Abstract
IgG autoantibodies, including antibodies to double-stranded DNA (dsDNA), are pathogenic in systemic lupus erythematosus, but the mechanisms controlling their production are not understood. To assess the role of invariant natural killer T (iNKT) cells in this process, we studied 44 lupus patients. We took advantage of the propensity of PBMCs from patients with active disease to spontaneously secrete IgG, in vitro. Despite the rarity of iNKT cells in lupus blood (0.002∼0.05% of CD3-positive T cells), antibody blockade of the conserved iNKT TCR or its ligand, CD1d, or selective depletion of iNKT cells, inhibited spontaneous secretion of total IgG and anti-dsDNA IgG by lupus PBMCs. Addition of anti-iNKT or anti-CD1d antibody to PBMC cultures also reduced the frequency of plasma cells, suggesting that lupus iNKT cells induce B cell maturation. Like fresh iNKT cells, expanded iNKT cell lines from lupus patients, but not healthy subjects, induced autologous B cells to secrete antibodies, including IgG anti-dsDNA. This activity was inhibited by anti-CD40L antibody, as well as anti-CD1d antibody, confirming a role for CD40L-CD40 and TCR-CD1d interactions in lupus iNKT mediated help. These results reveal a critical role for iNKT cells in B cell maturation and autoantibody production in patients with lupus.
Keywords: iNKT cells, lupus, autoantibodies, B cells
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by inflammation of multiple organs and uncontrolled production of autoantibodies [1]. The serological hallmark of this disease is the presence of high levels of autoantibodies against nuclear antigens. These autoantibodies, especially those directed against double-stranded DNA (dsDNA), are thought to contribute directly to the pathogenesis of SLE [2]. Studies in both humans and animal models have shown that T cells, especially CD4+ T cells, can promote pathogenic autoantibody production by “helping” B cells [3, 4]. The presumptive explanation for this helper activity is that conventional CD4+ T cells, recognizing a particular MHC class II associated autoantigen, interact with B cells that have receptors for the same autoantigen, resulting in reciprocal stimulation of the T and B cells and production of autoantibody. NK cells of the innate arm of the immune system have also been reported to help B cells produce IgG [5, 6] and it is possible that such cells may play a role in the development of pathogenic autoantibodies in lupus.
Invariant natural killer T (iNKT) cells in humans are a rare and distinct subset of lymphocytes that share characteristics of both conventional T cells (CD3 and TCRαβ) and NK cells (CD161). They express an invariant TCR α chain (Vα24Jα18) coupled with a variable Vβ11 TCR β chain, and recognize self and exogenous glycolipid antigens presented by CD1d, a nonclassical MHC class I-like antigen-presenting molecule [7]. The most widely studied iNKT cell agonist is α-galactosylceramide (αGalCer), a marine sponge-derived glycolipid that can be used to selectively activate iNKT cells in vitro and in vivo [8, 9]. Since iNKT cells display a constitutive effector memory phenotype, recognize endogenous glycolipids and rapidly produce immunoregulatory cytokines, including IL-4 and IFNγ, upon activation, they are regarded as members of both the innate and adaptive immune system [10]. After glycolipid activation, iNKT cells can also help B cell proliferation and antibody production in vitro and in vivo in a CD1d-restricted manner [11-14].
The role of iNKT cells in lupus is controversial, as studies in SLE animal models have yielded conflicting results. On the one hand, T cells expressing a transgenic anti-CD1d TCR induced lupus nephritis after transfer into Balb/c nude mice [15]. Treatment of NZBxNZW mice with anti-CD1d mAb or β-galactosylceramide to block iNKT cell function ameliorated lupus and decreased serum levels of IgG2a and anti-dsDNA antibodies [16-18]. Moreover, iNKT cells, but not conventional CD4+ T cells, from NZBxNZW mice with active disease helped B cells to secrete IgG anti-dsDNA antibody via recognition of CD1d on B cells [19]. On the other hand, CD1d-/- NZBxNZW mice developed more severe disease than their wild type littermates [20]. Similarly, in MRL-lpr/lpr mice CD1d deficiency led to exacerbation of skin disease [21], and recent studies in other models revealed that activated iNKT cells can inhibit autoreactive B cells and reduce IgG autoantibody production [22, 23]. Taken together, these findings suggest that iNKT cells may have different effects on lupus in mice, depending on the strain and type or stage of disease.
The relevance of murine lupus models to human SLE is uncertain. Because of their rarity in peripheral blood, human iNKT cells are difficult to study. The situation in SLE is especially challenging, as the frequency of iNKT cells in the blood of lupus patients is decreased relative to that in healthy subjects and the extent of the decrease is related to disease severity [24-27]. Nonetheless, iNKT cells can be extremely potent on a per cell basis, and in the current study we took advantage of this property to investigate their role in the regulation of immunoglobulin production in SLE. The results show that iNKT cells from lupus patients, but not conventional CD4+ T cells from the same patients, are potent inducers of IgG and anti-dsDNA IgG autoantibody production. The phenotype and function of these iNKT cells are similar to those of iNKT cells that promote autoantibody production and disease progression in mice [16-19].
Results
PBMCs from lupus patients with active disease spontaneously secrete immunoglobulin
Previous studies have demonstrated that freshly isolated PBMCs from lupus patients secrete immunoglobulin in the absence of exogenous stimuli [28-31]. In our initial studies we isolated PBMCs from 23 SLE patients and after culturing these cells for 10 days in the absence of human serum, we measured the level of IgG in the supernatant by ELISA. Significant amounts of IgG were detected in the culture supernatants from 11 of these patients, but not from any of 10 age and gender matched healthy subjects. There was no difference between lupus patients and healthy subjects in the viability of B cells and plasma cells at the beginning or end of the culture period (data not shown), ruling out dead or dying B cells as a significant source of IgG. There was a strong correlation between the amount of IgG secreted and the SLEDAI score (rs=0.6022, P=0.0024 by Spearman Rank Test) (Fig. 1A). A similar association could also be seen when comparing patients with active (SLEDAI ≥6) versus inactive or minimally active (SLEDAI <6) disease (P<0.01) (Fig. 1B) or when comparing patients receiving ≥10 mg per day of prednisone (who had more severe disease) versus those receiving lower doses or no prednisone (P<0.05) (Fig. 1C).
Fig. 1. Spontaneous immunoglobulin secretion by SLE patient PBMCs correlates with disease activity.
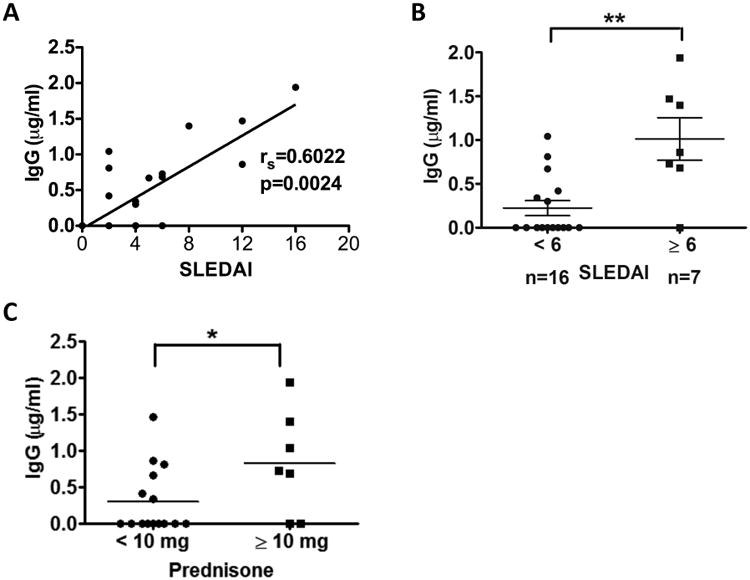
PBMCs from SLE patients (n=23) were cultured for 10 days in medium devoid of human serum, after which IgG was measured by ELISA in the culture supernatants. (A) Correlation between level of spontaneous IgG production and disease activity (SLEDAI score) was analyzed with the Spearman's rank correlation test (rs=0.6022, P=0.0024). (B) Comparison of spontaneous IgG production between patients with inactive disease (SLEDAI<6, n=16) and active disease (SLEDAI ≥6, n=7) using the Mann-Whitney test (**, P<0.01). Horizontal lines represent mean ± SD. (C) Comparison of spontaneous IgG levels between patients treated with no or low dose prednisone treatment (<10 mg/day, n=16) and higher dose treatment (≥10 mg/day, n=7) using the Mann-Whitney test. Each data point represents one individual sample. * indicates P<0.05.
