Abstract
Vaccines against mucosally invasive, intracellular pathogens must induce a myriad of immune responses in order to provide optimal mucosal and systemic protection, including CD4+ T cells, CD8+ T cells and antibody-producing B cells. In general, CD4+ T cells are known to provide important helper functions for both CD8+ T cell and B cell responses. However, the relative importance of CD4+ T cells, CD8+ T cells and B cells for mucosal protection is less clearly defined. We have studied these questions in detail using the murine model of Trypanosoma cruzi infection. Despite our initial hypothesis that mucosal antibodies would be important, we show that B cells are critical for systemic, but not mucosal, T. cruzi protective immunity. B cell deficient mice developed normal levels of CD8+ effector T cell responses early after mucosal T. cruzi infection and T. cruzi trans-sialidase vaccination. However, after highly virulent systemic challenge, T. cruzi immune mice lacking T. cruzi-specific B cells failed to control parasitemia or prevent death. Mechanistically, T. cruzi-specific CD8+ T cells generated in the absence of B cells expressed increased PD-1 and Lag-3 and became functionally exhausted after high-level T. cruzi systemic challenge. T. cruzi immune serum prevented CD8+ T cell functional exhaustion and reduced mortality in mice lacking B cells. Overall, these results demonstrate that T. cruzi-specific B cells are necessary during systemic, but not mucosal, parasite challenge.
Introduction
Trypanosoma cruzi is a protozoan parasite and the etiological agent of Chagas disease. Prevention and vector control practices throughout Latin America have reduced the current number of infected individuals to approximately 8-11 million people (1). However, movement of infected individuals to non-endemic areas poses an emerging public health problem. Up to forty percent of infected individuals develop serious cardiac and/or gastrointestinal problems 1-30 years after infection, leading to significant morbidity and mortality. T. cruzi is transmitted to both humans and animals by reduviid insects of the subfamily Triatominae. Infectious parasites are present in the excreta of infected Triatominae insects and can transmit via breaks in the skin, mucosal tissues associated with the eye and gastrointestinal tract, congenital transmission from mother to child, as well as blood and tissue donation from infected individuals.
T cells and B cells have been shown to play critical roles in protection against T. cruzi. CD8+ T cells are important in both primary (2) and secondary (3, 4) protective T. cruzi immunity. There are several highly immunodominant CD8+ epitopes encoded in the T. cruzi trans-sialidase (TS) family of genes. In the BALB/c background, up to 30-40% of CD8+ T cells during acute infection are responsive to the immunodominant H-2Kd restricted TS epitope IYNVGQVSI (TSKd1; (5, 6)). CD4 T cells have been shown to be necessary (7-9) but not sufficient (10) for generation of protective immunity against T. cruzi infection. B cells have also been shown to play an important role in systemic T. cruzi protection. Early work demonstrated that T. cruzi-specific antibodies can provide systemic protection via complement activation and lysis (11-13), opsonization (14), and antibody-dependent cellular cytotoxicity (ADCC) (15). These early results demonstrated that B cells play an important role in systemic T. cruzi protection through the production of T. cruzi-specific antibodies (16). Additionally, systemic infection of μMT B cell knockout mice with T. cruzi resulted in initial control of parasite replication but the mice eventually died due to increased parasitemia (16). Previous work by our lab demonstrated that T. cruzi mucosal infection induces protective immunity against subsequent challenge (17, 18). This mucosal protection was associated with increased levels of T. cruzi-specific IgG and IgA antibody secreting cells in the gastric mucosa (17), the initial site of invasion after oral parasite exposures. However, a causal role for B cells in mucosal T. cruzi protection has not been mechanistically defined.
In this current report, we have further examined the importance of B cells for both mucosal and systemic T. cruzi immunity. First, we demonstrate that in contrast to what we initially hypothesized, B cells are not required for mucosal T. cruzi protection. We predicted B cells producing secretory IgA would be very important in mucosal protection against an extracellular parasite life stage that invades through nasal and gastrointestinal epithelia, but this was found not to be the case. In contrast, we demonstrate that CD8+ T cells are critical for mucosal T. cruzi protection. We confirm that B cells are important for systemic T. cruzi protection in both knockout and transient depletion models. After virulent systemic challenge, B cell deficient/depleted mice are unable to control parasitemia and develop increased morbidity and mortality. We further demonstrate that T. cruzi-specific B cells are required for systemic protection, producing parasite-specific antibodies that reduce parasite load resulting in decreased CD8+ T cell exhaustion and mortality in B cell KO mice. Finally, we demonstrate that B cells play a critical role in development and maintenance of multifunctional CD8+ T cells, as well as in the prevention of CD8+ T cell exhaustion. Overall, our new data suggest that memory CD8+ T cells primed in mice lacking T. cruzi-specific B cells are sufficient to control low levels of tissue parasitism. However, these CD8+ T cells eventually become exhausted after high-level systemic challenge due to prolonged exposure to high levels of antigen.
Materials and Methods
Mice, parasites and infection/challenge protocols
BALB/c, SCID BALB/c, JhD B cell KO BALB/c and Hen-egg lysozyme (HEL)-specific BCR transgenic and C57BL/6 mice were used throughout this study. In some instances, female BALB/c mice were B cell depleted or not using α-CD20 (18B12) or IgG2a (2B8) mAb (kindly provided by Biogen Idec). Mice were treated with 250μg of antibody in PBS i.p. every 2-3 weeks. Mice were housed under pathogen free conditions. All studies were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC)/Animal Care Committee (ACC) in an AAALAC accredited facility at Saint Louis University. Tulahuèn strain culture-derived metacyclic tryptomastigotes (CMT) were used for mucosal infections/challenges and blood-form tryptomastigotes (BFT) were used for systemic challenges as described (18, 19) and detailed in Figure 1. Briefly, CMT can infect both mucosally (via oral, intranasal, conjunctival routes) and systemically whereas BFT can only infect systemically. These differences in infectivity are due to differential glycosylation resulting in increased inflammation induced by the BFT life stage, which is hypothesized to prevent infection at mucosal surfaces (20, 21). T. cruzi infection-induced immune (referred to as Tc immune throughout this paper) mice were generated by repeated low-dose infection of [(1-3×106) CMT intragastrically (i.g.)]. For i.g. infection of mice, mice were first given 0.5 ml 1.5% sodium bicarbonate in HBSS i.g. using a ball-ended 1.5-inch, 22 gauge animal feeding needle and rested for 15 minutes to neutralize stomach pH. Parasites were then diluted in PBS + 1% glucose, and 0.1ml was delivered i.g. These mice are referred to as Tc immune throughout this paper.
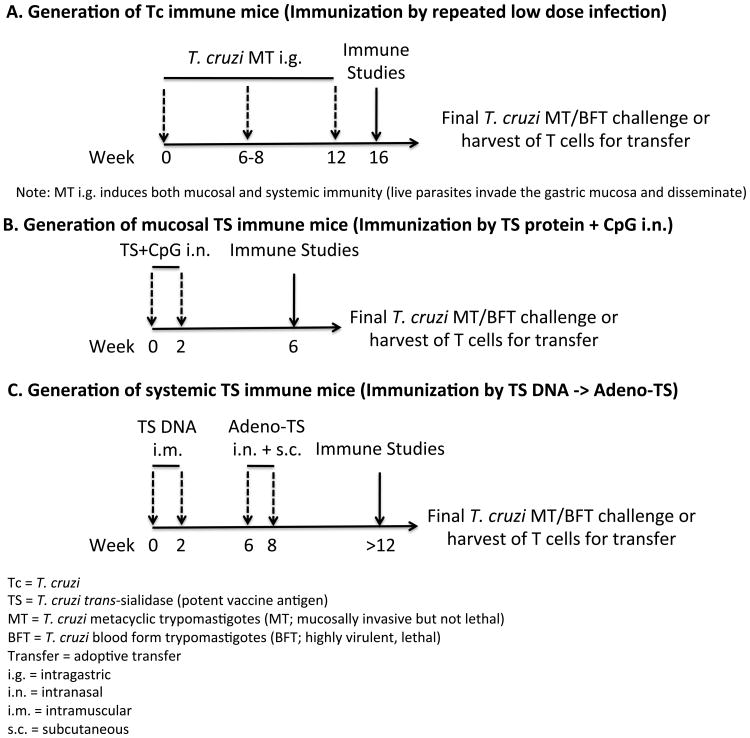
Figure 1. T. cruzi infection- and TS vaccine-induced memory models.
Shown are the major models utilized in this manuscript to demonstrate immunity induced by multiple low dose T. cruzi infections (Tc immune model), and by immunization with various trans-sialidase (TS) vaccines (TS immune models). (A) Generation of Tc immune mice. Mice are dosed at 0 and 6-8 weeks with low doses of T. cruzi metacyclic trypomastigotes (MT) intragastrically (i.g.). At least 4 weeks later, these mice are ready to be used for immune studies, sources of immune cells for use in adoptive transfer models, final mucosal challenge with high doses of MT i.g., or systemic T. cruzi challenge with blood form trypomastigotes (BFT). (B) Generation of mucosal TS immune mice. Mice are vaccinated at 0 and 2 weeks with CpG-adjuvanted recombinant TS intranasally (i.n.), and 4 weeks later mice are ready for use in various studies as described in (A). (C) Generation of systemic TS immune mice. Mice are vaccinated at 0 and 2 weeks with DNA-TS (intramuscular), and with adenovirus-expressing TS (subcutaneous and intranasal) on weeks 6 and 8. At least four weeks later, mice are ready for use in various studies as described in (A). It is important to note that Tc immune mice remain chronically infected with low levels of parasites and have so-called concomitant infection-induced natural immunity. In contract, TS immune mice are not infected until challenged later with T. cruzi.
