Abstract
Background.
Urinary tract infections (UTIs) are associated with significant morbidity and high frequency of antibiotic prescription. Diagnosing UTI is often difficult, particularly in the critically ill patient and in patients with unspecific and mild symptoms. The standard rapid tests have limited value, and there is a need for more reliable diagnostic tools. Heparin-binding protein (HBP) is released from neutrophils and has previously been studied as a diagnostic and predictive biomarker in different bacterial infections.
Methods.
This prospective survey enrolled adult patients at 2 primary care units and 2 hospital emergency departments, to investigate in urine HBP as a biomarker of UTI. In addition, urine levels of interleukin-6, white blood cells, and nitrite were analyzed and compared with HBP. Based on symptoms of UTI and microbiological findings, patients were classified into different groups, UTI (cystitis and pyelonephritis) and no UTI.
Results.
Three hundred ninety patients were evaluated. The prevalence of UTI in the study group was 45.4%. The sensitivity and specificity for HBP in urine as a marker for UTI were 89.2% and 89.8%, respectively. The positive and negative predictive values were 90.2% and 88.8%, respectively. Heparin-binding protein was the best diagnostic marker for UTI, with an area-under-curve value of 0.94 (95% confidence interval, 0.93–0.96). Heparin-binding protein was significantly better in distinguishing cystitis from pyelonephritis, compared with the other markers.
Conclusions.
An elevated level of HBP in the urine is associated with UTI and may be a useful diagnostic marker in adult patients with a suspected UTI.
Keywords: HBP, heparin-binding protein, urinary tract infection
Urinary tract infection (UTI) is one of the most common infections leading to hospitalization in the elderly population, and it accounts for nearly half of the antibiotic prescription in the primary care (PC) in Sweden [1]. The symptoms are sometimes nonspecific, and, in certain groups, such as the critically ill patient and those with cognitive impairments, they can be difficult to interpret. The diagnosis of UTI is usually based on the presentation of clinical symptoms combined with the result of the rapid dipstick test, which indicates presence of bacteria in the urine with the nitrite test and also measures semiquantitative levels of white blood cells (U-WBC). However, it has been shown that preliminary diagnoses have a relatively high error rate compared with the gold standard, the urine culture [2–7]. This fact often leads to excessive use of antibiotics with a risk of adverse reactions and negative ecological consequences [8, 9].
To improve diagnosis, alternative biomarkers have been evaluated. As a response to infection, leukocytes and epithelial cells in the urinary tract produce interleukin (IL)-6. It has been shown that measurement of IL-6 has a diagnostic value with an ability to discriminate between cystitis and pyelonephritis [10–12]. However, the clinical use of this marker is limited.
Heparin-binding protein (HBP) is a protein of 37 kDa stored in secretory and azurophilic granules of human neutrophils [13]. When released from activated neutrophils, this multifunctional inflammatory mediator [14] induces vascular leakage and acts as a (1) chemoattractant and (2) activator of monocytes [13]. In addition, HBP has a broad antimicrobial activity and may also contribute to bacterial clearance by direct opsonization [15].
Heparin-binding protein has been evaluated in clinical trials as a biomarker for different bacterial infections. Increased values of HBP in plasma, cerebrospinal fluid, and skin biopsies have been associated with severe sepsis, bacterial meningitis, and streptococcal skin infection, respectively [16–19]. Recently, a study measuring urine-HBP (U-HBP) in a pediatric population demonstrated that increased levels were associated with UTI. One of the most interesting results was that U-HBP had a higher specificity and sensitivity for UTI compared with U-WBC [20].
In the present study, U-HBP in adults were evaluated as a marker of UTI and compared with urine levels of IL-6 (U-IL-6) and the dipstick test in 2 different healthcare settings: the PC and the hospital emergency department (ED).
METHODS
Study Population
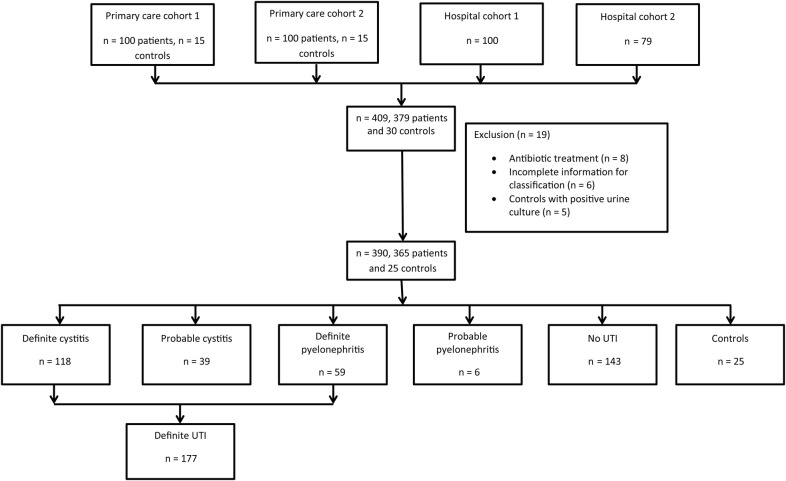
Urine samples were collected from 409 adult (≥18 years old) subjects (379 patients and 30 controls) from 4 different study cohorts, as illustrated in Figure 1. Nineteen patients were excluded from the analysis: 8 due to antibiotic treatment at inclusion, 6 with incomplete data for classification, and 5 controls due to positive urine culture. A final total of 390 patients were enrolled. Of these, 194 patients were enrolled at 2 different PC offices in Höganäs, Sweden between January and August 2012. The inclusion criteria in the PC cohorts were the suspicion of UTI based on symptoms such as: dysuria, frequency, urgency, suprapubic pain, haematuria, and/or flank pain. One hundred seventy-nine patients were enrolled at 2 different hospital EDs. At the Hospital of Helsingborg, Sweden, 100 patients were enrolled between January and August 2012, and at the Clinic for Infectious Diseases, Skåne University Hospital in Lund, Sweden, 79 patients were included between August 2010 and March 2012. The patients enrolled at hospital EDs were included if UTI was considered a possible diagnosis after a clinical evaluation by the attending physician. Finally, a control group, 25 patients without any suspicion of an infection, was included at the PC offices. The control subjects were matched for age and gender to the patients included at the PC. None of the control subjects had an underlying urogenital disease, signs of UTI symptoms during the last 3 months, or bacterial growth in the urine culture. The ethics committee of Skåne University Hospital approved the study. Informed consent was obtained from all patients.