Spontaneous immunoglobulin secretion by lupus PBMCs is dependent on iNKT cells
To assess the possibility that iNKT cells affect spontaneous IgG production in SLE, we selected patients with SLEDAI ≥6 who were positive for spontaneous IgG production and cultured their freshly obtained PBMC for 10 days in the presence of various blocking mAbs directed at molecules on B cells or iNKT cells, and measured Ig secretion in culture supernatants. Anti-CD1d mAb, but not neutralizing mAbs directed at other molecules on B cells (HLA Class I and HLA Class II) or the isotype control mAbs, inhibited IgG production by lupus PBMC (Fig. 2A). In the five lupus patients tested in this manner, anti-CD1d mAb inhibited IgG production by an average of 57%. The PBMCs of these patients produced spontaneous IgM and IgA in addition to IgG, and the secretion of all 3 classes of antibody was markedly inhibited by anti-CD1d antibody (Fig. 2A and B). A combination of anti-TCRVα24 and TCRVβ11 mAb, or a single antibody (clone: 6B11) specific for the conserved CDR3 domain of Vα24, or depletion of iNKT cells with anti-TCRVα24 antibody coated magnetic beads, all reduced spontaneous IgG secretion by >50% (Fig. 2C, D and E). By contrast, antibodies of the same subclass (IgG1) directed at CD2 and CD4, molecules that are expressed on both iNKT cells and conventional T cells, had no effect. FACS analysis confirmed that SLE B cells express CD1d, the main antigen-presenting molecule recognized by iNKT cells, at about the same level as B cells of healthy subjects (Supplementary Fig. S1). CD1c was also expressed on SLE B cells, but at a slightly lower level than that of healthy control B cells (Supplementary Fig. S1).
Fig. 2. Spontaneous immunoglobulin production by SLE PBMCs is dependent on iNKT cells.
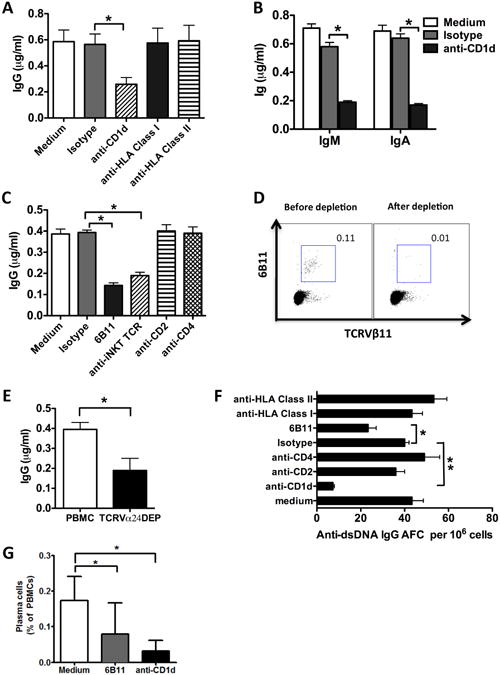
PBMCs from SLE patients were cultured for 10 days in the presence of various neutralizing antibodies or their isotypes, IgG (A and C, n=5), IgM and IgA (B, n=3) in culture supernatants was measured by ELISA (D) iNKT cells as a percentage of CD3+ T cells before and after anti-TCRVα24 depletion in one experiment representative of 5 performed. (E) iNKT cells were depleted from lupus PBMCs with anti-TCRVα24 antibody coated beads (TCRVα24DEP) and spontaneous IgG production by iNKT-depleted PBMCs was measured by ELISA (n=5). (F) B cell ELISPOT assay was used to detect anti-dsDNA IgG antibody forming cells (AFC) among SLE PBMCs in the presence or absence of the indicated antibody (n=4). The number of anti-dsDNA IgG AFC per 106 PBMCs is shown. (G) Frequency of plasma cells in lupus PBMCs cultured for one week in the presence or absence of anti-iNKT or anti-CD1d antibody (n=3). (A, C and E) Data are shown as the mean ± SD of five samples pooled from five independent experiments. (B) Data are shown as the mean ±SD of three samples pooled from three independent experiments. (F) Data are shown as the mean ± SD of four samples pooled from four independent experiments. * indicates P<0.05, ** indicates P<0.01. (Paired t-test used).
PBMCs of lupus patients with active disease may also spontaneously secrete anti-dsDNA antibody [28-31]. As shown in Figure 2F, PBMCs from patients with active SLE secreted IgG anti-dsDNA antibody. After the cells were cultured for 10 days in the presence of anti-CD1d or anti-iNKT (6B11) mAbs, the frequency of autoantibody secreting cells was reduced by >70% and 50%, respectively, whereas mAbs directed at CD2 and CD4 had no effect. MAbs directed at HLA-class I or HLA-class II, which are expressed on B cells, monocytes and dendritic cells, also did not affect the frequency of anti-dsDNA IgG secreting cells. Finally, when lupus PBMCs were cultured in the presence of anti-iNKT or anti-CD1d antibody, the development of plasma cells, defined as CD19dimCD20-CD27highCD138+ cells, was markedly reduced (Fig. 2G). These results suggest that interaction between the iNKT cell TCR and its CD1d ligand is required for maturation of B cells into plasma cells and spontaneous secretion of IgG and anti-dsDNA antibody by lupus PBMCs.
Lupus iNKT cells are rare in peripheral blood but secrete large amounts of Interferon-gamma (IFNγ)
Despite the potent B cell stimulatory activity of iNKT cells in our lupus cohort, the frequency of these cells in peripheral blood was decreased compared to those in healthy age- and gender-matched subjects (0.02% vs. 0.05% of CD3 T cells), which is in accord with prior reports (Fig. 3A). Nonetheless, CD4+CD8-, CD4-CD8- and CD4-CD8+ iNKT populations were present in similar proportions in lupus patients and healthy subjects (Fig. 3A). To study cytokine production by iNKT cells, we stimulated PBMCs with αGalCer and six days later performed intracellular staining of IFNγ, IL-4, IL-10, IL-17 and IL-21. The percentage of IFNγ+ iNKT cells in SLE patients (mean 16%) was higher than that in healthy controls (mean 4%) (Fig. 3B). There was no difference in the frequency of iNKT cells expressing several other cytokines between SLE patients and healthy controls (Fig. 3B). Despite the excess production of IFNγ by iNKT cells, addition of up to 20 ug/ml anti-IFNγ blocking antibody to SLE patient PBMC cultures had no effect on IgG production (Fig. 3C).
Fig. 3. iNKT cells are rare in lupus patients but expand after αGalCer stimulation.