Vaccinations
To generate mucosal immunity, naïve BALB/c mice (α-CD20/IgG2a mAb treated) were vaccinated with 50μg recombinant trans-sialidase (TS) plus 10μg CpG-containing ODN 1826 (Invivogen, San Diego, CA) intranasally twice, two weeks apart (TS/CpG). To generate systemic immunity, naïve WT, B cell KO or α-CD20/IgG2a mAb treated BALB/c mice were vaccinated with our heterologous TS vaccination protocol (100μg of TS-DNA on days 0 and 14 intramuscularly, and boosted on days 44 and 58 with 1×108 PFU adenovirus-expressing TS subcutaneously (base of tail) and intranasally) (4). These mice are referred to as TS immune mice throughout this paper.
Assessment of protective immunity
To assess protective mucosal immunity, mice were challenged with 1-2×107 CMT intragastrically. Twelve days later, which is the peak time for T. cruzi replication in the gastric mucosa (17), mice were sacrificed and gastric DNA used for quantitative qPCR as described (18). Briefly, 100-200ng of gastric DNA purified using QIAGEN DNeasy Blood and Tissue kits was added to each real time PCR reaction containing 900nM of each primer (5′ AACCACCACGACAACCACAA 3′ and 5′ TGCAGGACATCTGCACAAAGTA 3′), 250nM Taqman probe (FAM/TAM 5′TGCCCCAGGACCGTCCCCA 3′) and 1× Taqman PCR master mix. Thermocycling conditions using an Applied Biosystems 7500 Fast Real Time PCR instrument were 95°C, 10 minutes, followed by 40 cycles of 95°C, 15 seconds and 60°C, 1 minute. A standard curve was generated using DNA purified from a known number of T. cruzi epimastigotes.
To assess protective systemic immunity, mice were challenged with 5,000 T. cruzi (BFT) subcutaneously. Hind-limb paralysis was assessed via paralysis scores similar to that in experimental autoimmune encephalomyelitis (EAE) studies. 0 = No obvious changes in motor functions; 1 = Hind-limb weakness; 2 = Complete paralysis of hind limbs, 3 = Complete hind-limb paralysis and partial front leg paralysis; 4 = Death. Morbidity and mortality were assessed weekly for 60-200 days post systemic BFT challenge.
Adoptive transfers
To determine whether total lymphocytes from immune mice could transfer mucosal T. cruzi protection, one mouse equivalent (4-8×107) of spleen cells (SC) plus draining LN (dLN) cells from Tc immune and naïve BALB/c mice were transferred intravenously into SCID mice. Both spleen and draining lymph nodes were used for transfer experiments as Butcher et al have shown that there are more antigen-specific antibody-secreting cells (ASC) in the spleen after rotavirus infection (22). Furthermore, it was also shown that splenocytes from immune mice contain lymphocytes with mucosal homing capacity (α4β7) that can transfer mucosal protection against rotavirus intestinal infection (22, 23). To determine whether B cells were necessary for the transfer of mucosal T. cruzi protection, B cells were depleted using α-CD19 microbeads (Miltenyi Biotec, Auburn, CA) resulting in > 93% CD19+ depletion (supplemental figure 2). 2.4×107 CD19+ depleted cells from Tc immune or naïve BALB/c mice were transferred i.v. into naïve SCID mice. To determine whether CD8+ T cells alone were sufficient to transfer mucosal T. cruzi protection, CD8+ T cells were positively selected using α-CD8 microbeads (Miltenyi Biotec) resulting in > 98% CD8+ purity. 5.2-6.95×106 CD8+ T cells from Tc immune and naïve BALB/c mice were transferred intravenously into naïve SCID mice. One day after transfer, recipient mice were challenged with 1.2×107 T. cruzi (CMT) intragastrically. Twelve days later, recipient mice were sacrificed and T. cruzi DNA assessed in the gastric mucosa via real-time PCR. In some instances, CD8+ Thy1.2+ T cells (>95% CD8+) were purified from B cell KO, WT BALB/c Tc immune, and naïve BALB/c mice and transferred into naïve BALB/c Thy1.1+ mice. To normalize the number of CD8+TSKd1+ T cells transferred, purified CD8+ T cells were stained with a TSKd1 tetramer and analyzed with an LSR II flow cytometer (BD) and FlowJo flow cytometry software (Tree Star, Inc., Ashland, OR). Equivalent numbers of CD8+TSKd1+ tetramer positive cells from B cell KO and WT Tc immune mice were transferred i.v. into naïve BALB/c Thy1.1 mice. One day after transfer, recipient mice, along with naïve control mice, were challenged systemically with 5,000 T. cruzi (BFT) subcutaneously and followed for parasitemia and survival.
Passive serum transfers
Serum was collected from naïve and WT Tc immune (generated by repeated low-dose oral infection using CMT intragastrically) BALB/c mice. B cell KO BALB/c Tc immune mice were given either 300μl of WT BALB/c Tc immune serum, WT BALB/c naïve serum or PBS i.p. every week starting either two weeks prior to or at the time of T. cruzi (BFT) subcutaneous systemic challenge. T. cruzi-specific serum IgG endpoint titers were measured every one to two weeks.
T. cruzi-specific serum IgG ELISA
Serum was collected at different time points and assessed for trans-sialidase (TS)-specific serum IgG responses via ELISA as described (24). In some instances, 8M Urea was added to evaluate for the presence of high affinity antibodies. Following serum incubation, the ELISA plate was washed with PBS+0.05% Tween. 8M Urea was added for 5 min at RT, washed four times with PBS-Tween with the last wash incubated for 5 min at RT before removal. Endpoint titers were calculated using UNITWIN™ ELISA software (PhPlate AB, Stockholm, Sweden).
T. cruzi-specific IFN-γ, IgG and IgA ELISPOT
Millititer HA 96-well microtiter plates with nitrocellulose bases (Millipore, Bedford, MA) were coated with either purified rat α-mouse IFN-γ (clone R46A2; BD Pharmingen, San Diego, CA) or recombinant trans-sialidase (TS) as described (25). For IFN-γ ELISPOT analyses, spleen cells (2.5×105 cells/well) plus antigen presenting cells [1×105 A20J cells alone (Control A20; ATCC), A20J cells stably transfected with the TS gene (A20-TS), or A20J cells pulsed with 2.5 μg/mL of the immunogenic CD8+ H2Kd restricted TS peptide IYNVGQVSI; A20-TSKd1)] were cultured overnight at 37°C. TS-specific antibody ELISPOT analyses were done as previously described (24). Briefly, 1-5×105 spleen cells were added to TS-coated ELISPOT plates. Following overnight incubation at 37°C, anti-mouse IgG or IgA biotin was added to detect T. cruzi-specific IgG and IgA antibody secreting cells, respectively (SouthernBiotech). Results are represented as the number of spot-forming cells (SFC) or antibody-secreting cells (ASC) per million spleen cells or absolute number per spleen. Background responses to A20J cells were subtracted from experimental antigen responses in results shown from IFN-γ ELISPOT assays.
Flow cytometry and in vitro CD8+TSKd1+ expansion
All antibodies were purchased from BD Bioscience (San Diego, CA) with the exception of α-PD-1, α-Lag-3 and α-CD62L (eBioscience; San Diego, CA). The TSIYNVGQVSI/H2Kd-APC (TSKd1) tetramer was provided through the NIH Tetramer Core Facility (Atlanta, GA). In order to evaluate in vitro TSKd1 expansion, spleen cells were labeled with CFSE (Vybrant CFDA SE cell tracer, Invitrogen) and cultured with 1×105 irradiated (12,500 rads) APCs [A20-negative control (NC) or A20-TS] for 6 days at 37°C. Cells were then counted using Guava ViaCount reagent and the EasyCyte Flow cytometer (Millipore, Billerica, MA). Half of the cells were used for direct cell surface staining and the other half were stimulated with phorbol-12-myristate 13-acetate (PMA 10 ng/mL), ionomycin (500 ng/mL) and cultured with monensin (GolgiPlug; 1 μl/mL) and brefeldin A (GolgiStop; 0.67 μl/mL) for 3 hrs at 37°C. Cells were stained as described (4) and analyzed with an LSR-II Flow cytometer (BD) and FlowJo v7 software (Tree Star, Inc.). CD8+TSKd1+ stimulation indices were calculated by taking the absolute number of live CD19-CD3+CD4-CD8+tetramer+CFSEl° cells present after A20-TS stimulation divided by the number present after A20-negative control incubation. The percentages and absolute numbers of CD8+TSKd1+cytokine+ T cells were calculated by taking the percentages or numbers of live CD19-CD3+CD4-CD8+tetramer+CFSEl°cytokine+ cells present after stimulation with A20-TS subtracted by the number present after A20-negative control incubation.
Histopathology
Cardiac and skeletal muscle inflammation was assessed at days 30 or 87 days post systemic T. cruzi (BFT) challenge. The heart and skeletal muscles from individual mice were fixed in 10% formalin, paraffin embedded, sectioned and stained with H&E. Pathology slides were graded by a blinded pathologist. 1 = minimal, 2 = mild, 3 = moderate, 4 = marked.