Figure 1.
Flowchart describing the included and excluded patients in the primary care cohort and hospital cohort.
Patient Characteristics
Individual clinical data were registered for the patients. The final diagnoses were determined on the basis of complete patient charts and laboratory tests. Based on clinical symptoms of UTI, bacterial species, and bacterial concentrations in the urine culture, patients were classified into 3 groups (definite UTI, probable UTI, and no UTI). The criteria for UTI followed the guidelines from the European Confederation of Laboratory Medicine [21], with the exception of bacterial concentrations, where higher cutoff limits were used in the present study. Patients classified with definite UTI presented typical clinical symptoms and growth of a primary pathogen (Escherichia coli or Staphylococcus saprophyticus) of ≥104 colony-forming units (CFU)/mL or growth of a secondary pathogen of ≥105 CFU/mL in the urine culture from a midstream or indwelling catheter sample. Patients classified into the group of probable UTI also had typical clinical symptoms for UTI. However, they had less growth of bacteria in the urine (primary pathogens <104 CFU/mL, secondary pathogens <105 CFU/mL) or, in the absence of a positive urine culture, a positive nitrite test and/or elevated numbers of leukocytes in the urine. Patients with a final diagnosis other than UTI were classified into the no UTI group. In addition, patients with UTI were divided into 2 diagnostic groups of either cystitis or pyelonephritis. Pyelonephritis patients had typical UTI symptoms in addition to a body temperature registered at >37.5°C or a history of acute onset of fever and/or chills and/or elevated plasma C-reactive protein (P-CRP) related to UTI.
One-hundred sixty-four of the 179 patients (92%) in the hospital group had a serum creatinine (S-Cr) value measured and registered at enrollment, and the estimated glomerular filtration rates (eGFRs) were calculated [22]. The patients were divided into 3 different groups based on levels of eGFR, according to functional criteria from International Society of Nephrology [23]—(1) no kidney disease (NKD): GFR ≥60 mL/min per 1.73 m2, stable S-Cr; (2) acute kidney disease (AKD): increase in S-Cr by 50% within 7 days, or increase in S-Cr by 26.5 µmol/L within 2 days, or oliguria, or GFR <60 mL/min per 1.73 m2 for <3 months, or decrease in GFR by ≥35%, or increase in S-Cr by >50% for <3 months; and (3) chronic kidney disease (CKD): GFR <60 mL/min per 1.73 m2 for >3 months.
Urine and Blood Samples
Urine samples were collected in 10 mL plastic tubes (Sarstedt) at the time of inclusion. Tubes were centrifuged within 1 hour of sampling at 3000 rpm for 10 minutes, and separate aliquots of the supernatant were stored at −70°C until analysis. Noncentrifuged urine was subjected to analysis with a dipstick test (Combur 7 Test, Cobas, Roche), and bacterial culturing was performed at the Clinical Microbiological Laboratory, Skåne University Hospital. Four of the patients admitted for in-hospital antibiotic treatment were followed with daily urine samples during 4 consecutive days after enrollment.
Laboratory Analysis
The concentration of U-HBP was analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described [24, 25]. Urine samples were diluted 1:40. The level of U-HBP in each patient sample was determined by calculating the mean optical densities of the duplicates, which were correlated to the results from the standard curve. Levels of U-IL-6 were analyzed with a sandwich ELISA, according to the manufacturer's description (ELISA MAX, Deluxe Sets, Biolegend). Urine samples were diluted 1:5 and analyzed in duplicates. At this dilution, measurements of IL-6 concentrations below 1 pg/mL were not considered reliable. Thus, all obtained values below this level were estimated to 1 pg/mL. Standard analyses of CRP and creatinine were performed at the clinical chemistry laboratories, in Helsingborg and Lund, according to the manufacturer's instructions.
Statistical Analysis
Median, range, and interquartile range (IQR) were reported when appropriate. Comparisons between groups were made using the nonparametric tests: Mann-Whitney U test, χ2 test, and Fisher's exact test. The level of statistical significance was defined as a 2-tailed P value of 0.05. Correlations between variables were assessed using the Spearman's rank coefficient. Receiver operating characteristic curves were constructed, and areas under curves (AUC) were calculated to illustrate the diagnostic power of U-HBP, U-IL-6, U-WBC, and U-nitrite. Sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) were calculated. The SPSS software system, version 20.0, was used for statistical calculations. Figures were made using GraphPad Prism 6 software.