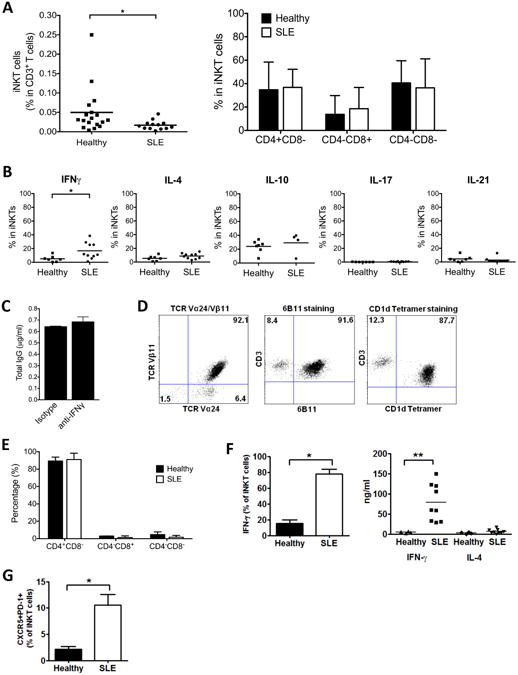
(A) Frequency of iNKT cells and CD4/CD8 distribution on fresh iNKT cells in SLE patients and healthy individuals (n=13 for SLE patients, n=18 for healthy subjects). (B) Cytokine production by iNKT cells was analyzed by intracellular staining of PBMCs stimulated with αGalCer for 6 days (n=7 for healthy subjects, n=10 for SLE patients except for IL-10 where n=4 for SLE patients). (C) Total IgG in the supernatant of SLE PBMCs cultured in the presence of blocking antibody against IFNγ. Data are representative of 4 independent experiments. (D) Characterization of expanded iNKT cells (n=3) by staining with anti-TCRVα24/TCRVβ11, anti-iNKT (clone 6B11) and human CD1d-αGalCer tetramer. Data are representative of 3 independent experiments. (E) Expression of CD4 and CD8 on expanded iNKT cells from healthy subjects (n=5) and SLE patients (n=5). Data are shown as the mean ± SD of five samples pooled from five independent experiments. (F) IFNγ and IL-4 expression in activated iNKT cells. Left: Expanded iNKT cells stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 hours were analyzed by FACS for intracellular IFNγ. Plots are representative of 5 independent experiments. Right: Expanded iNKT cells (5×104 cells/well) were stimulated with plate-bound anti-CD3 mAb for 24 hours. IFNγ and IL-4 in the supernatants were measured by ELISA. The results represent mean values of triplicate cultures from 9 lupus patients and 5 healthy subjects. Each symbol represents one individual sample. (G) Expression of CXCR5 and PD-1 in expanded iNKT cells by flow cytometric analysis. Data shown are representative of 3 independent experiments with SLE patients and healthy subjects. * indicates P<0.05, ** indicates P<0.01. (Paired t-test used).
Expanded iNKT cell lines from SLE patients help autologous B cells produce IgG and anti-dsDNA IgG
To study pure populations of iNKT cells, we stimulated PBMCs from lupus patients and healthy subjects with αGalCer and expanded the cells for 30 days in the presence of recombinant human (rh) IL-2, rhIL-15 and irradiated autologous PBMCs, and then FACS purified iNKT cells with antibodies to TCRVα24 and TCRVβ11. Using this approach we obtained expanded iNKT cell lines from 9 female SLE patients who were positive for serum anti-dsDNA IgG and 5 healthy female control subjects. All of the expanded iNKT cells from lupus patients and healthy controls stained positively with a CD1d-αGalCer tetramer as well as with 6B11 mAb and were comprised mainly of CD4+CD8- cells (Fig. 3D and E). iNKT lines from SLE patients produced more than 12 times the amount of IFNγ (mean 64.3 ng/ml) produced by iNKT cells from healthy controls (mean 4.9 ng/ml) (P<0.01), while there was no significant difference in IL-4 production between the two groups (mean 5.6 ng/ml in SLE patients versus mean 2.8 ng/ml in healthy controls) (P>0.1) (Fig. 3F). Analysis of intracellular cytokine staining shows that the majority of lupus iNKT cells (78%) produced IFNγ, indicating that the high level of IFNγ in the culture supernatant was due to the production of this cytokine by many, rather than few, of these cells. In contrast to the iNKT cells from lupus patients, only about 15% of iNKT cells from healthy subjects produced IFNγ after stimulation (Fig. 3F). Also, a higher proportion of the expanded iNKT cells from SLE patients expressed the follicular helper T cell markers, CXCR5 and PD-1 (Fig. 3G). To evaluate the capacity of the expanded iNKT cells to help autologous B cells secrete immunoglobulins and autoantibodies, we cultured these cells with freshly sorted autologous CD19+ B cells and measured the levels of IgM, IgA, IgG and anti-dsDNA IgG in the culture supernatants after 10 days. B cells from healthy subjects secreted little IgG and no IgG anti-dsDNA when cultured alone or with autologous iNKT cell lines, but secretion of IgM and IgA was significantly enhanced when B cells were cocultured with autologous iNKT cell lines (P<0.05) (Fig. 4A). IgM and IgA production promoted by iNKT cells was inhibited by anti-CD1d antibody. These results indicate that in the absence of exogenous T or B cell activators, iNKT cells from healthy individuals can help autologous B cells secrete IgM and IgA through their recognition of CD1d.
Fig. 4. Expanded iNKT cells from SLE patients, but not healthy subjects, can induce IgG production.
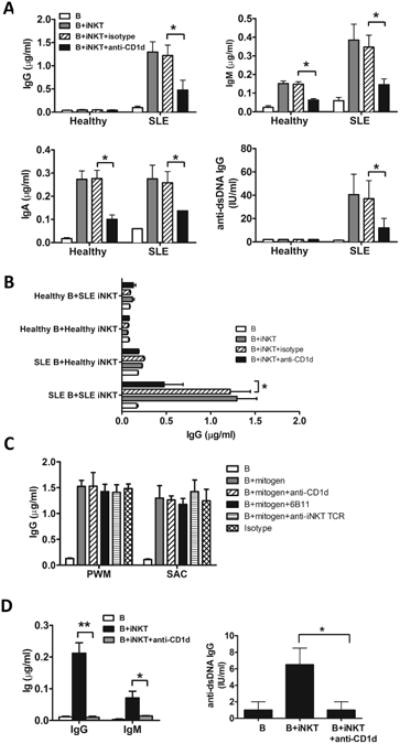
(A) B cells were cultured for 10 days with expanded autologous iNKT cells from healthy subjects (n=5) or lupus patients (n=5) in the presence or absence of anti-CD1d or isotype control antibody. Immunoglobulin in the culture supernatants was measured by ELISA. (B) B cells from healthy subjects were co-cultured with autologous iNKT cells or iNKT cells from SLE patients for 10 days in the presence of anti-CD1d or isotype control antibody. Alternatively, B cells from SLE patients were co-cultured with autologous iNKT cells or iNKT cells from healthy donors. IgG in the supernatant was measured by ELISA. (C) B cells from SLE patients were stimulated with PWM and SAC in the presence or absence of anti-CD1d, anti-iNKT (6B11) or anti-iNKT TCR (TCRVα24/TCRVβ11) antibodies for 10 days. (D) To assess the ability of iNKT cells from lupus patients to induce Ig class switching, sorted IgM+CD27-CD19+ naïve B cells were cultured with autologous iNKT cells for 10 days in the presence or absence of anti-CD1d antibody. Antibodies in the supernatant were measured by ELISA. Left panel shows total IgG and IgM, and right panel shows anti-dsDNA IgG. Antibodies in the supernatant were measured by ELISA. (A) Data are shown as mean ± SD of five samples pooled from five independent experiments. (B-D) Data are shown as mean ± SD of three samples pooled from three independent experiments. * indicates P<0.05, ** indicates P<0.01. (Paired t-test used).
