Abstract
Purification of small, native chromatin sections for proteomic identification of specifically bound proteins and histone posttranslational modifications is a powerful approach for studying mechanisms of chromosome metabolism. We detail a Chromatin Affinity Purification with Mass Spectrometry (ChAP-MS) approach for affinity purification of ~1 kb sections of chromatin for targeted proteomic analysis. This approach utilizes quantitative, high resolution mass spectrometry to categorize proteins and histone posttranslational modifications co-enriching with the given chromatin section as either “specific” to the targeted chromatin or “non-specific” contamination. In this way, the ChAP-MS approach can help define and re-define mechanisms of chromatin-templated activities.
Keywords: chromatin, purification, proteomics, epigenetics, histone, posttranslational modification
1. Introduction
Chromatin-associated proteins and epigenetic factors play key roles in the regulation of numerous chromosomal activities, such as gene transcription. The precise mechanisms by which these proteins function in the context of chromatin are largely unknown. One of the simplest questions one can ask is at what chromosomal location does a particular protein or histone posttranslational modification (PTM) exist. Most current methodologies address protein or histone PTM localization along chromosomes with chromatin immunoprecipitation (ChIP). ChIP involves using antibodies that recognize a specific protein or histone PTM to pull down the chromatin and then identifying the associating DNA (1–7). ChIP has several limitations, particularly that the researcher must know the protein/PTM of interest to target and also have a specific antibody for the protein/PTM. Therefore, ChIP is by nature a biased approach that requires target information. However, a biochemical approach that would provide for unbiased identification of proteins and histone PTMs at a given genomic locus could provide unprecedented insight into molecular mechanisms regulating chromosomal activities.
Here, we detail a method called Chromatin Affinity Purification with Mass Spectrometry (ChAP-MS) to help chromatin analysis proceed in an unbiased manner (8). ChAP-MS provides for the site-specific enrichment of a targeted ~1 kb section of a chromosome followed by unambiguous identification of associated proteins and histone PTMs using high resolution mass spectrometry. To validate this approach, we isolated a native genomic locus, a region 5′ to the GAL1 gene, from Saccharomyces cerevisiae and identified specifically bound proteins/PTMs in transcriptionally active and repressive conditions (8). The method uses homologous recombination to engineer a LexA DNA binding site at a desired chromosomal location and an ectopically expressed, PrA-tagged LexA fusion protein as the affinity handle for purification. Here we detail the methodology used to perform the ChAP-MS approach (8).
2. Materials
2.1. Cell Culture and Cryogenic Lysis
Synthetic complete media per liter: 6.7 g yeast nitrogen base without amino acids (Sigma Y0626), 2 g synthetic drop-out mix minus lysine and tryptophan (US Biological D9537-12), 900 mL dH2O. Media is sterilized by autoclaving. After cooling to room temperature, add 1 mL lysine (80 mg/mL in dH2O, sterile filtered) for isotopically light media, or add 1 mL of 13C615N2-lysine (80 mg/mL in dH2O, sterile filtered, Cambridge Isotope Laboratories CNLM-291-H-1) for isotopically heavy media. After adding light or heavy lysine, add 1 mL of ampicillin (100 mg/mL in 50% ethanol) and 100 mL of either autoclaved 20% glucose (w/v) or 30% galactose (w/v).
Glycine is a 2.5 M stock, autoclaved.
37% formaldehyde w/v (Fisher F79-500).
2.2 Affinity Purification
Dynabeads M270-epoxy (Invitrogen 143-02D) are suspended in N,N-dimethylformamide (Fisher D119) at 30 mg/mL and stored at 4°C.
Purified rabbit IgG (MP Biomedicals 55944) is re-suspended at 17 mg/mL in dH2O and stored at −80°C in 100 μL aliquots.
0.1 M sodium phosphate (pH 7.4) stock.
3 M ammonium sulfate stock
Solutions for washing IgG-coated Dynabeads: 100 mM glycine pH 2.5, 10 mM Tris pH 8.8, PBS, PBS + 0.5% TritonX-100.
Affinity purification buffer: 20 mM HEPES pH 7.5, 1 M NaCl, 1 M urea, 2 mM MgCl2, 0.1% Tween-20, 1/100 fungal protease inhibitor cocktail (Sigma P8215, stored at −20°C). Make buffer immediately prior to use and keep at 4°C.
Wash Buffer: 20 mM HEPES pH 7.5, 2 mM MgCl2, 10 mM NaCl, 0.1% Tween-20.
Fisher Tissuemiser (or any available probe tissue homogenizer that can operate at 30,000 RPMs).