Statistics
Statistical analyses were performed using Prism v4 software (GraphPad Software, Inc., La Jolla, CA). Mann-Whitney U tests or unpaired student t tests were used to compare responses between groups. Log-rank tests were used to compare survival between groups.
Results
CD8+ T cells, but not B cells, are Required for Protective Mucosal T. cruzi Immunity
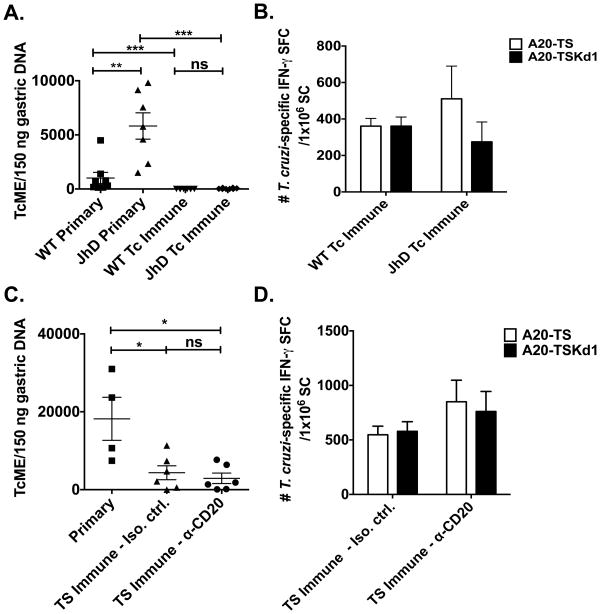
Previous work has shown that repeated T. cruzi mucosal infections result in chronically infected mice that develop concomitant immunity protective against subsequent mucosal challenges (Tc immune) (17). These Tc immune mice developed high numbers of T. cruzi-specific antibody secreting cells in the gastric mucosa, suggesting that B cells played an important role in mucosal T. cruzi protection (17). We first sought to directly address the potential causative role of B cells in protective mucosal T. cruzi immunity. Tc immune B cell knockout (KO; JhD) and wild-type (WT) BALB/c mice were generated as shown in Figure 1A. One month after the last infection, Tc immune and naïve B cell KO and WT BALB/c mice were challenged orally with a high-dose of T. cruzi. Mucosal protection was assessed 12 days later via real-time PCR as we have previously shown that the highest level of T. cruzi DNA can be measured in the gastric mucosa, the point of initial mucosal invasion after oral parasite inoculation, 10-14 days post-challenge (17) (Figure 2A). B cell KO mice had significantly higher levels of T. cruzi DNA in the gastric mucosa compared with WT mice after primary mucosal parasite infection (p=0.0006; Mann-Whitney U test), suggesting that B cells are important in the primary control of parasite replication. However, more relevant for vaccine development is the role of B cells in memory immune responses. Tc immune B cell KO mice did develop mucosal immunity, as they were significantly protected compared with the primary challenged B cell KO mice (Figure 2A, p<0.0005; Mann-Whitney U test). In addition, Tc immune B cell KO mice developed similar levels of mucosal protection compared with WT Tc immune mice (p=0.1719; Mann-Whitney U test). We also assessed the frequency of T. cruzi-specific T cells in B cell KO and WT Tc immune mice. There were similar frequencies of total T cells specific for the T. cruzi protein trans-sialidase (TS) (A20-TS response) and T cells reactive with an immunodominant CD8 T cell epitope (A20-TSKd1 response) in WT and B cell KO mice (Figure 2B). We measured immune responses in the spleen as it has been shown previously that immunity to mucosal pathogens can be efficiently measured in the spleen (22, 23). Antigen-specific cells generated in mucosal lymph nodes traffic through the spleen before migrating back to mucosal tissues. Additionally, higher total numbers of rotavirus-specific sIgA antibody secreting cells (ASC) have been recovered from spleens than from mucosal tissues (22), and splenic CD8+ T cells from rotavirus-immune mice can efficiently transfer mucosal protection (22, 23). Overall, our results demonstrate that B cells are not required for T. cruzi mucosal immunity.
Figure 2. B cells are not required for protective mucosal T. cruzi immunity.
(A,B) Tc immune JhD B cell KO and WT BALB/c mice were generated by repeated low-dose intragastric T. cruzi infections as described in Figure 1. One month following the last intragastric infection, naïve and Tc immune B cell KO and WT mice were challenged intragastrically with a high dose T. cruzi. Twelve days later (the point at which T. cruzi DNA is highest in the gastric mucosa (17)), mice were sacrificed and (A) gastric DNA isolated to assess the level of T. cruzi DNA via qPCR. n=7-8 mice/group. Data are derived from two independent experiments pooled together. (B&D) Splenocytes (SC) were isolated and the frequency of IFN-γ-producing TS or TSKd1-specific T cells measured via ELISPOT. Background responses were subtracted from the experimental values. (C,D) Mucosal TS immune mice were generated by intranasal vaccination with recombinant TS+CpG 1826 twice, two weeks apart. Mice were either B cell depleted (α-CD20) or not (IgG1κ) starting two weeks prior to vaccination. One month after vaccination, naïve and TS immune mice were challenged intragastrically with a high dose of T. cruzi. Twelve days later, mice were sacrificed and (C) gastric DNA isolated to assess the level of T. cruzi DNA via qPCR. n=5 mice/group. * p < 0.05, ** p < 0.01, *** p < 0.0005 Mann-Whitney U-test. TcME = T. cruzi molecular equivalents; SFC = spot forming cells. Error bars represent SEM.
Next, we wanted to determine whether B cells play an important role in trans-sialdiase (TS) vaccine-induced mucosal immunity (Figure 1B). TS is an enzyme on the surface of T. cruzi that is important for parasite infectivity as it has both neuraminidase and sialic acid transfer activity. We have previously shown that mice vaccinated with TS plus the TLR9 agonist CpG 1826 are protected against both mucosal and systemic T. cruzi challenges (26). B cells were first depleted in naïve wild type BALB/c mice using α-CD20 mAb (Biogen Idec). TS immune mice (Figure 1B) were intranasally vaccinated twice with recombinant TS + CpG 1826 two weeks apart, rested for one month, challenged with a high dose of T. cruzi orally (107) and assessed for mucosal protection via real-time PCR. Similar to the results seen in Tc immune mice, B cells were not required for TS vaccine-induced protective mucosal immunity (Figure 2C). TS- and TSKd1-specific T cell responses were comparable between B cell depleted and isotype control treated TS vaccinated mice (Figure 2D), demonstrating that B cells are not required for the development, maintenance and/or recall of TS- or TSKd1-specific T cells after mucosal T. cruzi challenge. Thus B cells are not required for TS-specific vaccine-induced mucosal immunity.
To confirm that B cells are not critical for mucosal T. cruzi protection, we performed adoptive transfer studies. We first determined whether cells from Tc immune mice or TS immune mice generated as shown in Figure 1C with a more potent vaccination regimen (heterologous TS DNA primed, adenovirus-TS boosted) could transfer mucosal protection, as this has not been shown previously. Due to the low numbers of cells recovered from the gastric lymph nodes, mixed populations of splenocytes plus gastric lymph node cells were utilized in order to transfer maximal numbers of parasite-specific cells. Previous work by others has shown that splenocytes from rotavirus-immune mice contain lymphocytes with mucosal homing capacity that can transfer mucosal protection (22, 23). Both Tc immune (Figure 3A) and TS immune (Figure 3B) donor cells were able to transfer mucosal protection to naïve SCID mice.
Figure 3. T. cruzi-specific CD8+ T cells generated by infection or vaccination can transfer mucosal T. cruzi protection.
(A-D) Subsets of immune cells from Tc immune or systemic TS immune mice were transferred into naïve SCID mice. Recipient mice were challenged the following day with a high-dose of T. cruzi intragastrically and 12 days later, mice were sacrificed and T. cruzi DNA assessed in the gastric mucosa via qPCR. (A) One mouse spleen cells (SC) + draining LN (dLN) cell equivalent from Tc immune or naïve BALB/c mice were transferred i.v. into naïve SCID mice. n=9-10 mice/group. Data presented are derived from two independent experiments pooled together. ** p < 0.01, Student t-test. (B) One mouse equivalent of splenocytes from TS immune mice (TS DNA prime, adenovirus-TS boost) or naïve BALB/c mice were transferred i.v. into naïve SCID mice. n=9-10 mice/group. * p < 0.05, Student t-test. Data presented are derived from two independent experiments pooled together. (C) One mouse equivalent of total splenocytes or CD19-depleted splenocytes populations from Tc immune or naïve BALB/c mice were transferred i.v. into naïve SCID mice. n=5 mice/group. * p < 0.05, ** p < 0.01 Mann-Whitney U test. Data are representative of two independent experiments. (D) One mouse spleen+gastric LN equivalent of highly purified CD8+ T cells (> 98% CD8+) from Tc immune or naïve BALB/c mice were transferred i.v. into naïve SCID mice. n=11 mice/group. *** p < 0.0001, Mann-Whitney U test. Data presented are derived from two independent experiments pooled together. Error bars represent SEM.