Isolated B cells from SLE patients spontaneously secreted slightly higher levels of IgG, IgM and IgA than healthy controls. When lupus B cells were cocultured with autologous iNKT cell lines, production of IgM, IgA, IgG was significantly increased (P<0.05), especially in the case of IgG where the increase was more than 10 fold (Fig. 4A). Moreover, the antibody production induced by iNKT lines was markedly inhibited by anti-CD1d mAb. More importantly, supernatants from co-cultures of lupus iNKT lines and B cells had significantly higher concentrations of anti-dsDNA IgG than the B cells alone, while the coculture of iNKT lines and B cells from healthy controls produced no detectable anti-dsDNA IgG (Fig. 4A). We also cultured iNKT lines from patients with freshly isolated B cells from healthy individuals, or iNKT lines from healthy subjects with B cells from lupus patients, but IgG production was not increased in either case compared to the B cells cultured alone (Fig. 4B), suggesting that both iNKT cells and B cells from SLE patients are required for antibody production under these conditions. To rule out nonspecific effects of the anti-CD1d and anti-TCR antibodies used in our studies, we activated purified B cells from SLE patients with Pokeweed Mitogen (PWM) or Staphylococcus Aureus Cowan Strain I (SAC) in the presence of these antibodies. The results showed that both PWM and SAC induced IgG production, and anti-CD1d or anti-iNKT TCR antibodies had no effect. (Fig. 4C).
B cells that have been activated by their specific antigens undergo maturation and become either memory IgM, IgA or IgG producing cells. This step is believed to be critical in the pathogenesis of SLE because IgG, but not IgM, anti-dsDNA antibodies can deposit in the kidney and induce destructive inflammation [32]. When purified IgM+CD27-CD19+ naïve B cells from active SLE patients were co-cultured with expanded autologous iNKT cells, they produced IgG antibody (Fig. 4D), suggesting that they had been induced to switch from IgM to IgG production by iNKT cells. We also found that expression of activation-induced cytidine deaminase (AID) in SLE B cells co-cultured with iNKT cells was much higher than in B cells cultured alone, confirming Ig class switch in SLE B cells co-cultured with iNKT cells (Supplementary Fig. S2). Anti-CD1d antibody inhibited IgG and IgM production, again demonstrating that iNKT helper activity in lupus patients is dependent on recognition of CD1d molecules on B cells (Fig. 4D).
Expanded conventional CD4+ T cells from SLE patients fail to induce B cells to secrete IgG
Conventional CD4+ T helper cells can augment antibody secretion based on specific interactions between their TCRs and peptides associated with MHC class II molecules on B cells. To evaluate the helper activity of conventional CD4+ T cells, we first stimulated iNKT-depleted CD4+ T cells from lupus patients with plate-bound anti-CD3 and anti-CD28 mAbs and then expanded the cells for 10 days with IL-2 prior to their coculture with fresh autologous B cells. Additionally, we obtained TCRVβ11- CD4+ T cells from the αGalCer stimulated SLE patient PBMC cultures that were used to generate expanded iNKT cells. As these cultures contained exogenous IL-2 and IL-15, in addition to αGalCer, it is not surprising that conventional non-NKT CD4+ cells were also expanded. The latter cells were purified by sorting for CD4+ TCRVβ11- T cells. As shown in Figure 5, both sources of activated TCRVβ11- CD4+ T cells failed to increase IgG, IgM or IgA production, as compared to cultures of B cells alone, and IgG anti-dsDNA was not detectable in the supernatants. By contrast, coculture of B cells with iNKT cell lines from the same patient resulted in markedly increased secretion of all isotypes of Ig, including anti-dsDNA IgG. Moreover, Ig secretion was inhibited by anti-CD1d mAb in the iNKT cocultures.
Fig. 5.
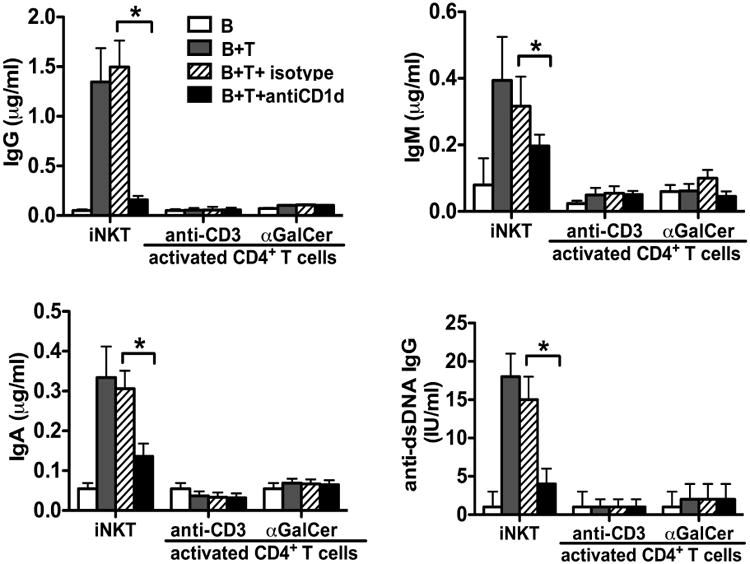
TCRVα24-TCRVβ11- conventional T cells from SLE patients fail to induce antibody production. Conventional CD4+ T cells were purified from two sources as described in Materials and Methods. These cells, or purified iNKT cells, were cultured with autologous B cells for 10 days in the presence or absence of anti-CD1d or isotype control antibody. The level of IgG, IgM, IgA and anti-dsDNA IgG immunoglobulins in the culture supernatants was determined by ELISA. Data are shown as mean ± SD of three samples pooled from three independent experiments. * indicates P<0.05. (Paired t-test used).
iNKT cell mediated B-helper activity is dependent on CD40L-CD40 interaction
CD4+ T helper cells can enhance B cell antibody secretion through CD40 engagement on B cells, and it has been shown that iNKT cells express CD40 ligand upon activation [33]. To determine whether CD40-CD40L interaction is required for lupus iNKT cells to help B cell antibody production, we cocultured expanded iNKT cells from SLE patients with freshly sorted autologous CD19+ B cells for 10 days in the presence or absence of anti-CD40L blocking antibody, and IgM, IgA, IgG and anti-dsDNA IgG were measured in the supernatants. Consistent with our previous data, lupus iNKT cell lines augmented production of IgM, IgA, IgG and anti-dsDNA IgG antibodies. Importantly, production of all 3 classes of antibody, as well as IgG anti-dsDNA, induced by iNKT lines was inhibited by anti-CD40L antibody (Fig. 6).
Fig. 6. Ability of expanded lupus iNKT cells to help B cells is dependent on CD40L-CD40 interaction.