RESULTS
Patient Characteristics
Three hundred sixty-five patients presenting with a clinical suspicion of UTI were included in the study. Of the 177 (48.5%) patients classified as having a definite UTI, 118 (66.7%) had cystitis and 59 (33.3%) had pyelonephritis. In addition, 45 (12.3%) had a probable UTI, and of these patients, 39 (86.7%) were classified as cystitis and 6 (13.3%) as pyelonephritis. One hundred and ninety-four (53.2%) of the study patients were included in the PC offices. In this group, a larger proportion of patients had definite UTI (60.3%), and 89.7% of these were diagnosed with cystitis. In the cohort recruited at the 2 hospital EDs, 35.1% had definite UTI and 78.3% of these had pyelonephritis. In addition to the enrolled patients with a tentative UTI diagnosis, 25 control subjects visiting the PC offices for reasons other than infection were included. Women were the overrepresented gender (90% at the PC group and 50.9% at the hospital group), and the median age was 61 years old for the entire study group. Patient demographics are presented separately for the PC and hospital cohort in Table 1 and Table 2. All patients with definite UTI had significant growth of a single bacterial species in the urine culture obtained at enrollment. Escherichia coli (n = 138) was the most prevalent pathogen in this group, followed by Klebsiella pneumoniae (n = 9) and Proteus mirabilis (n = 7).
Table 1.
Patient Characteristics and Laboratory Data in Primary Care Cohort (n = 219)
| Variables | Definite Cystitis (n = 105) | Definite Pyelonephritis (n = 12) | Probable Cystitis (n = 29) | Probable Pyelonephritis (n = 1) | No UTI (n = 47) | Controls (n = 25) | a | b | c |
|---|---|---|---|---|---|---|---|---|---|
| Gender (female), n (%) | 99 (94.3) | 12 (100) | 28 (96.6) | 1 (100) | 36 (76.6) | 21 (84) | NS | <.01 | NS |
| Age (years), (range) | 62 (19–93) | 45 (21–88) | 57 (18–88) | 71 | 57 (19–88) | 56 (46–70) | NS | <.01 | <.01 |
| U-HBP (ng/mL) | 121 (51–253) | 261 (170–606) | 85 (34–175) | 386 | 5 (3–12) | 7 (4–19) | .01 | <.01 | <.01 |
| U-IL-6 (pg/mL) | 17 (2–198) | 95 (6–649) | 9 (1–77) | 1008 | 1 (1–1) | 1 (1–1) | NS | <.01 | <.01 |
| U-WBC (WBC/µL)d | 3 (2–3) | 3 (3–3) | 3 (2–3) | 3 | 0 (0–0) | 0 (0–1.5) | NS | <.01 | <.01 |
| U-Nitrite (negative/positive)e | 0 (0–1) | 0 (0–1) | 0 (0–.0) | 0 | 0 (0–0) | 0 (0–0) | NS | <.01 | .01 |
| Urine culture positive, n (%)f | 105 (100) | 12 (100) | 11 (37.9) | 1 (100) | 10 (21.3) | 0 | |||
| Etiological agent, n (%) | |||||||||
| Escherichia coli | 83 (79) | 12 (100) | 8 (72.7) | 1 (100) | 6 (60.0) | ||||
| Proteus mirabilis | 4 (3.8) | ||||||||
| Klebsiella pneumoniae | 3 (2.9) | ||||||||
| Other gram-negative bacteria | 3 (2.9) | ||||||||
| Gram-positive bacteria | 12 (11.4) | 2 (18.2) | 2 (20) | ||||||
| Mixed culture | 1 (9.1) | 2 (20) |
Data are presented as median and interquartile range, unless otherwise stated. A value of P <.05 was considered significant.
Abbreviations: HBP, heparin-binding protein; IL, interleukin; NS, nonsignificant; U-WBC, urinary white blood cells.
a P values between (definite) cystitis and (definite) pyelonephritis.
b P values between (definite) cystitis and no UTI.
c P values between (definite) pyelonephritis and no UTI.
d Semiquantitative scale: 1 = 10–25 leukocytes/µL urine; 2 = approximately 75 leukocytes/µL urine; 3 = approximately 500 leukocytes/µL urine; 4 ≥ 500 leukocytes/µL urine.
e Positive nitrite was defined as 1, and negative nitrite as 0.
f Definition of positive culture is described under method section.
Table 2.