Next, we studied whether B cells are necessary and/or sufficient for the transfer of mucosal protection. We transferred highly purified CD19+ B cells from either naïve or Tc immune BALB/c mice into SCID mice. However, we could not confirm whether B cells were sufficient as SCID mice receiving purified B cells (Supplemental Figure 1A) alone did not develop T. cruzi-specific antibody responses (serum IgG by ELISA or ASC detected by ELISPOT; Supplemental Figures 1C and 1D, respectively), even though B cells were clearly present (Supplemental Figure 1B). Thus, we decided to perform B cell depletion studies to determine if B cells were necessary for the transfer of mucosal protection. B cells were depleted from Tc immune and naïve BALB/c splenocytes and gastric LN cells (Supplemental Figure 2). These B cell depleted fractions were transferred into naïve SCID mice and recipient mice were orally challenged the following day with a high dose of T. cruzi intragastrically. Twelve days after challenge, recipient mice were assessed for the presence of B cells and T. cruzi-specific serum IgG and IgG antibody secreting cells. Supplemental Figure 2 demonstrates that in SCID mice given CD19-depleted cells, B cells were undetectable. Figure 3C demonstrates that B cells are not necessary for the transfer of mucosal protection as SCID mice that received either total or B cell-depleted splenocytes+gLN cells from Tc immune mice were similarly protected (p<0.05, Mann-Whitney U test compared to transfer of cells from naïve mice). There was no significant difference in protection transferred by naïve total or naïve B cell depleted cell populations (p=0.22, Mann-Whitney U-test). These results confirm our earlier results detected in the B cell KO model that B cells are not required for mucosal T. cruzi protection.
Since B cells were not critical for mucosal T. cruzi protection, we examined whether CD8+ T cells alone from Tc immune mice could transfer mucosal protection. Previous work has shown that CD8+ T cells from Tc immune mice could transfer protection against normally lethal systemic parasite challenges (4, 27). However, in order for CD8+ T cells to mediate mucosal protection, these cells must be able to migrate into the gastric tissue and provide effector functions. Highly purified CD8+ T cells (Supplemental Figure 3A) from Tc immune and naïve BALB/c mice were transferred into naïve SCID mice one day prior to oral challenge. SCID mice that received Tc immune CD8+ T cells were significantly protected against oral T. cruzi challenge as compared to mice receiving naïve control CD8+ T cells (Figure 3D), despite the absence of detectable T. cruzi-specific serum IgG (Supplemental Figure 3B). Thus CD8+ T cells alone are able to confer mucosal T. cruzi protection.
B cells are Important for Systemic T. cruzi Immunity
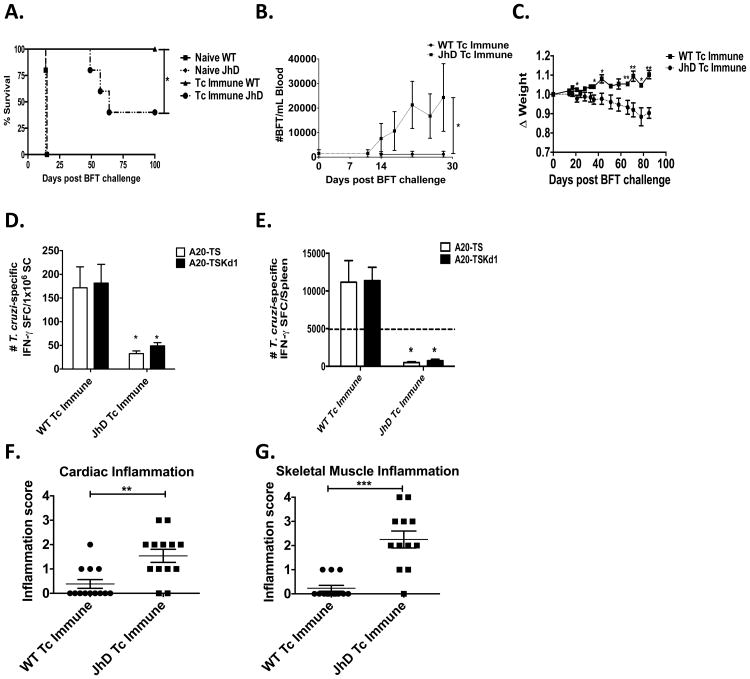
We next investigated the ability of B cells to contribute to systemic T. cruzi protection in our model systems. Previous work by Tarleton et al showed that B cell KO mice could initially control parasitemia after systemic infection with T. cruzi but eventually succumbed to the infection (16). First, we verified whether B cell KO mice could develop systemic protection. B cell KO and WT BALB/c mice were orally infected with a low-dose of T. cruzi twice, two months apart, to generate Tc immune mice (Figure 1A). These mice did not succumb to infection or require anti-parasitic drugs (data not shown). In contrast, we have previously shown that mice deficient in IL-12, IFN-γ or β2-microglobulin are unable to survive low-dose parasite infections even with anti-parasite drug intervention (19). We then challenged B cell KO and WT Tc immune mice systemically with blood-form tryptomastigotes (BFT), which are more virulent and induce higher levels of parasitemia in naïve mice (26, 28). B cell KO Tc immune mice survived significantly longer than their naïve cohorts but eventually succumbed to infection (Figure 4A), developing increased parasitemia (Figure 4B), weight loss (Figure 4C) and decreased TS- and TSKd1-specific CD8+ T cell responses (Figure 4D, 4E). Importantly, the frequencies and absolute numbers of IFN-γ-producing TSKd1-specific CD8+ T cells were significantly reduced. These results indicate that B cells are important for the maintenance of T. cruzi-specific, CD8+ T cell functional responses when systemic antigen levels are high. Previous work by our lab has shown that WT BALB/c mice require ∼5,000 TSKd1-specific IFN-γ-producing CD8+ T cells to be protected from death (Hoft et al, manuscript in preparation). Shown in Figure 4E, B cell KO Tc immune mice developed significantly lower numbers of TSKd1-specific IFN-γ-producing CD8+ T cells per spleen (738 ± 198) compared with WT Tc immune mice (11374 ± 1764). Thus, B cell KO Tc immune mice probably were not protected because they either did not develop or sustain sufficient numbers of IFN-γ-producing-TSKd1-specific CD8+ T cells. Consistent with the higher levels of parasitemia, Tc immune mice lacking B cells developed significantly higher levels of inflammation in cardiac and skeletal tissues, hallmarks of disease pathology induced by chronic T. cruzi infection (Figure 4F-G). Overall, these results demonstrate that B cells play a critical role in protective T. cruzi systemic immunity.
Figure 4. B cells are important for systemic T. cruzi immunity developing in response to parasite infection.
JhD B cell KO and WT Tc immune mice were generated as described in Figure 1. At least one month following the last mucosal infection, Tc immune and naïve B cell KO and WT BALB/c mice were challenged systemically with virulent T. cruzi BFT subcutaneously. (A) Survival was assessed for up to 100 days. * p < 0.05, Log-rank test. n=5/group. (B) Parasitemia was assessed prior to and up to 28 days after systemic T. cruzi (BFT) challenge. ** p < 0.01, area under the curve was assessed for each individual mouse and used to compare groups in Mann-Whitney U test. n=5/group. (C) Weight was measured prior to and through d85 post systemic challenge. * p < 0.05, ** p < 0.01, Mann-Whitney U test. Data in A-C are representative of two independent experiments with similar results. Thirty days after systemic challenge, some mice were sacrificed to assess immune and inflammatory responses. (D) The frequency and (E) absolute number of IFN-γ-producing TS- and TSKd1-specific T cells were measured via ELISPOT using total splenocytes. Background responses were subtracted from the experimental values. The dashed line in (E) represents 5000 TSKd1-specific CD8+ T cells, a number found in previous work to provide systemic protection (Hoft DF et al manuscript in preparation). * p < 0.05, Mann-Whitney U test. n=4 mice/group. Data are representative of two independent experiments with similar results. (F) Cardiac and (G) skeletal muscle inflammation were assessed at 30-87 days post systemic challenge. 1 = minimal, 2 = mild, 3 = moderate, 4 = marked. Naïve B cell KO and WT mice showed no signs of inflammation (data not shown). n=12-13 mice/group. ** p < 0.005, *** p < 0.0005, Mann-Whitney U test. Data presented are derived from two independent experiments with results pooled together. Error bars represent SEM.
Non-Specific B cells Cannot Reconstitute T. cruzi Immunity
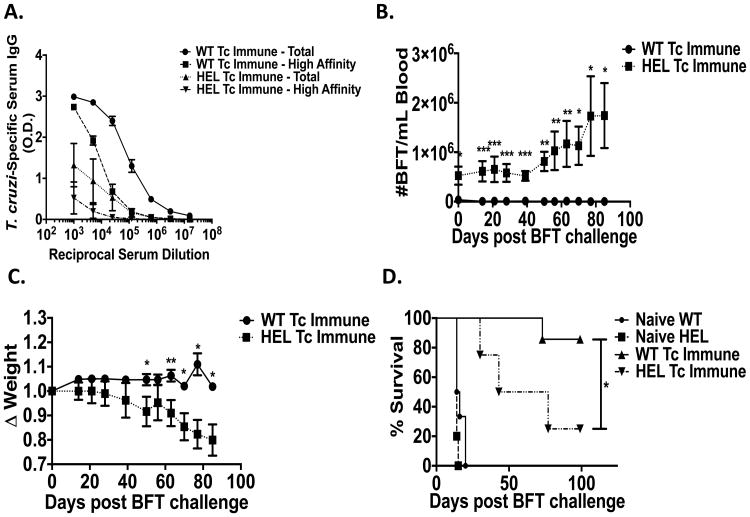
B cells provide many important functions such as antibody production, antigen-presentation, co-stimulation and cytokine production. Thus, B cell deficiency not only results in the absence of antibodies, but also could affect the development of T cell responses. In order to determine whether T. cruzi-specific B cells are critical for systemic protection, we studied hen-egg lysozyme (HEL)-specific BCR transgenic mice. Advantages of using these mice are that in contrast to complete B cell KO mice, lymphoid tissue development and cytokine production are relatively normal. However, these mice cannot generate B cells that produce high affinity T. cruzi-specific antibodies, which could be important for both direct protective effects as well as enhanced antigen presentation. HEL and WT Tc immune mice were generated by repeated low-dose T. cruzi oral infections (Figure 1A). Prior to and following systemic T. cruzi (BFT) challenge (at least one month after the second oral infection), mice were assessed for T. cruzi-specific IgG antibodies, parasitemia, weight loss and death (Figure 5).