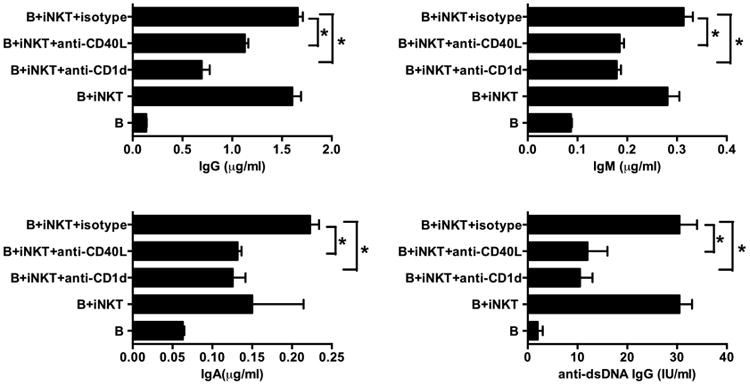
Purified expanded iNKT cells from lupus patients were cultured with autologous B cells for 10 days in the presence or absence of anti-CD40L, anti-CD1d or isotype control antibody. The level of IgG, IgM, IgA and anti-dsDNA IgG immunoglobulins in the culture supernatants was determined by ELISA. Data are shown as mean ± SD of three samples pooled from three independent experiments. * indicates P<0.05. (Paired t-test used).
Discussion
Spontaneous secretion of IgG by freshly isolated PBMCs is a characteristic finding in patients with clinically active SLE but not in patients with most other autoimmune diseases or healthy subjects [28-31]. This phenomenon is known to represent de novo IgG production and depend on T cell help, but the nature of the T helper cells has not been clearly defined. Our results point to iNKT cells as important inducers of B cell activation and maturation in lupus, and as the predominant source of T cell help for spontaneous IgG secretion, which is surprising given the low frequency of these cells. Previous studies have shown that CD4+ iNKT cell clones obtained from healthy persons can provide help to B cells, but only if they are stimulated with anti-CD3 antibody or αGalCer ex vivo [34-36]. A recent study showed that even in the absence of αGalCer stimulation, certain CD4+ iNKT cell clones from healthy subjects can help B cells produce IgG antibody[37]. Our study shows that expanded iNKT cell lines as well as fresh peripheral blood iNKT cells from lupus patients greatly enhance production of total IgG, IgM and IgA, as well as IgG, anti-dsDNA IgG antibody. It was not clear whether the iNKT cell enhancement of total IgG and anti-dsDNA IgG production resulted mainly from inducing naïve B cells to class-switch or from helping class switched memory B cells produce more IgG. However, when expanded iNKT cells were added to purified naïve B cells (IgM+CD27-CD19+), they induced IgG isotype class switching. A recent animal study confirmed that iNKT cells help B cells to produce class-switched antibodies in vivo [12].
The helper activity of the expanded iNKT cells in co-culture with purified B cells, like that of iNKT cells in fresh PBMC, did not require exogenous stimulation and was dependent on their recognition of CD1d on B cells. The nature of the CD1d associated antigen(s) on B cells recognized by lupus iNKT cells is unknown but presumed to be glycolipid rather than nucleic acid. Such glycolipids might be expressed on lupus B cells as a consequence of their exposure to other stimuli including agonists of toll-like receptors [38]. Although CD1d is expressed on cell types in addition to B cells (eg, dendritic cells), a role for these cells in the observed B cell help provided by iNKT cell lines can be ruled out because dendritic cells were absent from our cultures. This finding is consistent with published data showing that treatment of NZBxNZW mice with anti-CD1d antibody ameliorates lupus disease activity and simultaneously reduces the serum concentration of IgG2a anti-dsDNA antibodies [16, 17]. Moreover, recent studies demonstrated that iNKT cells can replace conventional CD4+ T helper cells with respect to the ability to induce antibody responses in MHC class II deficient mice immunized with a protein antigen and αGalCer [39]. In addition, iNKT cells, but not conventional CD4+ T cells, from NZBxNZW mice help B cells spontaneously secrete IgG and anti-dsDNA IgG antibodies in vitro [19, 40]. However, the results of the current human studies differ from those of recent studies in NZBxNZW mice (40), which show that IL-21 secreted by the CD4+ subset of iNKT cells plays an important role in spontaneous autoantibody secretion. In our studies, lupus iNKT cells produced only small amounts of IL-21 and neutralizing anti-IL21 antibody had no effect on spontaneous Ig secretion (data not shown).
Our findings indicate that iNKT cells from lupus patients produce large amounts of IFNγ. Previous studies in mouse models have shown that IFNγ can promote B cell activation during the initiation of antibody responses and is a key cytokine in murine lupus pathogenesis, promoting autoantibody production and glomerulonephritis [41-44]. In addition, αGalCer activated iNKT cells from lupus prone NZBxNZW mice secrete a high ratio of IFNγ to IL-4 as compared to iNKT cells from non-autoimmune mice [16, 17]. Harigai et al. reported that increased expression of IFNγ in SLE patients induces APCs to produce BLyS/BAFF, a well characterized B cell activating and survival factor that is the target of a drug approved for the treatment of SLE [45]. Given these findings, we were surprised that addition of up to 20 μg/ml of a neutralizing anti-IFNγ antibody failed to inhibit Ig secretion induced by lupus iNKT cells. This result suggests that IFNγ does not mediate the helper effect of these cells.
Additional studies of the mechanism by which lupus iNKT cells help B cells secrete antibodies revealed that IgM, IgA, IgG and IgG anti-dsDNA antibody levels were markedly reduced in co-cultures of iNKT cells and B cells to which anti-CD40L had been added. These results suggest that CD40/CD40L interactions are critical for iNKT cell help, just as these interactions are required for conventional T cell help. Previous studies have shown that WT mice immunized with a protein antigen and αGalCer had an increased frequency of plasma cells by comparison to mice immunized with antigen alone, indicating that iNKT cells enhance plasma cell differentiation and survival [39]. Our studies showed that lupus iNKT cells can induce B cells to differentiate into plasma cells in the absence of αGalCer, as demonstrated by the dramatic reduction of plasma cell frequency when lupus PBMCs were cultured in the presence of anti-iNKT or anti-CD1d antibody. Taken together, these data indicate that lupus iNKT cells induce B cell activation and maturation through CD40L-CD40 and TCR-CD1d interactions.
Although conventional CD4+ T cell subsets can promote antibody and autoantibody production via TCR recognition of endogenous protein antigens associated with MHC class II molecules on B cells, in our experiments in lupus patients such cells had little or no detectable helper activity for spontaneous Ig secretion. This could not be explained by the absence of a T cell stimulus, because CD4+ T cells depleted of iNKT cells and activated with anti-CD3 mAb failed to help B cell antibody secretion. One possibility is that pathogenic conventional helper T cells are sequestered in lymphoid organs or inflamed tissues. At least one subpopulation of CD4+ T cells with potent B cell helper capability resides mainly in lymph node germinal centers [46, 47] and is likely not present in large numbers in the circulation. Conceivably, that portion of spontaneous antibody production that could not be blocked with antibodies to iNKT cells or CD1d might depend on this or another T cell population [48, 49]. On the other hand, based on the finding that lupus-like abnormalities can be transferred to immunodeficient mice with PBMCs from SLE patients [50], it seems likely that T and B cells involved in the pathogenesis of SLE are at least partly represented in the circulation.