Patient Characteristics and Laboratory Data in the Hospital Cohort (n = 171)
| Variables | Definite Cystitis (n = 13) | Definite Pyelonephritis (n = 47) | Probable Cystitis (n = 10) | Probable Pyelonephritis (n = 5) | No UTI (n = 96) | a | b | c |
|---|---|---|---|---|---|---|---|---|
| Gender (female), n (%) | 11 (84.6) | 21 (44.7) | 7 (70) | 3 (60) | 45 (46.9) | <.01 | .01 | NS |
| Age (years), (range) | 55 (19–94) | 65 (19–93) | 45 (18–91) | 66 (24–79) | 65 (19–93) | NS | NS | NS |
| SIRS | 0.5 (0–1) | 2 (1–3) | 0.5 (0–1) | 2 (1.5–3) | 1 (1–3) | <.01 | NS | <.01 |
| Temperature (°C) | 36.6 (36.3–37.2) | 37.6 (37.2–38.7) | 36.9 (36.4–37.2) | 37.7 (36.8–38.4) | 37.5 (37.1–38.5) | <.01 | <.01 | NS |
| U-HBP (ng/mL) | 185 (27–399) | 223 (74–412) | 50 (22–204) | 78 (41–719) | 6 (4–13) | NS | <.01 | <.01 |
| U-IL-6 (pg/mL) | 1 (1–359) | 217 (7–719) | 107 (1–666) | 125 (48–1722) | 1 (1–10) | NS | NS | <.01 |
| U-WBC (WBC/µL)d | 3 (2–3) | 3 (3–3) | 3 (2–3) | 3 (2.5–3) | 0 (0–2) | NS | <.01 | <.01 |
| U-Nitrite (negative/positive)e | 1 (0–1) | 1 (0–1) | 0 (0–0.3) | 1 (0–1) | 0 (0–0) | NS | <.01 | <.01 |
| P-CRP (ng/mL) | 5 (3–24) | 110 (59–213) | 5 (4–7) | 108 (41–217) | 58 (7–117) | <.01 | <.01 | <.01 |
| S-Creatinine (µmol/L) | 80 (72–102) | 87 (73–116) | 81 (67–100) | 86 (63–137) | 79 (66–99) | NS | NS | .04 |
| Urine culture positive,f n (%) | 13 (100) | 47 (100) | 2 (20) | 2 (40) | 23 (24) | |||
| Etiological agent n (%) | ||||||||
| Escherichia coli | 11 (84.6) | 33 (70.2) | 2 (100) | 2 (100) | 5 (21.7) | |||
| Klebsiella pneumoniae | 2 (15.4) | 4 (8.5) | 1 (4.4) | |||||
| Proteus mirabilis | 5 (10.6) | 1 (4.4) | ||||||
| Mixed culture | 11 (47.8) | |||||||
| Other gram-negative bacteria | 5 (10.6) | 1 (4.4) | ||||||
| Gram-postive bacteria | 4 (17.4) |
Data are presented as median and interquartile range, unless otherwise stated. A value of P < .05 was considered significant.
Abbreviations: HBP, heparin-binding protein; IL, interleukin; NS, not significant; SIRS, systemic inflammatory response syndrome; U-WBC, urinary white blood cells.
aP values between (definite) cystitis and (definite) pyelonephritis.
bP values between (definite) cystitis and no UTI.
cP values between (definite) pyelonephritis and no UTI.
d Semiquantitative scale, 1 = 10–25 leukocytes/µL urine; 2 = approximately 75 leukocytes/µL urine; 3 = approximately 500 leukocytes/µL urine; 4 ≥ 500 leukocytes/µL urine.
e Positive nitrite was defined as 1, and negative nitrite as 0.
f Definition of positive culture is described under method section.
Distribution of Biomarkers in Relation to the Final Diagnosis of UTI
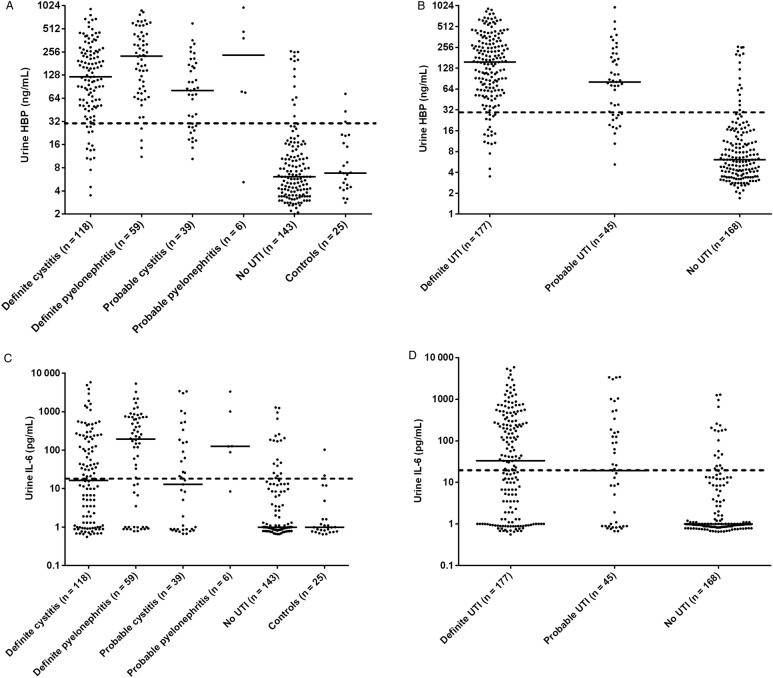
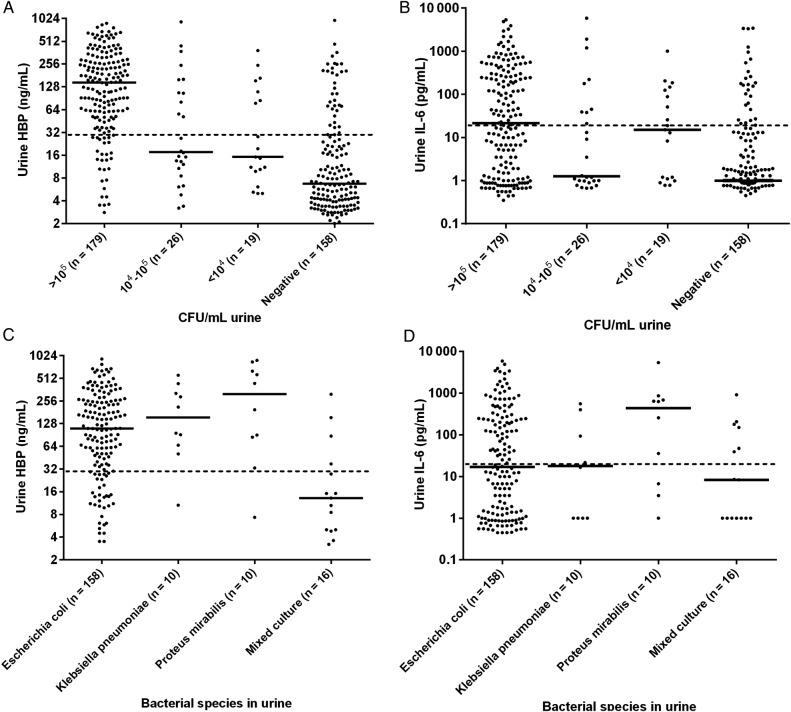