Diagenode Bioruptor UCD-200.
Rocking platform.
0.5 N ammonium hydroxide/0.5 mM EDTA solution. Make immediately prior to use.
2x SDS-PAGE loading buffer: 125 mM Tris-HCl pH 6.8, 20% glycerol, 4.1% SDS, 0.05% bromophenol blue, 4% 2-mercaptoethanol. Store at −20°C in 0.5 mL aliquots.
3. Methods
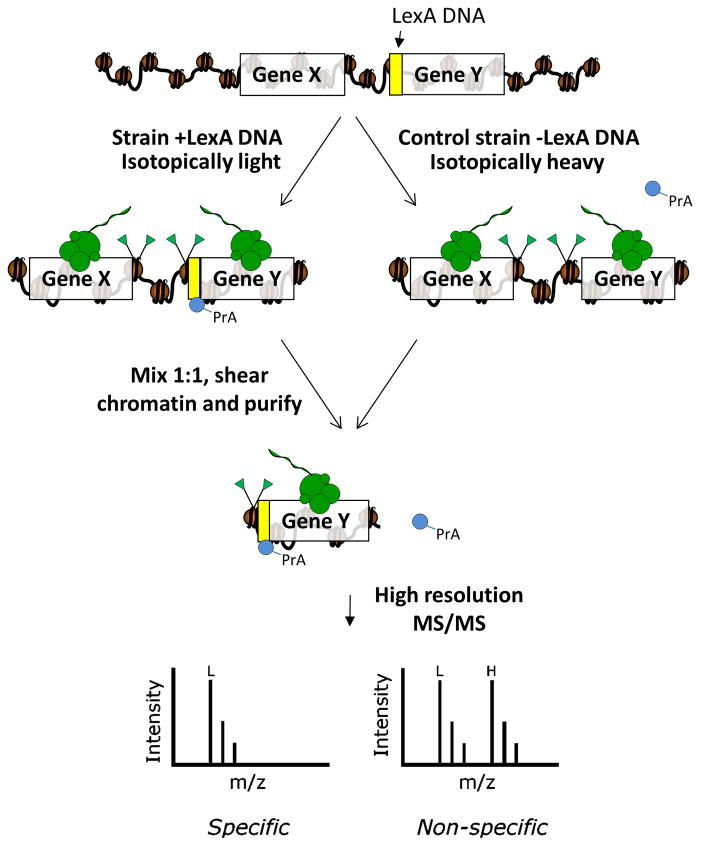
The methodological workflow for a ChAP-MS analysis is shown in Figure 1. The key components of this methodology are (1) a strain with an engineered LexA DNA binding site and an affinity-tagged LexA-PrA fusion protein expressed from a plasmid grown in isotopically light media and (2) a strain without the LexA DNA binding site also expressing LexA-PrA grown in isotopically heavy media. Both strains are grown in the absence of tryptophan to select for the pLexA-PrA (TRP1) plasmid. The two strains are grown to equivalent densities, subjected to in vivo chemical cross-linking with formaldehyde, and frozen independently. The cross-linking helps to stabilize the protein interactions and still allow for native chromatin purification (9). Frozen strains are mixed 1:1 prior to cryolysis with a ball mill maintained under liquid nitrogen temperature, which provides a method for generating cell lysate without thawing. The ability to differentiate “specific” and “nonspecific” protein interactions with the affinity-tagged chromatin occurs at the point of thawing the cell lysate and extends for the duration of the purification procedure. During the course of the purification, the specific protein interactions (which are exclusively isotopically light) with the affinity-tagged complex are maintained, while the nonspecific protein associations have a 1:1 likelihood to occur from either the light or heavy proteins. The readout for these stable and nonspecific protein interactions with the affinity-tagged chromatin section is high resolution mass spectrometry. When peptides are assigned to a given protein co-purifying with the affinity-tagged chromatin the type of protein interaction can be classified as stable if the peptides are ~100% isotopically light or nonspecific if the peptides are ~50% isotopically light. For simplicity, we detail below the overall approach used for the ChAP-MS analysis of chromatin at the 5′ end of the GAL1 gene in S. cerevisiae in transcriptionally active (+galactose) and inactive (+glucose) chromatin states (8).
Figure 1. Schematic representation of the ChAP-MS methodology.