Figure 5. Non-specific B cells cannot reconstitute T. cruzi induced immunity.
Tc immune hen-egg lysozyme (HEL) specific BCR transgenic or control C57BL/6 mice were generated by repeated low dose intragastric infections with T. cruzi. At least 1 month after the last infection, Tc immune and naïve HEL and WT mice were challenged systemically with virulent T. cruzi BFT subcutaneously. (A) Total and high affinity recombinant TS-specific serum IgG responses were measured 50 days post systemic challenge via ELISA. (B) Parasitemias were assessed at different time points pre- and post-final systemic challenge. n=7-10/group. ** p < 0.01, *** p < 0.005, Mann-Whitney U test. (C) Weight loss assessed at different time points after systemic challenge. n=7-10/group. ** p < 0.01 Mann-Whitney U test. (D) Mice were followed for survival for 100 days after systemic challenge. n=4-7 mice/group. * p < 0.05, Log-rank test. Data are representative of two independent experiments with similar results. Error bars represent SEM.
HEL Tc immune sera did have some background reactivity in our rTS-specific ELISA, but very little of this reactivity persisted in the presence of 8M urea demonstrating that minimal if any T. cruzi-specific, high affinity antibodies were present (Figure 5A). In contrast, WT Tc immune sera had markedly higher reactivity with rTS and much of this persisted despite 8M urea treatment.
Even before the high level T. cruzi BFT systemic challenge, parasitemia levels were increased in the HEL Tc immune mice (Figure 5B). These results could be interpreted to suggest that the absence of high affinity, T. cruzi-specific B cell responses resulted in a deficiency in protective mucosal memory immunity, despite our earlier results shown in Figures 2-3 demonstrating that B cells were not important for either infection- or vaccine-induced mucosal T. cruzi-specific protective immunity. However, parasitemia is a systemic endpoint largely controlled by systemic immunity. After oral T. cruzi challenges, the parasite drains to the gastric LN and disseminates throughout the body causing systemic infection. All Tc immune mice develop chronic systemic infection requiring systemic immunity to control parasitemia. The fact that HEL Tc immune mice had higher parasitemias prior to the high level BFT systemic challenge indicates that these mice have profound defects in systemic immunity, resulting in the inability to control systemic parasite infection even after low-dose T. cruzi mucosal challenge.
Consistent with the results presented in Figure 4, parasitemia levels increased further in HEL Tc immune mice after systemic challenge (Figure 5B), resulting in increased weight loss (Figure 5C) and death (Figure 5D). These results further demonstrate that T. cruzi-specific B cells are critical for systemic T. cruzi protection.
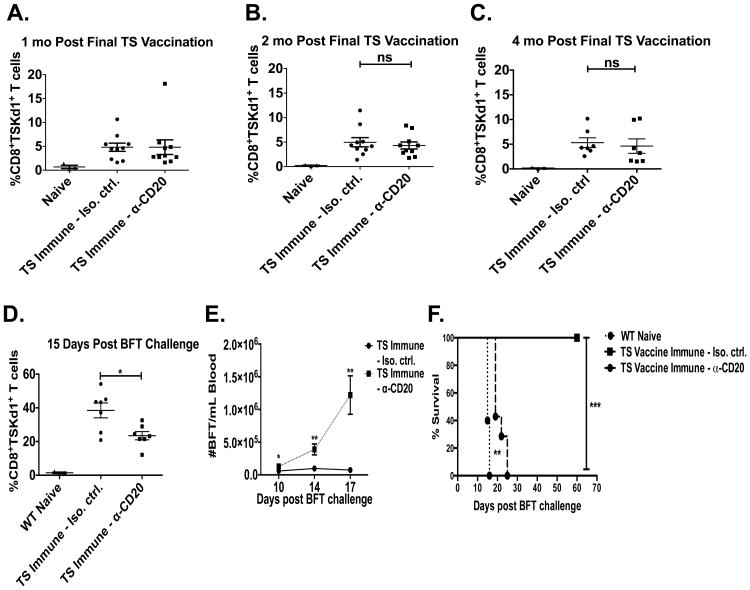
Similar Levels of Memory CD8+ T cells Develop in the Absence of Infection but Fail to Protect After Systemic Challenge
In our Tc immune model, mice are chronically infected, making it difficult to study the development of memory CD8+ T cell responses in the absence of persistently high levels of T. cruzi antigen. Thus, we examined the development of CD8+TSKd1+ T cells in our TS immune memory model (heterologous TS DNA primed, adenovirus-TS boosted, Figure 1C). As described earlier, B cells were depleted in vivo with α-CD20 antibody (or isotype control) throughout the vaccination/treatment regimen. TS immune B cell depleted and control mice developed similar levels of immunodominant CD8+TSKd1+ T cells detectable in blood (Figures 6A-C). Thus, antigen-specific CD8+ T cells can be primed normally in B cell depleted mice. These mice were then challenged systemically with T. cruzi and assessed for immune responses and protection. The percentages of the total CD8 populations that were CD8+TSKd1+ T cells expanded from approximately 5.3% ± 1.0% at 4 months post-boost (Figure 6C) to 38.5% ± 4.4% fifteen days after the systemic T. cruzi challenge in isotype control-treated TS immune mice (Figure 6D). Anti-CD20 treated TS immune mice had frequencies of CD8+TSKd1+ T cells similar to control mice prior to systemic challenge (4.62% ± 1.5%, Figure 6C). However, these cells did not expand optimally after systemic T. cruzi challenge (Figure 6D), resulting in significantly less CD8+TSKd1+ T cells after challenge than in control TS immune mice. These reduced numbers of CD8+TSKd1+ T cells in anti-CD20 treated TS immune mice were associated with increased parasitemia (Figure 6E) and death (Figure 6F). Although similar levels of immunodominant CD8+ T cells developed in response to TS vaccination in the absence of B cells, these CD8+ T cells were functionally deficient and failed to protect against T. cruzi systemic challenge. Thus, memory CD8+ T cells that develop in the absence of B cells were impaired in their ability to respond to and protect against virulent systemic T. cruzi challenges.
Figure 6. B cells are important for TS vaccine-induced systemic immunity.
B cell depleted (α-CD20) and isotype control treated BALB/c mice were vaccinated with our heterologous TS DNA prime, adeno-TS boost vaccination regimen (systemic TS immune mice). Mice were treated with α-CD20 or isotype control antibody every 2-3 weeks for the duration of the study and assessed for B cell depletion via flow cytometry and T. cruzi-specific serum IgG (data not shown). The percentages of CD8+TSKd1+ T cells were measured in blood via flow cytometry at (A) 1, (B) 2 and (C) 4 months after the final TS vaccination. TS immune and naïve BALB/c mice were challenged systemically with virulent T. cruzi BFT subcutaneously ∼ 4.5 months after the last TS vaccination. n=7-10/group. (D) 15 days after systemic challenge, the percentages of CD8+TSKd1+ T cells were measured in blood via flow cytometry. n=7/group. * p < 0.05, Mann-Whitney U test. (E) Parasitemia was measured through d17 post systemic challenge. n=7/group, * p < 0.05, ** p < 0.01, *** p < 0.005, Mann-Whitney U test. (F) Survival was assessed through d60 post systemic challenge. n=5-7/group. ** p < 0.01, *** p < 0.005, Log-rank test. Error bars represent SEM.