The levels of U-HBP were significantly higher (P < .01) in patients with definite UTI (median, 156 ng/mL; IQR, 61–313 ng/mL) compared to patients with no UTI (median, 6 ng/mL; IQR, 4–14 ng/mL) (Figure 2, A and B). There were no significant differences in biomarker levels between definite UTI and probable UTI. When a cut-off value of 30 ng/mL was used, the sensitivity and specificity for detection of definite UTI was 89.2% and 89.8%, respectively. On the basis of the prevalence of definite UTI of 48.5% in this study, the PPV was 90.2% and the NPV was 88.8% (Table 3). Urine levels of IL-6 were also significantly higher in the definite UTI group (median, 33 pg/mL; IQR, 1–297 pg/mL) compared to the no UTI group (median, 1 pg/mL; IQR, 1–4 pg/mL) (Figure 2, C and D). However, the sensitivity and the predictive values were lower than for HBP (Table 3). Analyses of dipstick tests showed that elevated levels of U-WBC and positive nitrite tests were more prevalent in patients with UTI. The sensitivity and specificity for U-WBC were 82.7% and 78%, respectively, when a cutoff level of 2 in a 4-graded scale (0–3) was used. In the group of patients with no UTI, 37 (25.8%) had a U-WBC over the proposed cutoff level (≥2). Among these, 22 patients (59.5%) had a low level of U-HBP (<30 ng/mL). The nitrite test had a sensitivity of 45.2% and a specificity of 89.3% for diagnosing definite UTI (Table 3). Receiver operating characteristics curve showed an AUC value of 0.94 for HBP in diagnosing definite UTI, which was significantly higher than for the other investigated parameters (Figure 3). Levels of HBP were also compared with the results of bacterial cultures. Patients with the highest concentration of bacteria in urine had significantly higher U-HBP levels compared with urine with lower concentration of bacteria. However, there were no differences between U-HBP levels among the different bacterial species found (Figure 4).
Figure 2.
Urine levels of heparin-binding protein (HBP) (A and B) and interleukin-6 (IL-6) (C and D) for the entire study population, n = 390 patients. The patient groups are described in the method section. Each dot represents the concentration in an individual of HBP and IL-6. Bars represent the median of the values. Dashed lines represent the proposed cutoff of 30 ng/mL for HBP and 30 pg/mL for IL-6.
Table 3.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value for U-HBP, U- IL-6, U-WBC, and Nitrite in Diagnosing Urinary Tract Infection
| Values Calculated for the Primary Care Cohort and Hospital Cohort Combined | |||||
|---|---|---|---|---|---|
| Cutoff Level | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| U-HBP | 30 ng/mL | 89.2 | 89.8 | 90.2 | 88.8 |
| U-IL-6 | 30 pg/mL | 52 | 92.9 | 88.5 | 64.7 |
| U-WBC | 2 | 82.7 | 78 | 73.9 | 85.6 |
| U-Nitrite | Positive | 45.2 | 89.3 | 81.6 | 60.7 |
| Values Calculated Separately for the Primary Care Cohort | |||||
| HBP | 30 ng/mL | 88.9 | 87.3 | 92 | 82.7 |
| U-IL-6 | 30 pg/mL | 46.2 | 98.6 | 98.1 | 53 |
| U-WBC | 2 | 85.5 | 83.3 | 89.3 | 77.9 |
| U-Nitrite | Positive | 39.3 | 93.1 | 90.2 | 48.6 |
| Values Calculated Separately for the Hospital Cohort | |||||
| HBP | 30 ng/mL | 89.8 | 91.7 | 86.9 | 93.6 |
| U-IL-6 | 30 pg/mL | 63.3 | 88.5 | 77.6 | 79.4 |
| U-WBC | 2 | 91.7 | 74 | 68.8 | 93.4 |
| U-Nitrite | Positive | 56.7 | 86.5 | 72.3 | 80.6 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value; U-HBP, heparin-binding protein in urine; U-IL-6, interleukin-6 in urine; U-WBC, urinary white blood cells.
Figure 3.