For the targeted purification of Gene Y for proteomic analysis, a LexA DNA binding site can be inserted by homologous recombination near the target gene. A wild type strain without the LexA DNA binding site serves as a control. Each strain contains an ectopically expressed PrA-tagged LexA fusion protein. The strain with the LexA DNA binding site is grown in isotopically light media, while the control strain is cultured in isotopically heavy media to label proteins. Cross-linked cells from each growth are mixed, chromatin is sheared by sonication to ~ 1 kb in length, and PrA-tagged LexA is collected on IgG-coated beads. Proteins and histones specifically enriched with the targeted Gene Y will be isotopically light as they arose only from the light culture. Proteins that are non-specifically enriched with the chromatin during purification will be a 1:1 mix of isotopically light and heavy. High resolution mass spectrometry is used to determine the level of isotopically light and heavy proteins, thus categorizing the enriched proteins and posttranslationally modified histones as “specific” or contaminant.
3.1. Engineering the S. cerevisiae LEXA::GAL1 pLexA-PrA Strain
The LEXA::GAL1 pLexA-PrA strain was designed to have a LexA DNA binding site upstream of the GAL1 start codon in the S. cerevisiae W303a background. First, the GAL1 gene was genomically replaced with URA3 using homologous recombination. Separately the GAL1 gene (+50 base pairs up- and downstream) was PCR amplified from genomic DNA with primers that incorporated a LexA DNA binding site (5′-CACTTGATACTGTATGAGCATACAGTATAATTGC) immediately upstream of the GAL1 start codon. The LEXA::GAL1 PCR product was transformed into the gal1::URA3 strain and selected for growth with 5-fluoroorotic acid, which is lethal in URA3 expressing cells. Positive transformants were sequenced to ensure homologous recombination of the cassette to create the LEXA::GAL1 strain.
A plasmid that constitutively expressed LexA-PrA fusion protein with TRP1 selection was created by amplification of the PrA sequence template pOM60 via PCR and subcloning into the Sac1/Sma1 ends of the expression plasmid pLexA-C. Transforming this plasmid into the LEXA::GAL1 strain gave rise to the LEXA::GAL1 pLexA-PrA strain.
A control strain was constructed by transforming the pLexA-PrA protein fusion plasmid into W303a S. cerevisiae.
3.2. Cell Culture and Cryogenic Lysis
S. cerevisiae LEXA::GAL1 pLexA-PrA and pLexA-PrA strains were grown in yeast synthetic media lacking tryptophan to mid-log phase (~3.0 × 107 cells/mL) at 30°C with shaking at 200 RPM. The LEXA::GAL1 pLexA-PrA strain was grown in isotopically light lysine, while the strain pLexA-PrA was cultured exclusively with isotopically heavy 13C615N2-lysine (9). Each strain was grown in 12 liters of media with either 2% glucose or 3% galactose to give ~5 × 1011 cells per growth condition. Large-scale growths are inoculated from 3 mL stationary phase cultures (in the respective synthetic media) with an estimated doubling time of 2.5 h.
Protein-protein interactions are partially trapped with in vivo chemical cross-linking. When the cultures reach ~3.0 × 107 cells/mL, remove the flasks from the incubator and add formaldehyde (37% stock concentration) to a final amount of 1.25% (see Note 1). Swirl the flask to mix and leave for 5 min at room temperature. Quench the cross-linking by adding 2.5 M glycine to a final concentration of 125 mM glycine. Swirl the flask to mix and leave for 5 min at room temperature.
Cells are collected by centrifugation (2,500 × g) at 4°C for 30 min in 1 L bottles, washed with 100 mL of ice cold dH2O, and re-collected by centrifugation. Add 20 mM HEPES (pH 7.5)/1.2% polyvinylpyrrolidone to the wet cell pellet (1 mL buffer/10 g of wet cells) and mix by pipetting.
Cells are frozen as a suspension in liquid nitrogen. Use scissors to cut ~1 cm off the end of a 1 mL pipette tip – increasing the size of the opening will provide for easier pipetting. Next, fill a 50 mL polypropylene conical tube with liquid nitrogen. Slowly pipette the cell suspension drop-wise into the liquid nitrogen. Liquid nitrogen should be added at intervals during the freezing procedure to keep the tube full. Once the cells are frozen as pellets, pour off the excess liquid nitrogen. Poke three holes in the cap of the conical tube with a needle and place it on the tube. The frozen cells are now stored at −80°C.
For cryolysis, the isotopically light LEXA::GAL1 pLexA-PrA and isotopically heavy pLexA-PrA cells are mixed 1:1 by cell weight. Cells are kept at liquid nitrogen temperature as much as possible during this process to avoid thawing. Weigh each set of pellets, mix 1:1 by weight, and add to a 50 mL polypropylene conical tube. Shake this tube thoroughly to ensure mixing of the pellets.
