Abstract
Hypervirulent strains of Klebsiella pneumoniae are associated with abscess formation, commonly hepatic, and metastatic spread, even in healthy patients. We describe a case of this clinical syndrome, genotypic and phenotypic features of the isolate, and briefly review epidemiology, clinical manifestations, and pathogenesis of this underappreciated syndrome.
Keywords: hypermucoviscous, hypervirulence, Klebsiella pneumoniae, virulence factors
CASE REPORT
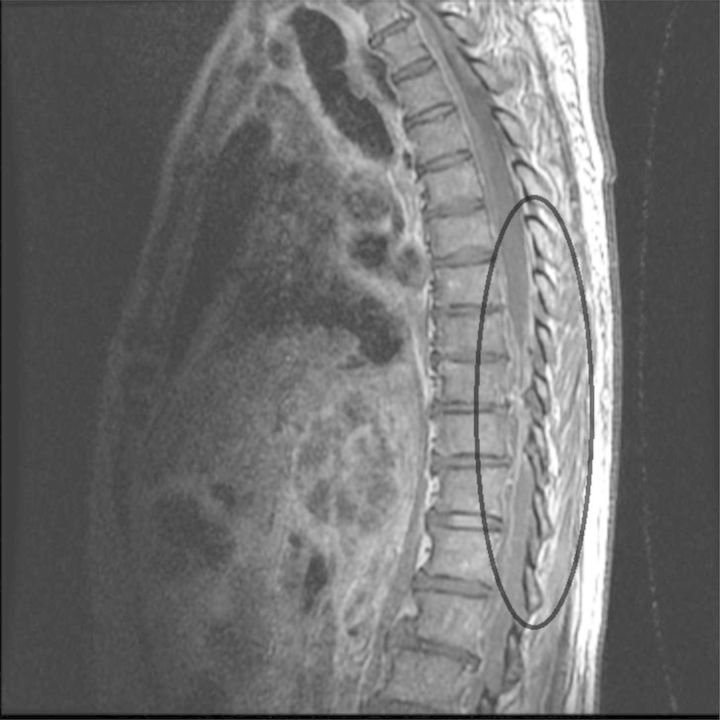
In November 2013, over the course of several weeks, a 74-year-old Vietnamese man with a history of hypertension and vitamin D deficiency developed subacute onset of abdominal pain, fevers, back pain, progressive weakness, and dyspnea on exertion. He was given empiric oseltamivir, which did not ameliorate his symptoms. He had previously been in a “re-education”/prison camp before moving to the United States in 1990. He last visited Ho Chi Minh City, Vietnam in 2011. At admission, he was febrile (103°F), tachycardic (103 beats per minute), dyspneic with conversation, and tender in the paramuscular area of the lumbar and thoracic spine. The white blood cell count was 13.7 × 103 cells/mm3; aspartate aminotransferase was 113 IU/L; the alanine aminotransferase was 101 IU/L; total bilirubin was 2.3 mg/dL; and the inflammatory markers C-reactive protein and erythrocyte sedimentation rate were 242 mg/L and 94 mm/hour, respectively. Blood cultures positive for Klebsiella pneumoniae prompted initiation of ceftriaxone. Imaging revealed multiple lesions in the liver, an epidural abscess that had a ventral and dorsal component, and involved the cervical, thoracic, and lumbar spine with concern for osteomyelitis at L1-L3 and adjacent bilateral psoas abscesses (Figure 1). He had a normal ophthalmologic exam. The epidural abscess was evacuated surgically, and the largest hepatic abscess was drained percutaneously. Cultures from the liver, thoracic, and lumbar abscesses also grew K. pneumoniae. A string test, a semiquantitative phenotypic test, to assess for hypermucoviscosity by stretching a bacterial colony on an agar plate with an inoculation needle, was positive. This finding, in conjunction with the clinical syndrome, suggested that a hypervirulent isolate of K. pneumoniae was responsible. Genotypic analyses supported this contention. Polymerase chain reaction generated amplicons for iucA and iroN (biosynthetic genes for the siderophores aerobactin and salmochelin, respectively), terB (a tellurite resistance gene), and rmpA (a regulatory gene whose product mediates increased capsule production and hypermucoviscosity). These genes are present on large virulence plasmids that appear to be critical for the increased pathogenic potential of hypervirulent K. pneumoniae [1, 2]. The patient received 8 weeks of intravenous ceftriaxone; thereafter, spinal and abdominal imaging showed resolution of his liver, psoas, and epidural abscesses.