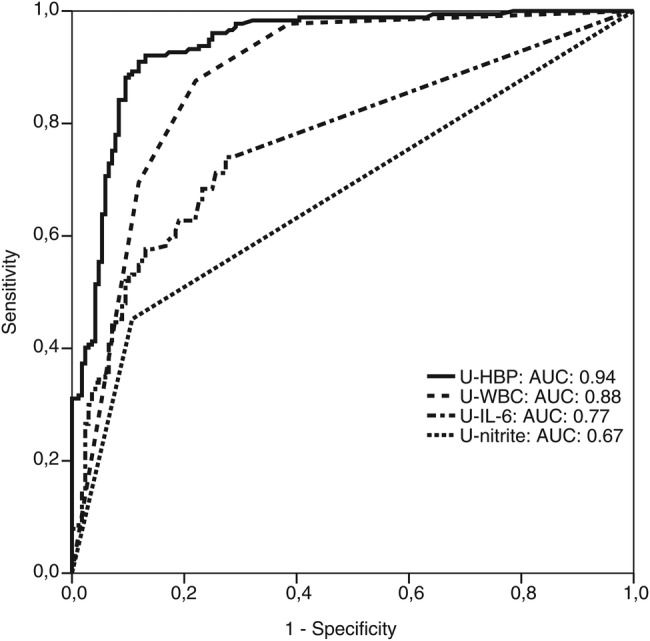
Receiver-operating characteristic curves of heparin-binding protein (HBP), interleukin-6 (IL-6), white blood cells (U-WBC), and nitrite in urine (U-nitrate) for patients with definite urinary tract infections (UTIs; n = 177) and no UTI (n = 168). Areas under the receiver-operating characteristics curves (AUC) were 0.94 (95% confidence interval [CI], 0.93–0.96) for HBP, 0.77 (95% CI, 0.72–0.82) for IL-6, 0.88 (95% CI, 0.84–0.92) for U-WBC, and 0.67 (95% CI, 0.62–0.73) for nitrite.
Figure 4.
Urine levels of heparin-binding protein (HBP) (A–C) and interleukin-6 (IL-6) (B–D) at different bacterial concentrations, and with the most prevalent bacterial species. Dashed lines represent the proposed cutoff of 30 ng/mL for HBP and 30 pg/mL for IL-6. CFU, colony-forming unit.
To get an indication of the clearance of U-HBP during infection, 4 patients with pyelonephritis were sampled consecutively over 4 days. Three of the patients had levels of U-HBP above cutoff (30 ng/mL) at inclusion. Urine-HBP decreased substantially in all 3 patients within 1 day of start of antibiotic treatment. The decrease preceded clinical improvement and a lowering of P-CRP (Figure 5). There was a significant correlation (Spearman's non-parametric test) between U-HBP and U-WBC (ρ = .79). The correlation between U-IL-6 and U-WBC was also significant (ρ = .48), as was the correlation between U-IL-6 and U-HBP (ρ = .57).
Figure 5.
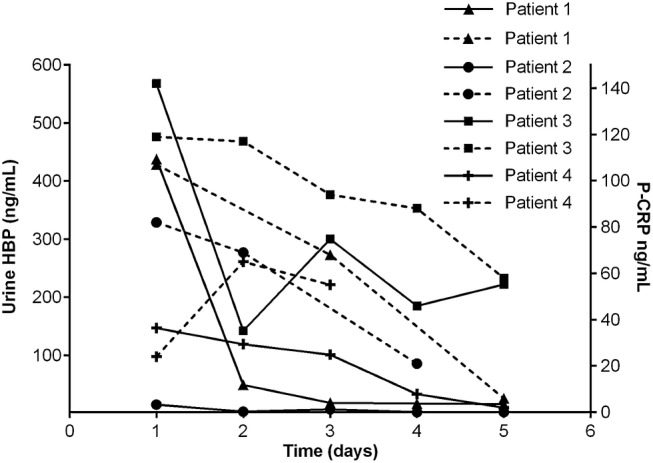
Urine levels of heparin-binding protein (HBP) and plasma C-reactive protein (P-CRP) in 4 patients during 5 consecutive days of treatment of antibiotics against pyelonephritis. Each dot represents an individual value of HBP and P-CRP. Each dashed line represents P-CRP. All values at day 1 represent the concentrations of HBP and P-CRP before treatment of antibiotics.
U-HBP and U-IL-6 in Patients With Pyelonephritis and Cystitis, in the Group of Definite UTI
Patients with febrile UTI (pyelonephritis) (n = 59) had a median HBP concentration of 227 ng/mL (IQR, 80–435 ng/mL), which was significantly higher than for the group of 118 patients with cystitis (median, 121 ng/mL; IQR, 51–262 ng/mL; P < .01). In addition, when the suggested cutoff value was used, the sensitivity of HBP for diagnosing pyelonephritis was 93.3%, which was higher than for cystitis 86.4%. Likewise, IL-6 was significantly higher among patients with pyelonephritis (median, 200 pg/mL; IQR, 7–719 pg/mL) than for patients with cystitis (median, 16 pg/mL; IQR, 1–197 pg/mL; P < .01). In the PC cohort, the levels of U-HBP were significantly higher in patients with pyelonephritis (n = 12) compared to patients with cystitis (n = 105) (P = .01); however, this finding was not true for U-IL-6 (P = .13) (Table 1). In the hospital cohort, there were no significant differences between cystitis and pyelonephritis for levels of U-HBP (P = .37) and IL-6 (P = .10).