B cells are Important for Multifunctional CD8+ T cell Responses and Prevention of CD8+ T cell Exhaustion
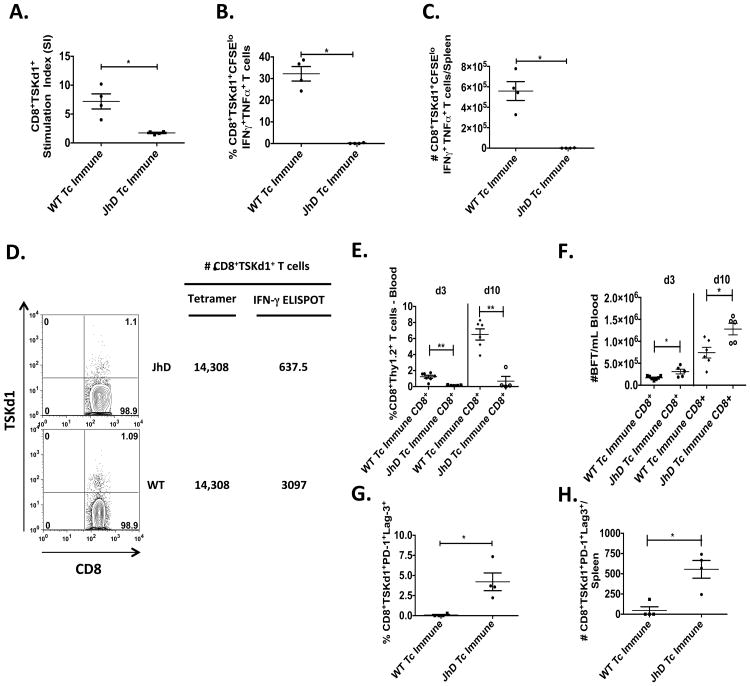
As we have shown in Figures 4-6, in the absence of B cells, mice are unable to develop immunity that prevents death after virulent T. cruzi systemic challenge. Interestingly, these mice are able to develop CD8+ T cell responses and survive longer than their naïve cohorts (Figure 4A). However, parasitemia increases over time in these B cell deficient immune mice after virulent systemic challenge, ultimately resulting in death. These data suggested that although similar numbers of T. cruzi-specific CD8+ T cells could be induced in B cell deficient mice, these CD8+ T cells were functionally deficient, especially when confronted with high antigen loads. To test this hypothesis, we first examined the ability of TSKd1-specific CD8+ T cells to expand and produce cytokines after ex vivo restimulation. B cell KO and WT Tc immune mice were challenged systemically with high doses of T. cruzi and immune responses evaluated 30 days later. Splenocytes were labeled with CFSE and co-cultured with either negative control APCs or APCs that were stably transduced with TS (28). Significant reductions in antigen-specific CD8+TSKd1+ T cell proliferative capacity (Figure 7A) and cytokine production (IFN-γ and TNF-α, Figures 7B-C) were detected in mice lacking B cells compared with WT mice. In order to evaluate CD8+TSKd1+ in vivo expansion, equivalent numbers of CD8+Thy1.2+TSKd1+-specific T cells from B cell KO and WT Tc immune mice (based on tetramer staining) were transferred into naïve Thy1.1+ BALB/c mice with normal B cell numbers and function (Figures 7D-H). Recipient mice were then challenged systemically one day later. CD8+Thy1.2+ T cells from B cell KO Tc immune mice failed to expand normally in vivo compared with WT infection-immune CD8+ T cells (Figure 7E), resulting in a reduced ability to control parasitemia (Figure 7F). These results identify intrinsic functional defects in T. cruzi-specific CD8+ T cells generated in the absence of B cells.
Figure 7. B cells prevent T. cruzi-specific CD8+ T cell exhaustion.
At least one month following the last sublethal mucosal T. cruzi infection, Tc immune and naïve B cell KO and WT BALB/c mice were systemically challenged with virulent T. cruzi BFT. Thirty days later, mice were sacrificed and T. cruzi-specific CD8+ T cell responses measured. (A-C) Splenocytes were stained with CFSE and co-cultured with either control A20 or A20-TS APCs for 6 days. Some cells were used to assess CD8+TSKd1+ proliferation via flow cytometry (A). CD8+TSKd1+ stimulation indices were calculated by taking the number of live CD19-CD3+CD4-CD8+TSKd1+CFSEl° present after A20-TS stimulation divided by the number after control A20 incubation. (B-C) PMA/Ionomycin, Golgi stop and Golgi plug were added to the remaining cells and placed back in culture for 3h prior to surface and intracellular cytokine staining. The percentages (B) and absolute numbers (C) of CD8+TSKd1+cytokine+ T cells were calculated by taking the number of live CD19-CD3+CD4-CD8+TSKd1+CFSEl°cytokine+ cells after stimulation with A20-TS divided by the number present after control A20 incubation. (A-C) * p < 0.05, Mann-Whitney U test. Data are representative of two independent experiments with similar results. (D) CD8+ T cells were purified from the spleens of Tc immune B cell KO and WT BALB/c mice 87 days after systemic T. cruzi (BFT) challenge, and then equivalent numbers of TSKd1+ T cells were transferred i.v. into naïve Thy1.1+ BALB/c mice. One day later, these mice were challenged systemically with virulent T. cruzi BFT subcutaneously. (E) Three and ten days after systemic challenge, recipient mice were bled to assess for the percentage of CD8+Thy1.2+ T cells in the blood via flow cytometry. n=4-7 mice/group. ** p < 0.01, Mann-Whitney U test. (F) Ten and fourteen days after systemic challenge, parasitemia was assessed in recipient mice. n=5-7 mice/group. * p < 0.05, Student t-test. (G-H) Splenocytes were directly stained on day 30 post BFT challenge and assessed for the percentages (G) and absolute numbers (H) of CD8+TSKd1+PD-1+Lag-3+ T cells via flow cytometry. n=4 mice/group. Data are representative of two independent experiments with similar results. Error bars represent SEM.
To further examine the mechanism responsible for CD8+ T cell dysfunction in B cell deficient mice, we evaluated expression of the inhibitory receptors PD-1 and Lag-3 on tetramer positive CD8+ T cells. There were significant increases in the percentages (Figure 7G) and absolute numbers (Figure 7H) of CD8+TSKd1+PD-1+Lag-3+ T cells in the spleens of B cell KO Tc immune mice compared with WT Tc immune mice. We also found significant increases in CD8+TSKd1+Lag-3+ T cells in the blood of TS vaccinated and B cell depleted mice 15 days after systemic T. cruzi challenge (Supplemental Figure 4). Overall, these results demonstrate that B cells play a critical role for both the induction of optimally functional CD8+ T cell responses and the prevention of CD8+ T cell exhaustion.
Passive Immune Serum Transfer Prevents CD8+ T cell Exhaustion and Increases Survival Post-challenge
Previous work by others has shown that passive serum transfers from Tc immune into naïve mice can transfer systemic protection (11-15). We hypothesized that the lack of T. cruzi-specific antibodies in B cell KO Tc immune mice resulted in increased tissue parasitism and death due to the inability of functionally exhausted CD8+ T cells to properly control T. cruzi infection. To test this hypothesis, we performed passive serum transfers. Prior to and after systemic T. cruzi challenge, B cell KO Tc immune mice received WT Tc immune serum, naïve serum or PBS. As shown in Figure 8A, T. cruzi-specific antibody responses were present in B cell KO mice after transfer of WT Tc immune serum. The immune serum protected B cell KO mice from increased morbidity and mortality (Figures 8B-C). This protection was associated with increased TSKd1-specific CD8+ T cell numbers (Figure 8D) and total TS-specific and TSKd1-specific T cell function (Figure 8E). Finally, Tc immune serum transfer resulted in reduced expression of the T cell exhaustion markers PD-1 and Lag-3 on TSKd1-specific CD8+ T cells (Figure 8F). Overall, these results demonstrate that T. cruzi-specific antibodies could prevent death and CD8+ T cell exhaustion.
Figure 8. Tc immune serum can rescue CD8-mediated systemic protection in B cell KO mice.
JhD B cell KO Tc immune mice were generated as described in Figure 1 and the materials and methods section. Beginning at least one month following the last mucosal infection, mice were injected i.p. weekly with 300μl of either WT naïve or WT Tc immune serum. (A) Passive serum transfers reconstitute T. cruzi TS-specific IgG levels. Serum-treated Tc immune B cell KO mice were then challenged systemically with virulent T. cruzi BFT and monitored for morbidity (B) and mortality (C). (B) Hind-limb paralysis was measured on days 30 and 87 post systemic T. cruzi challenge, see experimental procedures for scoring system, n=10 mice/group, ** p = 0.0115, Mann-Whitney U test, data are representative of two independent experiments with similar results. (C) n=5 mice/group, data are representative of two independent experiments with similar results. * p < 0.05, Log-rank test. (D) Five weeks after systemic challenge, spleen cells from serum-rescued B cell KO mice were assessed by flow cytometry and ELISPOT. (D) Percentage of CD8+TSKd1+ T cells measured via flow cytometry. (E) Frequency of IFN-γ-producing TS and TSKd1-specific T cells measured by ELISPOT. Background responses were subtracted from the experimental values. (F) Frequencies of PD-1+LAG-3+ TSKd1-specific CD8+ T cells. Error bars represent SEM.
Discussion
In this report, we evaluated the importance of B cells in mucosal and systemic T. cruzi protection. We first examined whether B cells were important for mucosal T. cruzi protection. Previous results suggested that B cells would play a critical role in mucosal T. cruzi protection (18, 26). In fact, B cells have been shown to play important roles in mucosal protection against several mucosal pathogens such as helminthes (29, 30) and rotavirus (31, 32). Although we failed to identify a critical role for B cells in mucosal T. cruzi protection, this does not rule out the possibility that B cell responses could provide some level of mucosal protection. However, using both B cell deficient mice and adoptive transfer studies, we showed that CD8+ T cells, but not B cells, are critical for mucosal T. cruzi immunity.