The reduced frequency of circulating iNKT cells in patients with lupus, combined with the ability of iNKT cells to down regulate immunity in other settings, has led to the suggestion that activation or enhancement of these cells should be a goal of therapy [24-26, 51, 52]. This view was reinforced by a report that lupus iNKT cells, which had been stored frozen prior to being thawed and stimulated with αGalCer, produced large amounts of IL-10 but not IFNγ [27]. Importantly, the latter study was performed with cells that had been cryopreserved, whereas we studied only freshly isolated cells. In our experience, frozen-thawed peripheral blood iNKT cells from both lupus patients and healthy subjects often differ in their cytokine secretion from fresh iNKT cells obtained from the same individuals (Supplementary Fig. S3), and this could explain the difference between our data and the prior study. Our findings clearly argue against the view that reduced iNKT cell activity plays a pathogenic role in lupus and instead suggest that excessive activation of iNKT cells likely contributes to disease development. In this regard, the reduced frequency of iNKT cells in lupus blood could be due to their migration to inflamed tissues. Indeed, IFNγ-secreting iNKT cells have recently been shown to be enriched at sites of cutaneous inflammation in lupus patients [53]. Regardless of their frequency in blood, our data suggest that iNKT cells play an important role in the induction of autoantibody production in patients with SLE. New therapies directed at neutralizing or antagonizing these cells could prove useful in the treatment of this disease.
Materials and Methods
Patients and healthy donors
This study included 44 patients who fulfilled the American College of Rheumatology criteria for the classification of SLE and 32 healthy subjects. Clinical and demographic features of the participating lupus patients are described in Table 1. The disease activity of patients with SLE was quantified by SLE Disease Activity Index (SLEDAI). Patients were recruited from the Immunology and Rheumatology Clinic at Stanford Hospital and the Oklahoma Research Center with approval of the administrative panels on Human Subjects in Medical Research from both institutions. Healthy control PBMCs were obtained from blood donors at the Stanford Blood Center.
Table 1. Clinical and demographic features of the participating lupus patients.
| Patient | Age/Gender | SLE DAI | Drug treatment | Anti-dsDNA | ANA | Complement C3 or C4 |
|---|---|---|---|---|---|---|
| 1 | 42/F | 8 | Prednisone/antimalarials | + | + | Low |
| 2 | 67/F | 8 | Prednisone/azathioprine | + | + | Low |
| 3 | 44/F | 4 | Antimalarials | + | + | Normal |
| 4 | 35/F | 2 | Prednisone/antimalarials | + | + | Normal |
| 5 | 26/F | 12 | Prednisone/antimalarials | + | + | Low |
| 6 | 38/F | 12 | Prednisone/antimalarials | + | + | Normal |
| 7 | 45/F | 5 | Prednisone | + | + | Low |
| 8 | 32/F | 6 | Prednisone | + | + | Normal |
| 9 | 67/F | 4 | Antimalarials/mycophenolate | + | + | Normal |
| 10 | 35/F | 2 | Azathioprine/antimalarials | + | + | Normal |
| 11 | 54/F | 4 | Prednisone | - | + | Low |
| 12 | 38/F | 16 | Prednisone | + | + | Low |
| 13 | 24/F | 0 | Prednisone/antimalarials | - | + | Normal |
| 14 | 58/F | 4 | Prednisone/azathioprine | - | - | Normal |
| 15 | 22/F | 2 | Prednisone/antimalarials | + | + | Low |
| 16 | 47/F | 4 | Prednisone/antimalarials | + | + | Low |
| 17 | 29/F | 4 | Prednisone/antimalarials | + | + | Low |
| 18 | 40/F | 0 | Prednisone/antimalarials | + | + | Normal |
| 19 | 46/F | 2 | Prednisone/antimalarials | + | + | Normal |
| 20 | 26/F | 6 | Prednisone/mycophenolate | + | + | Normal |
| 21 | 22/F | 4 | Antimalarials/azathioprine | + | + | Normal |
| 22 | 82/F | 2 | Antimalarials | + | + | Normal |
| 23 | 34/F | 2 | Prednisone/antimalarials | - | + | Low |
| 24 | 33/F | 6 | Prednisone/antimalarials | + | + | Normal |
| 25 | 38/F | 6 | Prednisone/antimalarials | - | + | Normal |
| 26 | 46/F | 2 | Prednisone/antimalarials | + | + | Normal |
| 27 | 22/F | 4 | Prednisone/mycophenolate | + | + | Low |
| 28 | 21/F | 2 | Azathioprine | - | + | Low |
| 29 | 63/F | 4 | Prednisone/antimalarials | - | + | Normal |
| 30 | 42/F | 6 | Antimalarials/azathioprine | + | + | Low |
| 31 | 23/F | 9 | Prednisone/antimalarials/mycophenolate | + | + | Low |
| 32 | 30/F | 2 | Antimalarials | - | + | Low |
| 33 | 49/F | 0 | Prednisone/antimalarials | - | + | Normal |
| 34 | 26/F | 0 | Antimalarials/azathioprine | - | + | Normal |
| 35 | 31/F | 12 | Prednisone/antimalarials/mycophenolate | + | + | Low |
| 36 | 47/F | 11 | Antimalarials/azathioprine | + | + | Low |
| 37 | 35/F | 12 | Prednisone/mycophenolate | + | + | Low |
| 38 | 52/F | 0 | - | + | Normal | |
| 39 | 30/F | 5 | Antimalarials | + | + | Low |
| 40 | 63/F | 2 | Prednisone/antimalarials | + | + | Normal |
| 41 | 18/M | 8 | Prednisone/antimalarials/mycophenolate | + | + | Low |
| 42 | 49/M | 2 | Antimalarials | - | + | Normal |
| 43 | 33/F | 8 | Mycophenolate | - | + | Normal |
| 44 | 24/F | 15 | Prednisone/mycophenolate | + | + | Low |
Antibodies and cell staining
Antibodies against human CD19, IgM, CD27, NKT (6B11), CD3, CD4, CD8, IFNγ, IL-4, IL-10, IL-17, IL-21, CD28 and CD1d (blocking) were from BD Biosciences. Antibodies against human CD20, CD38, CD69, CD138, HLA-ABC, HLA-DR, CD1d, CD1c, CD4 (blocking), CD2 (blocking), CD40L (blocking) and isotype control mIgG1 or mIgG2a were purchased from Biolegend. Antibodies against human Vα24 and Vβ11 were from Beckman Coulter. PE-conjugated CD1d–αGalCer tetramer was provided by the US National Institutes of Health Tetramer Core Facility. For surface staining, cells were maintained in the dark at 4 °C throughout. Cells were incubated for 20 min with each antibody in FACS buffer (PBS with 2mM EDTA and 1% FCS) and were washed thoroughly with FACS buffer. For intracellular cytokine staining, cells were stimulated with PMA (50 ng/ml) and ionomycin (1 μg/ml) for 4 hours in the presence of Brefeldin A. Then cells were fixed and permeabilized for 20 min at 4 °C with Cytofix/Cytoperm buffer (BD Biosciences) and washed twice with Perm/Wash buffer (BD Biosciences). Anti-cytokine antibodies were added to the cells and incubated in the dark at 4 °C for 30 min. LSR II and FACSDiva software (BD) were used for the acquisition of flow cytometry data, and FlowJo software (TreeStar) was used for analysis.
Cell isolation
PBMCs were prepared from human peripheral blood using Ficoll density gradient centrifugation. B cells and CD4+ T cells were purified from PBMCs by MACS with anti-CD19 and anti-CD4 microbeads (Miltenyi Biotec). Naïve B cells (CD19+CD27-IgM+) were sorted by FACS Aria II. Activated conventional CD4+ T cells were obtained from two sources. 1) CD4+ T cells which had been stimulated with plate-bound anti-CD3 (2 μg/ml) and anti-CD28 (0.5 μg/ml) antibodies in the presence of rhIL-2 (10 ng/ml) for 10 days and then depleted of iNKT cells using biotinylated anti-TCRVα24 antibody and anti-biotin microbeads (Miltenyi Biotec). 2) PBMCs which had been stimulated with αGalCer and expanded with rhIL-2/15 for 30 days, after which iNKT were depleted as above and conventional CD4+ T cells were then purified by FACS.