Urine-HBP Levels and Kidney Failure
To evaluate the impact of kidney failure on U-HBP levels, patients were divided into 3 different groups described in the method section: NKD (n = 120), AKD (n = 19), and CKD (n = 25). There was a significant difference in the levels of U-HBP between the patients with NKD and CKD (P = .01), but not between the other groups. Urine IL-6 levels did not differ significantly between the groups. In patients with UTI median values of U-HBP in the 3 groups, NKD (n = 47), AKD (n = 10), and CKD (n = 15) were 92, 275, and 562 ng/mL, respectively. Urine HBP levels were significantly higher in patients with CKD compared to NKD (P = .01). There were no significant differences between the other groups. However, all 3 groups had an HBP level above the suggested cutoff (30 ng/mL). All groups with no UTI (NKD n = 73, AKD n = 9, CKD n = 10) had lower median U-HBP levels (6 ng/mL, 9 ng/mL, and 8 ng/mL, respectively). There was no significant difference for U-IL-6 between the groups.
DISCUSSION
This study represents the first evaluation of HBP as a marker of UTI in adults. In this relatively large cohort of 390 patients from 4 different healthcare institutions, the diagnostic test characteristic of HBP was superior to that of U-WBC, U-IL-6 and nitrite. We studied HBP in 2 different populations; PC patients and patients at the hospital ED, which enabled inclusion of patients with both cystitis and pyelonephritis. This enabled inclusion of patients with both cystitis and pyelonephritis. The prevalence of UTI and cystitis was higher in the PC cohort than in the more complex patient group at the hospital ED. These differences were expected and may explain the higher PPV and lower NPV for UTI in the PC group. Compared with HBP, which had high sensitivity and specificity for diagnosing UTI in both patient groups, the diagnostic sensitivity for IL-6 was lower in the PC group. This result (1) probably reflects the low prevalence of pyelonephritis among these patients and perhaps (2) that IL-6 needs a stronger inflammatory stimulus to be initiated than is needed for the release of the prefabricated HBP from neutrophils. Similar to the present study, others have found higher levels of U-IL-6 in patients with pyelonephritis than in patients with cystitis [10]. In this study, U-HBP (P = .01) was better than U-IL-6 (P = .13) in distinguishing cystitis from pyelonephritis, especially in the PC setting.
Urine HBP levels in the no UTI group were not affected by the presence of acute or chronic renal failure, indicating that the cutoff level for U-HBP and diagnosing UTI is applicable regardless of kidney function. However, U-HBP was significantly higher in patients with UTI and CKD compared to those with no or acute renal failure. The reason for this outcome is unclear, but the data suggest that it is more complicated to discriminate between cystitis and pyelonephritis in patients with impaired kidney function.
A strong correlation was detected between U-HBP and U-WBC (r = 0.79). This result was expected because HBP is secreted from neutrophils. However, not all patients with elevated levels of U-WBC had high levels of U-HBP in this study. Notably, among patients with no UTI but still positive for U-WBC in the dipstick test (false positives), 59.5% had a U-HBP below cutoff, making HBP a more specific marker than U-WBC.
Higher levels of U-HBP were noticed in patients with higher concentrations of bacteria in urine compared to patients with lower concentrations. This finding is in line with previous studies showing the direct effect of bacterial structures on the release of HBP [26].
The strength of the study is the large sample size and the inclusion of patients in 2 different healthcare settings, which made it possible to enroll a substantial number of patients with both cystitis and pyelonephritis. The main limitation of the study is the risk of misclassification of some patients. Independent clinically evaluated patients and symptoms may have been misinterpreted or not completely registered. In addition, results from urine cultures may have been influenced by recent antibiotic treatment or short bladder incubation.
In conclusion, U-HBP was the best diagnostic marker for UTI and could also discriminate between cystitis and pyelonephritis. The results suggest that HBP may add to the diagnostic accuracy of tests presently used in the PC and the hospital ED. Further studies of HBP as a candidate UTI biomarker are warranted and should also be expanded to patient groups such as asymptomatic carriers and those with neutropenic fever and urogenital pathology.
Notes
Acknowledgments We thank the staff at the ED in Helsingborg and Lund and at the PC units in Höganäs for their assistance in including patients to the study. We also thank the staff at Biomedical Centre in Lund for the support in technical assistance and Dick Nelson for statistical support.
Financial support. This work was funded by Swedish Research Council (projects 7480 and 13413), the Royal Physiographic Society, Lund, the Swedish Government Funds for Clinical Research (ALF), Svenska Läkarsällskapet, Svenska Vetenskapsrådet, Anna-Lisa, and Sven-Eric Lundgrens stiftelse, and CIHR IMPACT strategic training Post-doctoral Fellowship.
Potential conflicts of interests. A. L. and P. Å. are listed as inventors on a pending patent application on the use of HBP as a diagnostic tool in sepsis filed by Hansa Medical AB.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. SWEDRES 2010: a report on Swedish antibiotic utilisation and resistance in human medicine. Available at: http://www.strama.se/uploads/docs/Swedres%202010%20final.pdf . Accessed 26 November 2013.