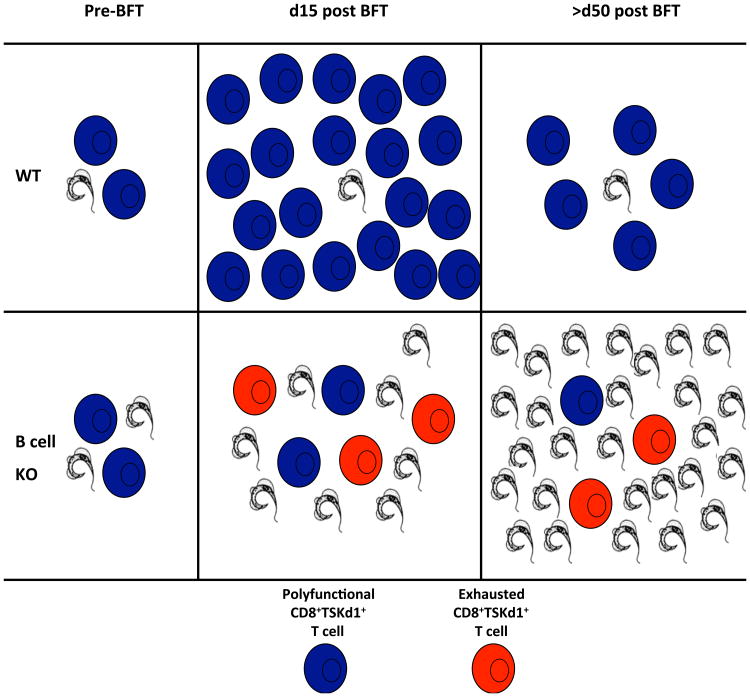
Next, we examined the importance of B cells in systemic protection induced by multiple low dose parasite infections or highly potent vaccine regimens (TS DNA prime/adenovirus-TS boost). B cell deficient mice infected orally with T. cruzi developed higher levels of parasitemia compared with WT mice but this did not result in increased morbidity and mortality. These results indicate that B cells are not required when systemic antigen load is low after an oral infection. In contrast, we did show that B cell responses are absolutely required for protection after systemic challenge with the more virulent blood-form trypomastigote (BFT) that leads to higher systemic antigen loads. Tc immune B cell deficient mice survived longer than their naïve cohorts after virulent systemic parasite challenge. However, Tc immune B cell deficient mice developed increased parasitemia over time compared with WT Tc immune mice, resulting in significant morbidity and eventual death. We also determined that T. cruzi-specific B cells, not polyclonal nonspecific B cells, are critically important for T. cruzi systemic protection using HEL BCR transgenic mice. Decreased survival in B cell knockout μMT-/- mice after T. cruzi systemic challenge has been shown previously (16, 26). In addition, our results are similar to recently published data demonstrating that after disseminating virus infection (LCMV-t1b), μMT knockout and anti-CD20 treated mice could not control viral replication, resulting in weight loss and decreased LCMV-specific CD8+ T cell functionality (33). Overall, these previous results combined with our new data suggest that memory CD8+ T cells primed in mice lacking T. cruzi-specific B cells are sufficient to control low levels of tissue parasitism, but eventually become exhausted after systemic challenge due to prolonged exposure to high levels of antigen. These findings are consistent with previous data showing that high antigen load results in CD8+ T cell exhaustion (34-36). The role of B cells in preventing CD8+ T cell exhaustion is nicely shown when examining the CD8+ T cell response after TS vaccination. B cell deficient mice developed similar frequencies of TSKd1-specific CD8+ T cells compared with WT mice after our heterologous TS vaccine regimen. These results confirm that B cells are not required for the initial priming of CD8+ T cell memory. However, after systemic challenge (resulting in high antigen load), CD8+TSKd1+ T cells from B cell deficient TS immune mice did not expand as well as CD8+ T cells primed in WT mice. This suboptimal CD8+TSKd1+ T cell secondary expansion capacity in B cell deficient, TS vaccinated mice was associated with increased parasitemia and death. Thus, although T. cruzi-specific CD8+ T cells can be primed in the absence of B cells, they become functionally deficient severely impairing protective immunity when antigen level is high (Figure 9).
Figure 9. Proposed model of T. cruzi-specific CD8+ T cell dysfunction in the absence of B cells.
In both WT and JhD B cell KO mice, T. cruzi TSKd1-specific CD8+ T cells are generated after low-dose mucosal T. cruzi challenge. Prior to systemic T. cruzi BFT challenge, there is higher parasitemia in the blood of B cell KO Tc immune mice, suggesting the early stages of CD8+ T cell dysfunction. After systemic T. cruzi BFT challenge, polyfunctional TSKd1-specific CD8+ T cells expand to high numbers in WT Tc immune mice by 15 days post-challenge. These T cells contract and are maintained at a higher number through d50 post systemic challenge. This typical memory T cell response in WT infection-immune mice results in higher numbers and better quality T cells associated with limited parasite persistence. In B cell KO Tc immune mice, however, CD8+ T cell expansion is significantly impaired. By d15 post systemic challenge, TSKd1-specific CD8+ T cells are markedly reduced in B cell deficient mice, associated with increased parasitemia levels. By d50, most TSKd1-specific CD8+ T cells appear to be functionally exhausted due to limited proliferative and cytokine-producing capacity, and express increased levels of the inhibitory receptors PD-1 and Lag-3. This functional exhaustion of CD8+TSKd1+ T cells in mice lacking B cells results in increased parasitemia and death.
In order to determine whether the defect in memory CD8+ T cell secondary expansion present in Tc immune B cell KO mice was due to poor priming or deficient support for re-expansion, we studied TSKd1-specific memory CD8+ T cells generated in B cell KO and WT Tc immune mice after subsequent transfer into immunocompetent BALB/c mice (Figure 7D-H). Memory CD8+ T cells from B cell KO mice failed to expand in response to systemic parasite challenge after transfer into normal hosts containing polyclonal B cells, demonstrating an intrinsic defect in CD8+ T cell responses generated in mice lacking B cells. This failure of expansion resulted in significantly reduced protection. We then examined expression of the inhibitory receptors PD-1 and Lag-3 by CD8+TSKd1+ T cells after TS vaccination and T. cruzi infection. After systemic challenge of Tc immune and TS immune B cell deficient mice, significant increases in CD8+TSKd1+ T cells expressing PD-1+ and Lag-3+ were observed. Overall, the lack of both direct ex vivo and in vivo expansion capacity, decreased cytokine production, increased inhibitory receptor expression and reduced protection associated with memory T cells primed in the absence of B cells, all indicate an important role for B cells in the prevention of CD8+ T cell exhaustion. To our knowledge, these results are the first to mechanistically demonstrate the importance of B cells for prevention of T. cruzi CD8+ T cell exhaustion.
Few studies have evaluated the role of B cells in the development of CD8+ T cell memory. During primary responses to an acute LCMV-Armstrong infection, CD8+ T cell responses in mice lacking B cells were normal at early timepoints (37), but reduced at 154 days post infection (37, 38). In a vaccination model using Listeria monocytogenes, B cells were not required for the initial activation and expansion of antigen-specific CD8+ T cell responses (39). However, in the absence of B cells, a more profound contraction phase occurred leading to lower numbers of persisting antigen-specific memory CD8+ T cells (39). Another group showed that lack of B cells affected the long-term maintenance of CD8+ T cell memory (40). Previous T. cruzi studies have also indicated that B cells might be important in T. cruzi-specific memory T cell development as lack of mature B cells (μMT-/- mice) resulted in increased susceptibility to primary parasite infection and failure to develop robust vaccine-induced protection (16, 26). However, none of these previous studies investigated the specific role of CD8 exhaustion in the absence of B cells. Our present results confirm these previous data and further demonstrate that B cells are critically important for both the development of fully functional CD8+ T cell responses as well as the prevention of CD8+ T cell exhaustion.
B cells may provide help for optimal CD8+ T cell memory responses by several mechanisms. First, B cells can present antigen and produce cytokines. Prior to high-level systemic challenge, the frequencies of CD8+TSKd1+ T cells were similar in Tc immune B cell knockout and WT mice, suggesting that general T cell priming is not affected by the absence of B cells. However, CD8+TSKd1+ T cells induced in the absence of B cells and restimulated in the presence of high systemic antigen load in vivo were functionally deficient, producing reduced multifunctional cytokine responses (IFN-γ+TNF-α+). It has been reported previously that B cells amplify IFN-γ production by T cells in a contact-dependent manner (41). Our data also indicate that the production of antigen-specific antibodies is another function of B cells critical for protective T. cruzi systemic immunity. Thus in the absence of T. cruzi-specific IgG, increases in parasitemia and subsequent increases in overall antigen load occurred. Furthermore, passive transfer of T. cruzi immune sera known to reduce systemic parasite burden, also reduced CD8+ T cell exhaustion and mortality in B cell KO Tc immune mice after virulent systemic challenge.
Although we did not study T. cruzi-specific CD4+ T cells, we do not believe that CD4+ T cells are irrelevant for inducing protective CD8+ T cells. We and others have shown that CD4+ T cell help is required for effective CD8+ T cell memory (10, 42) and can rescue exhausted CD8+ T cells (43). Work recently published showed that CD4+ T cells are dysfunctional in the absence of B cells in the LCMV model (33). These data support the importance of our work, which for the first time demonstrates the principle that B cell responses are important for preventing the exhaustion of T cells directed against a major human pathogen.
Our work clearly demonstrates that previously activated, memory CD8+ T cells alone can protect against mucosal T. cruzi challenge. However, after highly virulent T. cruzi systemic challenges, CD8+ T cells failed to provide optimal protection in the absence of B cells because CD8+ T cells became functionally exhausted. We further showed that HEL-specific B cells cannot prevent CD8+ T cell exhaustion, indicating that T. cruzi-specific B cells/antibody are needed for protection. Using both infection- and vaccine-induced memory immune models, we demonstrate that the absence of B cells leads to CD8+ T cell exhaustion in mice with high antigen load. Overall, these data show that B cells are important for two interrelated aspects of antigen-specific CD8+ T cell responses. First, antigen-specific B cells are critically important for the optimal development of multifunctional CD8+ T cell memory. Second, T. cruzi-specific antibodies can directly reduce overall parasite load, preventing CD8+ T cell exhaustion. In conclusion, our results demonstrate that although B cells are not directly important for mucosal immunity, they do play critical roles for the induction and functional responses of CD8+ memory T cells, which are absolutely essential for both mucosal and systemic protective T. cruzi immunity. These studies identify an important role for B cells in the prevention of CD8+ T cell exhaustion, providing critical new information for vaccine design.