In vitro expansion of iNKT cells
PBMCs were cultured in RPMI 1640 medium supplemented with 10% normal human serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine. During the first week PBMCs (1×106 cells/ml) were maintained in 12-well plates in a 37°C humidified incubator with 5% CO2. αGalCer (100 ng/ml) was added at the start of culture, and rhIL-2 (50 ng/ml) and rhIL-15 (10 ng/ml) (PeproTech) were added 16 hours later. Cultures were restimulated every 10 days with 1×106 cells/ml αGalCer-pulsed, irradiated (3000 rad) autologous PBMCs in medium containing rhIL-2 in addition to 50% AIM V, 45% RPMI and 5% human serum. After two rounds of restimulation, TCRVα24Vβ11 double positive cells were sorted with mAbs to TCRVα24 and TCRVβ11 (Beckman Coulter) using a FACSAria II.
Cytokine production by expanded iNKT cells
Sorted iNKT cells were cultured in anti-CD3 (5 μg/ml) and anti-CD28 (2 μg/ml) antibody coated plates for 24 hours. The concentrations of IL-4 and IFNγ in the supernatant were determined by ELISA (BD Bioscience). To measure intracellular cytokines, sorted iNKT cells were stained with APC-conjugated anti-IFNγ and PE-conjugated anti-IL-4 antibodies (BD Biosciences) and analyzed by flow cytometry.
Assays of antibody production
To assess spontaneous antibody production, fresh PBMCs (2×106 cells/ml) were cultured in RPMI medium containing 5% FCS in the presence or absence of the indicated antibodies. The effect of isolated T or iNKT cells on immunoglobulin production was assessed by culturing purified B cells (1×105 cells/well) alone or with autologous iNKT cell lines (1×105 cells/well) or iNKT-depleted CD4+ T cells in U-bottom 96-well plates. In some experiments, isolated B cells were cultured with pokeweed mitogen (PWM) (Life Technologies) at a final dilution of 1:100, or were cultured with Staphylococcus aureus Cowan I strain (SAC) (Sigma, St. Louis, MO) at a final concentration of 1:10,000 (v/v) and IL-2 (10ng/ml) from PeproTech. After 10 days of culture, supernatants were harvested and measured by ELISA for total IgG, IgM, IgA (Bethyl Laboratories) and anti-dsDNA IgG (Signosis). In blocking experiments, anti-human mAbs to CD1d, NKT TCR (clone 6B11), Vα24, Vβ11, HLA-ABC, HLA-DR, CD4, CD2 and isotype control Ig were used at 20 μg/ml. Although plate-bound 6B11 is mitogenic for iNKT cells [54], in its soluble form this mAb does not activate these cells (data not shown).
Detection of antibody-forming cells
IgG anti-dsDNA antibody-forming cells were detected by enzyme-linked immunosorbent spot-forming cell assays (ELISPOT). MultiScreen-IP sterile white plates (Millipore) were pre-wet with 50 μl of 70% ethanol per well before washing five times with water. Plates were then coated with 100 μl of 100 μg/ml calf thymus dsDNA (Sigma) in PBS (PH 7.4) overnight at 4°C. Coated plates were blocked with culture media for 1 hour at room temperature (RT). For detection of IgG anti-dsDNA–producing cells, 5×105 fresh PBMCs were added to each well. The plates were incubated for 72 hours at 37°C and the cells were washed away with PBS. Biotinylated anti-IgG at 1 μg/ml (MABTECH) was added to the wells and incubated for 2 hours at RT, followed by incubation with alkaline phosphatase-labeled streptavidin (MABTECH) diluted at 1:1000 for 1 hour at RT. Specific antibody-binding spots were visualized with substrate 5-bromo-4-chloro-3-indolyl phosphate/NBT-plus (MABTECH). Developed plates were evaluated and read by ZellNet Consulting (Fort Lee, NJ). Results are expressed as the number of IgG anti-dsDNA antibody forming cells (AFC) per 106 PBMCs.
Analysis of AID expression
SLE B cells were cultured alone or co-cultured with autologous iNKT cells for 7 days. Total RNA was extracted from B cells and B and iNKT cocultures using RNeasy Micro Plus kit (Qiagen). RNA was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Primers: AID (Forward, AGACACTCTGGACACCACTAT, Reverse, CTTAGCCCAGCGGACATTT), CD79B (Forward-CCAGGCTGGCGTTGTCTCCTG, Reverse-AGGCGCTGTTCATGTAGCAGTG). Quantitative RT-PCR was performed using SYBR green (Applied Biosystems) on a QuantStudio 6 Flex machine (Applied Biosystems). Results were normalized to CD79B gene.
Statistical analysis
Results are expressed as mean ± SD. Statistical significance between groups was determined by an unpaired t test or a Mann-Whitney U test. Correlations between variables were evaluated by Spearman's rank correlation test. A p value of <0.05 was considered significant. Graphs and statistical analyses were performed using Prism software.
Supplementary Material
Acknowledgments
We thank Lorna Tolentino for expert assistance with flow cytometry. These studies were supported in part by National Institutes of Health grants R01 AR051748 (EGE) and R01 DK082537 (SS).
Nonstandard abbreviations used
- AFC
antibody forming cells
- αGalCer
α-galactosylceramide
- iNKT
invariant natural killer T
- SLEDAI
SLE Disease Activity Index
Footnotes
Author contributions: LS, CB, and EE designed the research; LS and CB performed the research; EC provided patient samples; LS, EE, SS, and EC analyzed data; and LS and EE wrote the paper.
Conflict of Interest Disclosure: The authors declare no commercial or financial conflict of interest.
