs of adult Drosophila melanogaster. Chem. Senses. 2010;35:183–193. doi: 10.1093/chemse/bjp095. [DOI] [PubMed] [Google Scholar]
- Bredendiek N, Hutte J, Steingraber A, Hatt H, Gisselmann G, Neuhaus EM. Go alpha is involved in sugar perception in Drosophila. Chem. Senses. 2011;36:69–81. doi: 10.1093/chemse/bjq100. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors – a molecular-basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Cavarra MS, Assef YA, Kotsias BA. Effects of ionomycin and thapsigargin on ion currents in oocytes of Bufo arenarum. J. Exp. Zool. 2003;297A:130–137. doi: 10.1002/jez.a.10237. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Roman G, Hardin PE. Go contributes to olfactory reception in Drosophila melanogaster. BMC Physiol. 2009;9:22. doi: 10.1186/1472-6793-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhang W, Farhat K, Oberland S, Gisselmann G, Neuhaus EM. The stimulatory Galpha(s) protein is involved in olfactory signal transduction in Drosophila. PLoS ONE. 2011;6:e18605. doi: 10.1371/journal.pone.0018605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- Getahun MN, Olsson SB, Lavista-Llanos S, Hansson BS, Wicher D. Insect odorant response sensitivity is tuned by metabotropically autoregulated olfactory receptors. PLoS ONE. 2013;8:e58889. doi: 10.1371/journal.pone.0058889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol. Therapeut. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244:790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- Kain P, Chakraborty TS, Sundaram S, Siddiqi O, Rodrigues V, Hasan G. Reduced odor responses from antennal neurons of Gq, phospholipase C, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J. Neurosci. 2008;28:4745–4755. doi: 10.1523/JNEUROSCI.5306-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G-J, Gong Z-J, Cheng J-A, Mao C-G, Zhu Z-R. Cloning and expression analysis of a G-protein subunit- in the rice water weevil Lissorhoptrus oryzophilus Kuschel. Arch. Insect Biochem. 2011;76:43–54. doi: 10.1002/arch.20403. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, Tomlinson A. Multiple roles of a trimeric G protein in Drosophila cell polarization. Cell Cycle. 2006;5:2464–2472. doi: 10.4161/cc.5.21.3410. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Sémériva M, TomLinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Katanayeva N, Kopein D, Portmann R, Hess D, Katanaev VL. Competing activities of heterotrimeric G proteins in Drosophila wing maturation. PLoS ONE. 2010;5:e12331. doi: 10.1371/journal.pone.0012331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat. Rev. Neurosci. 2010;11:188–200. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- Kopein D, Katanaev VL. Drosophila GoLoco-protein pins is a target of Galpha(o)-mediated G protein-coupled receptor signaling. Mol. Biol. Cell. 2009;20:3865–3877. doi: 10.1091/mbc.E09-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: specificity of activation and role in transduction. J. Neurosci. 1998;18:5630–5639. doi: 10.1523/JNEUROSCI.18-15-05630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40:551–561. doi: 10.1016/s0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- Lundin C, Kall L, Kreher SA, Kapp K, Sonnhammer EL, Carlson JR, Heijne G, Nilsson I. Membrane topology of the Drosophila OR83b odorant receptor. FEBS Lett. 2007;581:5601–5604. doi: 10.1016/j.febslet.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MJ, Grinstein S. Ionomycin activates electrogenic Ca2+ influx in rat thymic lymphocytes. Biochem. J. 1993;296:33–39. doi: 10.1042/bj2960033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Miura N, Atsumi S, Tabunoki H, Sato R. Expression and localization of three G protein alpha subunits, Go, Gq, and Gs, in adult antennae of the silkmoth (Bombyx mori. J. Comp. Neurol. 2005;485:143–152. doi: 10.1002/cne.20488. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Seven-transmembrane proteins as odorant and chemosensory receptors. Science. 1999;286:707–711. doi: 10.1126/science.286.5440.707. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Jacob R. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem. J. 1994;300:665–672. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Pellegrino M, Sato K, Vosshall LB, Touhara K. Amino acid residues contributing to function of the heteromeric insect olfactory receptor complex. PLoS ONE. 2012;7:e32372. doi: 10.1371/journal.pone.0032372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Störtkuhl K, Hatt H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 2005;8:15–17. doi: 10.1038/nn1371. [DOI] [PubMed] [Google Scholar]
- Nichols AS, Chen S, Luetje CW. Subunit contributions to insect olfactory receptor function: channel block and odorant recognition. Chem. Senses. 2011;36:781–790. doi: 10.1093/chemse/bjr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pask GM, Jones PL, Rutzler M, Rinker DC, Zwiebel LJ. Heteromeric Anopheline odorant receptors exhibit distinct channel properties. PLoS ONE. 2011;6:e28774. doi: 10.1371/journal.pone.0028774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz D, Roeske T, Syed Z, de Bruyne M, Galizia CG. The molecular receptive range of an olfactory receptor in vivoDrosophila melanogaster Or22a) J. Neurobiol. 2006;66:1544–1563. doi: 10.1002/neu.20333. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, Haughey NJ, Greenway SC, Yacono PW, Golan DE, Geiger JD. Expression of ryanodine receptors in human embryonic kidney (HEK293) cells. Biochem. J. 1998;334:79–86. doi: 10.1042/bj3340079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PJ, Thurmond RL, Khorana HG. Structure and function in rhodopsin: high level expression of a synthetic bovine opsin gene and its mutants in stable mammalian cell lines. Proc. Natl. Acad. Sci. USA. 1996;93:11487–11492. doi: 10.1073/pnas.93.21.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nässel DR, Lee C-H, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutzler M, Lu T, Zwiebel LJ. Galpha encoding gene family of the malaria vector mosquito Anopheles gambiae: expression analysis and immunolocalization of AGalphaq and AGalphao in female antennae. J. Comp. Neurol. 2006;499:533–545. doi: 10.1002/cne.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargsyan V, Getahun MN, Llanos SL, Olsson SB, Hansson BS, Wicher D. Phosphorylation via PKC regulates the function of the Drosophila odorant co-receptor. Front. Cell. Neurosci. 2011;5:5. doi: 10.3389/fncel.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J. Neurosci. 2007;27:11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, Christie DL, Chen C, Newcomb RD, Warr CG. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem. Molec. 2008;38:770–780. doi: 10.1016/j.ibmb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Sutko JL, Airey JA, Welch W, Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol. Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- Tsitoura P, Andronopoulou E, Tsikou D, Agalou A, Papakonstantinou MP, Kotzia GA, Labropoulou V, Swevers L, Georgoussi Z, Iatrou K. Expression and membrane topology of Anopheles gambiae odorant receptors in Lepidopteran insect cells. PLoS ONE. 2010;5:e15428. doi: 10.1371/journal.pone.0015428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wicher D, Schäfer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Yao CA, Carlson JR. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J. Neurosci. 2010;30:4562–4572. doi: 10.1523/JNEUROSCI.6357-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Plant S. Mechanism of release of Ca2+ from intracellular stores in response to ionomycin in oocytes of the frog Xenopus laevis. J. Physiol. 1992;458:307–318. doi: 10.1113/jphysiol.1992.sp019419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Calcium from intracellular stores contributes to the odor-mediated calcium response in HEK293T cells.
Fig. S2. Classification of responders and non-responders.
Fig. S3. Expression levels of dORs were unaltered by over-expression of Gαo/i.
Fig. S4. Adapted and tonic responses are less variable than phasic responses.