Supplementary Material
Acknowledgments
We would like to thank Robert Dunn and Biogen Idec for providing the α-CD20/isotype control antibodies; Paul Garside at the University of Glasgow for providing us with the HEL BCR transgenic mice; Joy Eslick, Sherri Koehm, Erin Touchette, Kelly O'Shea and Stella Hoft for technical assistance; Andreas Wieland at Emory University for critical reading of this manuscript; and the NIH tetramer core facility at Emory University for providing us with the TSIYNVGQVSI/H2Kd-APC tetramer.
This work was supported by grants from the National Institutes of Health (R01 AI040196 and R56A104019 to D.F.H.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 2.Tarleton RL, Koller BH, Latour A, Postan M. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356:338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 3.Hoft DF, Eickhoff CS. Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges. Infection and immunity. 2002;70:6715–6725. doi: 10.1128/IAI.70.12.6715-6725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eickhoff CS, Vasconcelos JR, Sullivan NL, Blazevic A, Bruna-Romero O, Rodrigues MM, Hoft DF. Co-administration of a plasmid DNA encoding IL-15 improves long-term protection of a genetic vaccine against Trypanosoma cruzi. PLoS neglected tropical diseases. 2011;5:e983. doi: 10.1371/journal.pntd.0000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasconcelos JR, Bruna-Romero O, Araujo AF, Dominguez MR, Ersching J, de Alencar BC, Machado AV, Gazzinelli RT, Bortoluci KR, Amarante-Mendes GP, Lopes MF, Rodrigues MM. Pathogen-induced proapoptotic phenotype and high CD95 (Fas) expression accompany a suboptimal CD8+ T-cell response: reversal by adenoviral vaccine. PLoS pathogens. 2012;8:e1002699. doi: 10.1371/journal.ppat.1002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg CS, Martin DL, Tarleton RL. CD8+ T cells specific for immunodominant trans-sialidase epitopes contribute to control of Trypanosoma cruzi infection but are not required for resistance. J Immunol. 2010;185:560–568. doi: 10.4049/jimmunol.1000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarleton RL. The role of T cells in Trypanosoma cruzi infections. Parasitology today. 1995;11:7–9. doi: 10.1016/0169-4758(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 8.Rottenberg ME, Bakhiet M, Olsson T, Kristensson K, Mak T, Wigzell H, Orn A. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infection and immunity. 1993;61:5129–5133. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarleton RL, Grusby MJ, Postan M, Glimcher LH. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. International immunology. 1996;8:13–22. doi: 10.1093/intimm/8.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Hoft DF, Schnapp AR, Eickhoff CS, Roodman ST. Involvement of CD4(+) Th1 cells in systemic immunity protective against primary and secondary challenges with Trypanosoma cruzi. Infection and immunity. 2000;68:197–204. doi: 10.1128/iai.68.1.197-204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mota I, Umekita LF. The effect of C3 depletion on the clearance of Trypanosoma cruzi induced by IgG antibodies. Immunology letters. 1989;21:223–225. doi: 10.1016/0165-2478(89)90108-9. [DOI] [PubMed] [Google Scholar]
- 12.Brodskyn CI, Silva AM, Takehara HA, Mota I. IgG subclasses responsible for immune clearance in mice infected with Trypanosoma cruzi. Immunology and cell biology. 1989;67(Pt 6):343–348. doi: 10.1038/icb.1989.50. [DOI] [PubMed] [Google Scholar]
- 13.Spinella S, Liegeard P, Hontebeyrie-Joskowicz M. Trypanosoma cruzi: predominance of IgG2a in nonspecific humoral response during experimental Chagas' disease. Experimental parasitology. 1992;74:46–56. doi: 10.1016/0014-4894(92)90138-z. [DOI] [PubMed] [Google Scholar]
- 14.Scott MT, Moyes L. 75Se-methionine labelled Trypanosoma cruzi blood trypomastigotes: opsonization by chronic infection serum facilitates killing in spleen and liver. Clinical and experimental immunology. 1982;48:754–757. [PMC free article] [PubMed] [Google Scholar]
- 15.Lima-Martins MV, Sanchez GA, Krettli AU, Brener Z. Antibody-dependent cell cytotoxicity against Trypanosoma cruzi is only mediated by protective antibodies. Parasite immunology. 1985;7:367–376. doi: 10.1111/j.1365-3024.1985.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Tarleton RL. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite immunology. 1998;20:207–216. doi: 10.1046/j.1365-3024.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 17.Hoft DF, Farrar PL, Kratz-Owens K, Shaffer D. Gastric invasion by Trypanosoma cruzi and induction of protective mucosal immune responses. Infection and immunity. 1996;64:3800–3810. doi: 10.1128/iai.64.9.3800-3810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giddings OK, Eickhoff CS, Smith TJ, Bryant LA, Hoft DF. Anatomical route of invasion and protective mucosal immunity in Trypanosoma cruzi conjunctival infection. Infection and immunity. 2006;74:5549–5560. doi: 10.1128/IAI.00319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoft DF, Eickhoff CS. Type 1 immunity provides both optimal mucosal and systemic protection against a mucosally invasive, intracellular pathogen. Infection and immunity. 2005;73:4934–4940. doi: 10.1128/IAI.73.8.4934-4940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eickhoff CS, Eckmann L, Hoft DF. Differential interleukin-8 and nitric oxide production in epithelial cells induced by mucosally invasive and noninvasive Trypanosoma cruzi trypomastigotes. Infection and immunity. 2003;71:5394–5397. doi: 10.1128/IAI.71.9.5394-5397.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camargo MM, I, Almeida C, Pereira ME, Ferguson MA, Travassos LR, Gazzinelli RT. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 22.Williams MB, Rose JR, Rott LS, Franco MA, Greenberg HB, Butcher EC. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, alpha4beta7. J Immunol. 1998;161:4227–4235. [PubMed] [Google Scholar]
- 23.Rose JR, Williams MB, Rott LS, Butcher EC, Greenberg HB. Expression of the mucosal homing receptor alpha4beta7 correlates with the ability of CD8+ memory T cells to clear rotavirus infection. Journal of virology. 1998;72:726–730. doi: 10.1128/jvi.72.1.726-730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan NL, Eickhoff CS, Zhang X, Giddings OK, Lane TE, Hoft DF. Importance of the CCR5-CCL5 axis for mucosal Trypanosoma cruzi protection and B cell activation. J Immunol. 2011;187:1358–1368. doi: 10.4049/jimmunol.1100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoft DF, Eickhoff CS, Giddings OK, Vasconcelos JR, Rodrigues MM. Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic Trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. J Immunol. 2007;179:6889–6900. doi: 10.4049/jimmunol.179.10.6889. [DOI] [PubMed] [Google Scholar]
- 26.Hoft DF, Eickhoff CS, Giddings OK, Vasconcelos JR, Rodrigues MM. Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. J Immunol. 2007;179:6889–6900. doi: 10.4049/jimmunol.179.10.6889. [DOI] [PubMed] [Google Scholar]
- 27.Bustamante JM, Bixby LM, Tarleton RL. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nature medicine. 2008;14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pereira-Chioccola VL, Costa F, Ribeirao M, Soares IS, Arena F, Schenkman S, Rodrigues MM. Comparison of antibody and protective immune responses against Trypanosoma cruzi infection elicited by immunization with a parasite antigen delivered as naked DNA or recombinant protein. Parasite immunology. 1999;21:103–110. doi: 10.1046/j.1365-3024.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 29.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, Mohrs K, Mohrs M, Randall T, Lund FE. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwell NM, Else KJ. B cells and antibodies are required for resistance to the parasitic gastrointestinal nematode Trichuris muris. Infection and immunity. 2001;69:3860–3868. doi: 10.1128/IAI.69.6.3860-3868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanCott JL, McNeal MM, Flint J, Bailey SA, Choi AH, Ward RL. Role for T cell-independent B cell activity in the resolution of primary rotavirus infection in mice. European journal of immunology. 2001;31:3380–3387. doi: 10.1002/1521-4141(200111)31:11<3380::aid-immu3380>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Kuklin NA, Rott L, Feng N, Conner ME, Wagner N, Muller W, Greenberg HB. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J Immunol. 2001;166:1894–1902. doi: 10.4049/jimmunol.166.3.1894. [DOI] [PubMed] [Google Scholar]
- 33.Misumi I, Whitmire JK. B cell depletion curtails CD4+ T cell memory and reduces protection against disseminating virus infection. J Immunol. 2014;192:1597–1608. doi: 10.4049/jimmunol.1302661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. The Journal of experimental medicine. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. Journal of virology. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Current opinion in immunology. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asano MS, Ahmed R. CD8 T cell memory in B cell-deficient mice. The Journal of experimental medicine. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen H, Whitmire JK, Fan X, Shedlock DJ, Kaech SM, Ahmed R. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 40.Thomsen AR, Johansen J, Marker O, Christensen JP. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 41.Menard LC, Minns LA, Darche S, Mielcarz DW, Foureau DM, Roos D, Dzierszinski F, Kasper LH, Buzoni-Gatel D. B cells amplify IFN-gamma production by T cells via a TNF-alpha-mediated mechanism. J Immunol. 2007;179:4857–4866. doi: 10.4049/jimmunol.179.7.4857. [DOI] [PubMed] [Google Scholar]
- 42.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 43.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.