- 2.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287:2701–10. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 3.Little P, Turner S, Rumsby K, et al. Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study. Health Technol Assess (Winchester, England) 2009;13:1–73. doi: 10.3310/hta13190. iii–iv, ix–xi. [DOI] [PubMed] [Google Scholar]
- 4.Deville WL, Yzermans JC, van Duijn NP, Bezemer PD, van der Windt DA, Bouter LM. The urine dipstick test useful to rule out infections. A meta-analysis of the accuracy. BMC Urol. 2004;4:4. doi: 10.1186/1471-2490-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St John A, Boyd JC, Lowes AJ, Price CP. The use of urinary dipstick tests to exclude urinary tract infection: a systematic review of the literature. Am J Clin Pathol. 2006;126:428–36. doi: 10.1309/C69RW1BT7E4QAFPV. [DOI] [PubMed] [Google Scholar]
- 6.Hurlbut TA, 3rd, Littenberg B. The diagnostic accuracy of rapid dipstick tests to predict urinary tract infection. Am J Clin Pathol. 1991;96:582–8. doi: 10.1093/ajcp/96.5.582. [DOI] [PubMed] [Google Scholar]
- 7.Knottnerus BJ, Geerlings SE, Moll van Charante EP, Ter Riet G. Toward a simple diagnostic index for acute uncomplicated urinary tract infections. Ann Fam Med. 2013;11:442–51. doi: 10.1370/afm.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 2004;38(Suppl 4):S341–5. doi: 10.1086/382690. [DOI] [PubMed] [Google Scholar]
- 9.Gillings MR. Evolutionary consequences of antibiotic use for the resistome, mobilome and microbial pangenome. Front Microbiol. 2013;4:4. doi: 10.3389/fmicb.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanda N, Juthani-Mehta M. Novel biomarkers for the diagnosis of urinary tract infection-a systematic review. Biomark Insights. 2009;4:111–21. doi: 10.4137/bmi.s3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otto G, Braconier J, Andreasson A, Svanborg C. Interleukin-6 and disease severity in patients with bacteremic and nonbacteremic febrile urinary tract infection. J Infect Dis. 1999;179:172–9. doi: 10.1086/314534. [DOI] [PubMed] [Google Scholar]
- 12.Rodhe N, Löfgren S, Strindhall J, Matussek A, Mölstad S. Cytokines in urine in elderly subjects with acute cystitis and asymptomatic bacteriuria. Scand J Prim Health Care. 2009;27:74–9. doi: 10.1080/02813430902757634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautam N, Olofsson AM, Herwald H, Matussek A, Mölstad S. Heparin-binding protein (HBP/CAP37): a missing link in neutrophil-evoked alteration of vascular permeability. Nat Med. 2001;7:1123–7. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- 14.Linder A, Soehnlein O, Åkesson P. Roles of heparin-binding protein in bacterial infections. J Innate Immun. 2010;2:431–8. doi: 10.1159/000314853. [DOI] [PubMed] [Google Scholar]
- 15.Soehnlein O. Direct and alternative antimicrobial mechanisms of neutrophil-derived granule proteins. J Mol Med (Berl) 2009;87:1157–64. doi: 10.1007/s00109-009-0508-6. [DOI] [PubMed] [Google Scholar]
- 16.Linder A, Christensson B, Herwald H, Björck L, Åkesson P. Heparin-binding protein: an early marker of circulatory failure in sepsis. Clin Infect Dis. 2009;49:1044–50. doi: 10.1086/605563. [DOI] [PubMed] [Google Scholar]
- 17.Linder A, Åkesson P, Inghammar M, Treutiger CJ, Linner A, Sunden-Cullberg J. Elevated plasma levels of heparin-binding protein in intensive care unit patients with severe sepsis and septic shock. Crit Care. 2012;16:R90. doi: 10.1186/cc11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linder A, Åkesson P, Brink M, Studahl M, Björck L, Christensson B. Heparin-binding protein: a diagnostic marker of acute bacterial meningitis. Crit Care Med. 2011;39:812–7. doi: 10.1097/CCM.0b013e318206c396. [DOI] [PubMed] [Google Scholar]
- 19.Linder A, Johansson L, Thulin P, et al. Erysipelas caused by group A streptococcus activates the contact system and induces the release of heparin-binding protein. J Invest Dermatol. 2010;130:1365–72. doi: 10.1038/jid.2009.437. [DOI] [PubMed] [Google Scholar]
- 20.Kjölvmark C, Åkesson P, Linder A. Elevated urine levels of heparin-binding protein in children with urinary tract infection. Pediatr Nephrol. 2012;27:1301–8. doi: 10.1007/s00467-012-2132-x. [DOI] [PubMed] [Google Scholar]
- 21.European Confederation of Laboratory M. European urinalysis guidelines. Scand J Clin Lab Invest Suppl. 2000;231:1–86. [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of internal medicine. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Kidney International Supplements. 2012;2:19–36. doi: 10.1038/kisup.2011.32. Section 2: AKI Definition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tapper H, Karlsson A, Mörgelin M, Flodgaard H, Herwald H. Secretion of heparin-binding protein from human neutrophils is determined by its localization in azurophilic granules and secretory vesicles. Blood. 2002;99:1785–93. doi: 10.1182/blood.v99.5.1785. [DOI] [PubMed] [Google Scholar]
- 25.Lindmark A, Garwicz D, Rasmussen PB, Flodgaard H, Gullberg U. Characterization of the biosynthesis, processing, and sorting of human HBP/CAP37/azurocidin. J Leukoc Biol. 1999;66:634–43. doi: 10.1002/jlb.66.4.634. [DOI] [PubMed] [Google Scholar]
- 26.Herwald H, Cramer H, Mörgelin M, et al. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell. 2004;116:367–79. doi: 10.1016/s0092-8674(04)00057-1. [DOI] [PubMed] [Google Scholar]