Figure 1.
Magnetic resonance imaging scan performed upon admission demonstrated increased signal at the L1-L3 disc level and in the adjacent vertebral bodies concerning for discitis or osteomyelitis. A large anterior epidural abscess extends down from at least T10. Canal narrowing, which is highlighted, appears to be most severe at L4-L5 from disc bulge and epidural abscess.
Epidemiology
In the mid-1980s, case reports from Taiwan described healthy patients with community-acquired K. pneumoniae liver abscesses and serious concomitant end-organ manifestations such as endophthalmitis and meningitis [3]. This pathotype has become known as “hypervirulent” to differentiate it from “classical” K. pneumoniae, which is commonly isolated from infected patients in Western countries [1]. In contrast to the usual healthcare-associated classical K. pneumoniae infections, hypervirulent K. pneumoniae can cause serious organ and life-threatening infections in younger, healthy individuals from the community. In the last decade, 813 cases of the invasive liver abscess syndromes associated with hypervirulent K. pneumoniae were reported [4]. This result is likely an underestimation due to underreporting, limited methods for differentiating hypervirulent from classical pathotypes, and unfamiliarity of this syndrome outside of Asia [4]. Klebsiella pneumoniae is now the most common cause of pyogenic liver abscess in Asia and possibly in North America as well [4–6]. Colonization rates of the serotypes K1 and K2, which are more likely to have the hypervirulence plasmid, are higher in Asia, likely leading to a higher prevalence of cases in the Pacific Rim, although there is still some question as to whether Asian hosts have an undetermined increased susceptibility to this infection [1, 7]. In addition to Asian ethnicity, some epidemiological studies have showed a higher incidence of disease at 55–60 years and a male dominance [4]. Diabetes mellitus has also been identified as a risk factor for this clinical syndrome [1, 4].
Clinical Manifestations
It is becoming increasingly clear that the initially described pyogenic liver abscess in the absence of biliary tract disease represents just one of many primary infections due to this organism. Other presentations include pneumonia, endophthalmitis, meningitis, nonhepatic abscess at a variety of sites, and necrotizing fasciitis [1]. These patients are commonly bacteremic. Metastatic spread from a site of infection is a defining, and potentially devastating, characteristic. Of 512 cases reviewed in Taiwan, the country with the highest prevalence of this syndrome, 15% of patients developed metastatic infection [4]. Other studies have shown up to 13% of patients with hypervirulent K. pneumoniae liver abscess developed concomitant central nervous system manifestations such as meningitis or endophthalmitis [8]. Metastatic infection most commonly involves the lung, causing pneumonia or empyema [1, 4]. Other sites of metastatic infection include bone and the genitourinary system, and there are case reports of endocarditis and a Lemierre syndrome variant thought to be secondary to hypervirulent K. pneumoniae [1].
Virulence
Phenotypically, hypervirulent K. pneumoniae is more resistant to complement and neutrophil-mediated bactericidal activity in vitro and virulent in vivo than classical strains [9]. Virulence is associated with the acquisition of a 200- to 220-kilobase plasmid that contains rmpA and siderophore biosynthetic genes. Increased capsule production, which is mediated at least in part by RmpA, enhances virulence in animal infection models. In contrast to classical K. pneumoniae, the hypervirulent isolates secrete greater amounts of siderophores that mediate iron acquisition from the host. This phenotype enhances growth and survival in human ascites ex vivo [10]. Other potential virulence factors for hypervirulent K. pneumoniae have been recently reviewed [1]. However, significant knowledge gaps exist, and undoubtedly numerous additional factors yet to be described will prove to be critical in the pathogenesis of infection. It remains unclear what features of hypervirulent K. pneumoniae enable metastatic spread. Furthermore, although intestinal colonization is almost certainly a prerequisite for disease, the portal of entry leading to infection and the mechanism by which this occurs is also unknown.