References
- 1.La Cava A, Fang CJ, Singh RP, Ebling F, Hahn BH. Manipulation of immune regulation in systemic lupus erythematosus. Autoimmun Rev. 2005;4:515–519. doi: 10.1016/j.autrev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Singh RR. SLE: translating lessons from model systems to human disease. Trends Immunol. 2005;26:572–579. doi: 10.1016/j.it.2005.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando DG, Sercarz EE, Hahn BH. Mechanisms of T and B cell collaboration in the in vitro production of anti-DNA antibodies in the NZB/NZW F1 murine SLE model. J Immunol. 1987;138:3185–3190. [PubMed] [Google Scholar]
- 4.Singh RR, Kumar V, Ebling FM, Southwood S, Sette A, Sercarz EE, Hahn BH. T cell determinants from autoantibodies to DNA can upregulate autoimmunity in murine systemic lupus erythematosus. J Exp Med. 1995;181:2017–2027. doi: 10.1084/jem.181.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan D, Wilder J, Dang T, Bennett M, Kumar V. Activation of B lymphocytes by NK cells. Int Immunol. 1992;4:1373–1380. doi: 10.1093/intimm/4.12.1373. [DOI] [PubMed] [Google Scholar]
- 6.Gray JD, Horwitz DA. Activated human NK cells can stimulate resting B cells to secrete immunoglobulin. J Immunol. 1995;154:5656–5664. [PubMed] [Google Scholar]
- 7.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 8.Van Kaer L. alpha-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 11.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. blood. 2008;111:2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, Besra GS, Cerundolo V, Batista FD. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, Besra GS, Brenner MB. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci U S A. 2008;105:8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tazawa H, Irei T, Tanaka Y, Igarashi Y, Tashiro H, Ohdan H. Blockade of invariant TCR-CD1d interaction specifically inhibits antibody production against blood group A carbohydrates. Blood. 2013;122:2582–2590. doi: 10.1182/blood-2012-02-407452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng D, Dick M, Cheng L, Amano M, Dejbakhsh-Jones S, Huie P, Sibley R, Strober S. Subsets of transgenic T cells that recognize CD1 induce or prevent murine lupus: role of cytokines. J Exp Med. 1998;187:525–536. doi: 10.1084/jem.187.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng D, Lee MK, Tung J, Brendolan A, Strober S. Cutting edge: a role for CD1 in the pathogenesis of lupus in NZB/NZW mice. J Immunol. 2000;164:5000–5004. doi: 10.4049/jimmunol.164.10.5000. [DOI] [PubMed] [Google Scholar]
- 17.Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest. 2003;112:1211–1222. doi: 10.1172/JCI17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morshed SR, Takahashi T, Savage PB, Kambham N, Strober S. beta-galactosylceramide alters invariant natural killer T cell function and is effective treatment for lupus. Clin Immunol. 2009 doi: 10.1016/j.clim.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur J Immunol. 2008;38:156–165. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, Van Kaer L, Singh RR. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 2007;56:1219–1233. doi: 10.1002/art.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang JQ, Chun T, Liu H, Hong S, Bui H, Van Kaer L, Wang CR, Singh RR. CD1d deficiency exacerbates inflammatory dermatitis in MRL-lpr/lpr mice. Eur J Immunol. 2004;34:1723–1732. doi: 10.1002/eji.200324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang JQ, Wen X, Kim PJ, Singh RR. Invariant NKT cells inhibit autoreactive B cells in a contact- and CD1d-dependent manner. J Immunol. 2011;186:1512–1520. doi: 10.4049/jimmunol.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wermeling F, Lind SM, Jordo ED, Cardell SL, Karlsson MC. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J Exp Med. 2010;207:943–952. doi: 10.1084/jem.20091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojo S, Adachi Y, Keino H, Taniguchi M, Sumida T. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 2001;44:1127–1138. doi: 10.1002/1529-0131(200105)44:5<1127::AID-ANR194>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 25.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer T cells in families of patients with systemic lupus erythematosus: their possible role in regulation of IGG production. Arthritis Rheum. 2007;56:303–310. doi: 10.1002/art.22326. [DOI] [PubMed] [Google Scholar]
- 26.Cho YN, Kee SJ, Lee SJ, Seo SR, Kim TJ, Lee S, et al. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology. 2011;50:1054–1063. doi: 10.1093/rheumatology/keq457. [DOI] [PubMed] [Google Scholar]
- 27.Bosma A, Abdel-Gadir A, Isenberg DA, Jury EC, Mauri C. Lipid-antigen presentation by CD1d(+) B cells is essential for the maintenance of invariant natural killer T cells. Immunity. 2012;36:477–490. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobi AM, Rohde W, Volk HD, Dorner T, Burmester GR, Hiepe F. Prolactin enhances the in vitro production of IgG in peripheral blood mononuclear cells from patients with systemic lupus erythematosus but not from healthy controls. Ann Rheum Dis. 2001;60:242–247. doi: 10.1136/ard.60.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki N, Sakane T, Engleman EG. Anti-DNA antibody production by CD5+ and CD5- B cells of patients with systemic lupus erythematosus. Journal of Clinical Investigation. 1990;85:238–247. doi: 10.1172/JCI114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jasin HE, Ziff M. Immunoglobulin synthesis by peripheral blood cells in systemic lupus erythematosus. Arthritis & Rheumatism. 1975;18:219–228. doi: 10.1002/art.1780180305. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi T. Spontaneous production of antibodies to deoxyribonucleic acids in patients with systemic lupus erythematosus. Clinical Immunology and Immunopathology. 1985;35:47–56. doi: 10.1016/0090-1229(85)90077-7. [DOI] [PubMed] [Google Scholar]
- 32.Budhai L, Oh K, Davidson A. An in vitro assay for detection of glomerular binding IgG autoantibodies in patients with systemic lupus erythematosus. J Clin Invest. 1996;98:1585–1593. doi: 10.1172/JCI118952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu TY, Uemura Y, Suzuki M, Narita Y, Hirata S, Ohyama H, Ishihara O, Matsushita S. Distinct subsets of human invariant NKT cells differentially regulate T helper responses via dendritic cells. Eur J Immunol. 2008;38:1012–1023. doi: 10.1002/eji.200737838. [DOI] [PubMed] [Google Scholar]
- 34.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. Innate immune responses support adaptive immunity: NKT cells induce B cell activation. Vaccine. 2003;21 Suppl 2:S48–54. doi: 10.1016/s0264-410x(03)00200-7. [DOI] [PubMed] [Google Scholar]
- 36.Rossignol A, Barra A, Herbelin A, Preud'homme JL, Gombert JM. Freshly isolated Valpha24+ CD4+ invariant natural killer T cells activated by alpha-galactosylceramide-pulsed B cells promote both IgG and IgE production. Clin Exp Immunol. 2007;148:555–563. doi: 10.1111/j.1365-2249.2007.03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng SG, Ghnewa YG, O'Reilly VP, Lyons VG, Atzberger A, Hogan AE, Exley MA, Doherty DG. Human invariant NKT cell subsets differentially promote differentiation, antibody production, and T cell stimulation by B cells in vitro. J Immunol. 2013;191:1666–1676. doi: 10.4049/jimmunol.1202223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang X, Zhang B, Jarrell JA, Price JV, Dai H, Utz PJ, Strober S. The helper iNKT cell subset expressed the CD4+Ly108+NK1.1- phenotype. J Autoimmun. In Press. [Google Scholar]
- 41.Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol. 2003;170:3915–3925. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- 42.Peng SL, Moslehi J, Craft J. Roles of interferon-gamma and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–1946. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson-Leger C, Hasbold J, Holman M, Klaus GG. The effects of IFN-gamma on CD40-mediated activation of B cells from X-linked immunodeficient or normal mice. J Immunol. 1997;159:1150–1159. [PubMed] [Google Scholar]
- 44.Engleman EG, Sonnenfeld G, Dauphinee M, Greenspan JS, Talal N, McDevitt HO, Merigan TC. Treatment of NZB/NZW F1 hybrid mice with Mycobacterium bovis strain BCG or type II interferon preparations accelerates autoimmune disease. Arthritis Rheum. 1981;24:1396–1402. doi: 10.1002/art.1780241110. [DOI] [PubMed] [Google Scholar]
- 45.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 46.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989;143:103–112. [PubMed] [Google Scholar]
- 49.Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8- T helper cell lines that express the gamma delta T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990;87:7020–7024. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duchosal MA, McConahey PJ, Robinson CA, Dixon FJ. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med. 1990;172:985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 52.Chatenoud L. Do NKT cells control autoimmunity? J Clin Invest. 2002;110:747–748. doi: 10.1172/JCI16625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hofmann SC, Bosma A, Bruckner-Tuderman L, Vukmanovic-Stejic M, Jury EC, Isenberg DA, Mauri C. Invariant natural killer T cells are enriched at the site of cutaneous inflammation in lupus erythematosus. J Dermatol Sci. 2013;71:22–28. doi: 10.1016/j.jdermsci.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Exley MA, Hou R, Shaulov A, Tonti E, Dellabona P, Casorati G, Akbari O, Akman HO, Greenfield EA, Gumperz JE, Boyson JE, Balk SP, Wilson SB. Selective activation, expansion, and monitoring of human iNKT cells with a monoclonal antibody specific for the TCR alpha-chain CDR3 loop. Eur J Immunol. 2008;38:1756–1766. doi: 10.1002/eji.200737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