Cryogenic lysis is performed with a Retsch MM301 mixer mill using stainless steel cylinders (Retsch 25 mL screw top grinding jars) and ball bearings (Retsch 20 mm stainless steel). Cell pellets and cell powder are kept at liquid nitrogen temperature as much as possible during this process to avoid thawing. Stainless steel cylinders and ball bearings are precooled in liquid nitrogen (the cylinders and ball bearings are cooled once the nitrogen stops “boiling”). Tongs should be used to retrieve the cylinders from the liquid nitrogen. Approximately 3 g of mixed cell pellets are added to a cylinder with a ball bearing and then placed into liquid nitrogen. Once the cylinder with ball bearing and yeast pellets is cooled, it is attached to the mixer mill, processed for 3 min at 30 Hz, and then returned to the liquid nitrogen. The cycle of cryolysing is repeated five times. Following the final cycle, the cylinder is opened and the cell powder is scooped out into a 50 mL polypropylene conical tube that is in a bath of liquid nitrogen (no liquid nitrogen in the tube). The tube is sealed with a cap containing three holes made with a needle and placed at −80°C for storage.
Cryogenic lysis with a mixer mill is the preferred method for lysing and blending the cells. One should avoid methods, such as lysis with glass beads as the samples will thaw during the procedure, which precludes uniform blending of the samples prior to thawing. If a mixer mill is not readily available, a reasonable alternative for cryogenic lysis is manual grinding of the cells in the presence of liquid nitrogen with a mortar and pestle. When manually grinding, the cells should be covered with liquid nitrogen during the lysis process. The cells should be ground into a fine powder. Grinding should continue until >75% lysis is visually observed with a light microscope. After the cells are ground, the cells are stored at −80°C as described above immediately after allowing the liquid nitrogen to evaporate.
3.3 Affinity Purification
-
For affinity purification via the PrA-tag on LexA, 80 mg of M270-epoxy Dynabeads are coated with 6 mg of rabbit IgG (see Note 2). The following mixture is incubated overnight at 30°C with rocking: 80 mg of M270-epoxy Dynabeads, 6 mg of rabbit IgG, 1 M ammonium sulfate, 60 mM sodium phosphate (pH 7.4).
Following the overnight coupling, the beads are collected with a magnet and washed with: 1 mL of 100 mM glycine pH 2.5, 1 mL of 10 mM Tris pH 8.8, 4 times with 1 mL of PBS, 1 mL of PBS/0.5% TritonX-100, 1 mL of PBS. All washes are done quickly except for the PBS/tritionX-100 wash that should be done for 15 min. For each chromatin purification, the beads should be freshly coupled.
For a typical purification, we use 10 g of cryogenically lysed cell powder. All steps are performed at 4°C. To 10 g of cell powder containing isotopically light LEXA::GAL1 pLexA-PrA and isotopically heavy pLexA-PrA cell lysates, add 50 mL of affinity purification buffer (see Note 3). The cell lysate is suspended by gentle inversion. Cell lysate is thoroughly blended with a handheld Fisher Tissuemiser (or any available probe tissue homogenizer) for 20 s at 30,000 RPMs. Chromatin is sheared with a Diagenode Bioruptor UCD-200 using the low setting and 30 seconds on/off cycle for 12 min total time in 20 mL aliquots to yield ~1 kb chromatin fragments. The number of cycles and duration of sonication will vary between sonicators, thus parameters for sonication of chromatin to ~1000 bp should be empirically determined. The supernatant is collected by centrifugation at 2,500 × g for 10 min. The 80 mg of IgG-coated Dynabeads are added for ~4 h with gentle inversion on a rocking platform (see Note 4). Beads are collected with a magnet and washed five times with 1 mL of affinity purification buffer (without protease inhibitors) and three times with wash buffer. Proteins are eluted from the beads with 0.5 mL of 0.5 N ammonium hydroxide/0.5 mM EDTA for 5 min at room temperature and the eluant is lyophilized with a Savant SpeedVac Concentrator. This elution procedure minimizes the amount of heavy and light chain antibody that is released from the resin. The lyophilized proteins are re-suspended in 20 μL SDS-PAGE loading buffer and heated to 95°C for 20 min.
Proteins collected from the affinity purification are resolved by 4–20% SDS-PAGE. In our laboratory, SDS-PAGE is performed with the pre-cast Invitrogen Tris–glycine gel system, and the gel is stained with the Thermo GelCode Blue Stain (a colloidal Coomassie stain) prior to imaging for documentation. A colloidal Coomassie stain is recommended, as this stain is sensitive for imaging purposes and easily removed during processing for mass spectrometry. All handling of the gel should be done with powder-free gloves and clean labware. Cleaning items with a commercial glass cleaner such as Windex is good for minimizing keratin contamination, which is the major source of contamination during processing. The gel lane is sliced into 2 mm bands in preparation for mass spectrometry. The easiest way to slice the gel is to place it on a clean glass plate with a ruler underneath and use a clean razor to excise 2 mm sections. The excised gel bands are placed into 1.7 mL microcentrifuge tubes and stored at −20°C.