DISCUSSION
Our case demonstrates some classic features of infection due to hypervirulent K. pneumoniae. The infection was community-acquired and in a previously healthy patient. Metastatic spread, one of the defining features, was observed in the epidural space, spine, and psoas muscles. This clinical syndrome connotes important implications for management. Unfortunately, a rapid and reliable test to identify hypervirulent K. pneumoniae is still lacking. Although the string test is widely considered to be reliable for distinguishing hypervirulent from classical K. pneumoniae strains, its sensitivity is not optimal and a significant minority of classical K. pneumoniae strains are positive [11, 12]. These performance issues are especially problematic in regions of lower hypervirulent K. pneumoniae prevalence, such as the United States. Nonetheless, when suspected, a prompt ophthalmological exam should be performed because of the rapid course and high degree of morbidity associated with hypervirulent K. pneumoniae endophthalmitis [4, 8]. Vigilance for other metastatic manifestations should also be emphasized because there may be a need for source control as demonstrated by this case. Although diabetes mellitus has been identified as a risk factor, many patients with the syndrome have no underlying immunodeficiency or comorbidities. Strict glycemic control in diabetic patients was shown to decrease metastatic complications in one study [13].
Over the last few decades, mortality rates have declined with dual approach of percutaneous drainage and antibiotic therapy [4, 14]. However, percutaneous drainage can be challenging with hypervirulent K. pneumoniae due to its hypermucoviscous nature. Unlike other etiologies of hepatic abscess, hypervirulent K. pneumoniae liver abscess is typically monomicrobial [6]. Antimicrobial susceptibility patterns of hypervirulent K. pneumoniae remain largely pan-sensitive for now, usually only resistant to ampicillin [8]. Classical K. pneumoniae, however, has increasingly acquired genes for extended spectrum β-lactamases and carbapenemases.
Treatment of these infections with a third-generation cephalosporin is preferred, although there is some debate as to whether first-generation cephalosporins could be equally effective [4, 6, 14]. Duration of therapy has not been established in controlled trials. In general, a period of 2–6 weeks has been used, with a lengthier duration used for larger abscesses that resolve slowly or in the presence of metastatic disease that requires a more prolonged duration [4]. A conservative approach would be to treat until radiographic evidence of infection is resolved. However, even with this approach, anecdotal reports of recurrences have been described [1].
In summary, infection caused by hypervirulent K. pneumoniae is associated with notable morbidity and potential mortality. To provide optimal care and minimize these untoward consequences requires prompt recognition of this pathogen. This distinction would facilitate attention to key aspects of treatment such as control of hyperglycemia in patients with diabetes mellitus and aggressively looking for metastatic spread of infection. Although progress has been made in understanding the virulence factors driving this clinical syndrome, rapid diagnostic tests and an increased understanding of the epidemiology of this unique pathogen are still needed. These needs are becoming even more pressing with recent disconcerting reports describing acquisition of multidrug resistance by hypervirulent K. pneumoniae isolates [15].
Acknowledgments
Financial support. This work was supported by in part by the National Institutes of Health Grant 1R21AI088318-01A1 (to T. A. R.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4:1–12. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo TA, Olson R, Macdonald U, et al. Aerobactin mediates virulence and accounts for the increased siderophore production under iron limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun. 2014;82:2356–67. doi: 10.1128/IAI.01667-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913–6. [PubMed] [Google Scholar]
- 4.Siu LK, Yeh KM, Lin JC, et al. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–7. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 5.Rahimian J, Wilson T, Oram V, et al. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 6.Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100:322–31. doi: 10.1111/j.1572-0241.2005.40310.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol. 2012;12:13. doi: 10.1186/1471-2180-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang CT, Lai SY, Yi W, et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–93. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 9.Pomakova DK, Hsiao CB, Beanan JM, et al. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumoniae: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis. 2012;31:981–9. doi: 10.1007/s10096-011-1396-6. [DOI] [PubMed] [Google Scholar]
- 10.Russo TA, Shon AS, Beanan JM, et al. Hypervirulent K. pneumoniae secretes more and more active iron-acquisition molecules than “classical” K. pneumoniae thereby enhancing its virulence. PLoS One. 2011;6:e26734. doi: 10.1371/journal.pone.0026734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HC, Chuang YC, Yu WL, et al. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med. 2006;259:606–14. doi: 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 12.Fang CT, Chuang YP, Shun CT, et al. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199:697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab. 2006;91:3084–7. doi: 10.1210/jc.2005-2749. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Chen YS, Tsai HC, et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis. 2008;47:642–50. doi: 10.1086/590932. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Sun G, Yu Y, et al. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014;58:225–32. doi: 10.1093/cid/cit675. [DOI] [PubMed] [Google Scholar]