Gel bands are submitted to a proteomics facility for protein identification and high resolution analysis of tryptic peptides (see Note 5). The proteomics facility performs the following: destaining of the gel bands, in-gel trypsin digestion and tandem mass spectrometric identification of the tryptic peptides. A high resolution mass analyzer should be used to collect the mass spectra. Tandem mass spectra do not necessarily need to be collected with a high resolution mass analyzer. Proteins and posttranslationally modified histones will be identified with database searching of the tandem mass spectrometric data. Peak areas of peptides corresponding to the assigned proteins will be extracted from the mass spectra. Note that peak areas only need to be extracted for peptides that will contain the heavy amino acid(s). The percent isotopically light peptide is calculated: (light area/(heavy area + light area)) × 100. Percent light peptide values are averaged together for a given protein and the standard deviation is calculated. A typical representation of this data is a bar graph with percent light on the y-axis and the given protein on the x-axis (8). Specific protein interactions are seen as ~100% isotopically light. Nonspecific protein associations are seen as ~50% isotopically light.
After identification of stable and nonspecific interactions, the investigator must select an appropriate system to explore the functional significance of the identified associating proteins. Systems for knocking out or knocking down a particular protein provide a good method for studying the significance of the protein interaction. qPCR-ChIP assays can also be performed to validate the proteomic findings (8).
Acknowledgments
We would like to acknowledge mass spectrometric support from the UAMS Proteomics Facility and support from NIH grants R01GM106024, R33CA173264, UL1RR029884, P30GM103450, and P20GM103429.
Footnotes
Several concentrations of formaldehyde cross-linking were tested to determine the amount needed to both trap protein-protein interactions and allow the chromatin to be soluble (10).
4 mg of coupled Dynabeads are used for each gram of cell powder.
The affinity purification buffer described in this section is an empirically determined buffer that provides good yields for chromatin associated protein complexes. The components of the buffer can be varied in accordance to the protein complex under study. Some of the components typically varied include NaCl and TritonX-100.
The time of incubation with coated Dynabeads is empirically determined. Shorter affinity isolation times (e.g., 1–4 h) can be explored and may result in fewer nonspecific interactions.
The mass spectrometric processing and data analysis are standard procedures for a proteomics facility. Proteomics facilities are available at many universities and are also available commercially.
References
- 1.Dedon PC, Soults JA, Allis CD, Gorovsky MA. Formaldehyde cross-linking and immunoprecipitation demonstrate developmental changes in H1 association with transcriptionally active genes. Mol Cell Biol. 1991;11:1729–33. doi: 10.1128/mcb.11.3.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren B, Robert F, Wyrick JJ, Aparicio O, Jennings EG, Simon I, Zeitlinger J, Schreiber J, Hannett N, Kanin E, Volkert TL, Wilson CJ, Bell SP, Yound RA. Genome-wide location and function of DNA binding proteins. Science. 2000;290:2306–9. doi: 10.1126/science.290.5500.2306. [DOI] [PubMed] [Google Scholar]
- 3.Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, Bell GW, Walker K, Rolfe PA, Herbolsheimer E, Zeitlinger J, Lewitter F, Gifford DK, Young RA. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Robertson G, Hirst M, Bainbridge M, Bilenky M, Zhao Y, Zeng T, Euskirchen G, Bernier B, Varhol R, Delaney A, Thiessen N, Griffith OL, He A, Marra M, Snyder M, Jones S. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods. 2007;4:651–7. doi: 10.1038/nmeth1068. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 6.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–37. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–60. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrum S, Raman A, Taverna SD, Tackett AJ. ChAP-MS: A Method for Identification of Proteins and Histone Posttranslational Modifications at a Single Genomic Locus. Cell Reports. 2012;2:198–205. doi: 10.1016/j.celrep.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrum SD, Smart SK, Larson S, Tackett AJ. Analysis of Stable and Transient Protein-Protein Interactions. Methods in Mol Biol. 2012;833:143–52. doi: 10.1007/978-1-61779-477-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrum SD, Taverna SD, Tackett AJ. Quantitative analysis of histone exchange for transcriptionally active chromatin. J Clin Bioinforma. 2011;1:17. doi: 10.1186/2043-9113-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]