In this case-control study, HTLV-1 infection increased risk of bronchiectasis 1.84 times. HTLV-1 proviral loads for bronchiectasis patients were significantly higher than those of controls. HTLV-1 proviral loads correlated with the extent of radiologically determined pulmonary injury.
Keywords: Australia, bronchiectasis, HTLV-1, HTLV-1 proviral load, Indigenous, pulmonary disease, Strongyloides stercoralis
Abstract
Background.
We previously suggested that infection with the human T-lymphotropic virus type 1 (HTLV-1) subtype C is associated with bronchiectasis among Indigenous Australians. Bronchiectasis might therefore result from an HTLV-1-mediated inflammatory process that is typically associated with a high HTLV-1 proviral load (PVL). Human T-lymphotropic virus type 1 PVL have not been reported for Indigenous Australians.
Methods.
Thirty-six Indigenous adults admitted with bronchiectasis from June 1, 2008, to December 31, 2009 were prospectively recruited and matched by age, sex, and ethno-geographic origin to 36 controls. Case notes and chest high-resolution computed tomographs were reviewed, and pulmonary injury scores were calculated. A PVL assay for the HTLV-1c subtype that infects Indigenous Australians was developed and applied to this study. Clinical, radiological, and virological parameters were compared between groups and according to HTLV-1 serostatus.
Results.
Human T-lymphotropic virus type 1 infection was the main predictor of bronchiectasis in a multivariable model (adjusted risk ratio [aRR], 1.84; 95% confidence interval [CI], 1.19–2.84; P = .006). Moreover, the median HTLV-1c PVL (interquartile range) for cases was >100-fold that of controls (cases, 0.319 [0.007, 0.749]; controls, 0.003 [0.000, 0.051] per 100 peripheral blood lymphocytes; P = .007), and HTLV-1c PVL were closely correlated with radiologically determined pulmonary injury scores (Spearman's rho = 0.7457; P = .0000). Other predictors of bronchiectasis were positive Strongyloides serology (aRR, 1.69; 95% CI, 1.13–2.53) and childhood skin infections (aRR, 1.62; 95% CI, 1.07–2.44). Bronchiectasis was the major predictor of death (aRR, 2.71; 95% CI, 1.36–5.39; P = .004).
Conclusions.
These data strongly support an etiological association between HTLV-1 infection and bronchiectasis in a socially disadvantaged population at risk of recurrent lower respiratory tract infections.
The human T-lymphotropic virus type 1 (HTLV-1) is an oncogenic retrovirus that infects at least 5–10 million people, most of whom reside in developing countries [1]. Among the many clusters of high HTLV-1 endemicity worldwide is a small endemic focus in central Australia [1], where 7.2%–13.9% of the Indigenous population is infected [2, 3]. In <10% of HTLV-1 carriers, infection is complicated by noninfective sequelae including adult T-cell leukemia/lymphoma [4, 5] and inflammatory disorders, the prototype of which is HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [5]. In resource-limited areas, infectious complications also contribute to HTLV-1-related morbidity and mortality [5]. People infected with HTLV-1 are at increased risk of symptomatic and life-threatening complicated strongyloidiasis [6], and a severe exudative eczema, infective dermatitis, predominantly affects HTLV-1 carriers from a lower socioeconomic background [5, 7].
Human T-lymphotropic virus type 1-associated inflammatory syndromes may affect organ systems that are remote from the central nervous system [5]. Pulmonary involvement, for example, affects all lung compartments [8–11] and has been reported in as many as 30% of asymptomatic people with HTLV-1 infection [10]. However, clinically significant pulmonary disease is uncommon among people infected with HTLV-1 in developed countries [12, 13]. In contrast, we recently suggested that HTLV-1 infection in an impoverished Indigenous population in central Australia was associated with multilobar bronchiectasis, and bronchiectasis was a major cause of early mortality among Indigenous adults [14]. In this retrospective study, differences in presentation and outcomes were compared among patients with established bronchiectasis according to HTLV-1 serostatus. Consequently, we were unable to determine risk relative to a control group without bronchiectasis or to study the possible pathological basis of disease.
Among socially disadvantaged Indigenous Australians, bronchiectasis might result from an HTLV-1-mediated inflammatory process, a predisposition to repeated lower respiratory tract infections (LRTIs), or a combination of these factors. A major determinant of HTLV-1-associated inflammatory diseases, such as HAM/TSP, is thought to be a high HTLV-1 antigen load [15]. The HTLV-1 antigen load is reflected in the integration of HTLV-1 provirus into peripheral blood lymphocytes (PBLs), the quantification of which is defined as the HTLV-1 proviral load (PVL). Unfortunately, we were unable to quantify HTLV-1 PVL for subjects whose clinical details were described in our previous study [14]. The highly divergent HTLV-1c strains that infect Indigenous Australians have since been better characterized [16]. A method for determining the PVL of these HTLV-1c strains has subsequently been developed, and this method was applied to the present study. Therefore, the purpose of this case-control study was to further define the risk of bronchiectasis among Indigenous adults who were infected with HTLV-1c and to determine whether higher HTLV-1c PVL or more frequent LRTIs contribute to this risk.
METHODS
The Study Setting
Most Indigenous residents of central Australia reside in conditions of considerable socioeconomic disadvantage in isolated remote communities or overcrowded “town camps,” which have poor amenities and limited refuse disposal. A single, well resourced, community-based hospital, Alice Springs Hospital (ASH), serves this region of 1 000 000 km2. The hospital operates in a complex cross-cultural environment; rates of self-discharge are the highest reported worldwide, and many patients seek help from traditional healers before attending ASH [17]. No attempt has been made to control HTLV-1 transmission among Indigenous Australians.
Recruitment
Cases were Indigenous adults (age ≥15 years) with bronchiectasis who were admitted to ASH for >48 h with an infective exacerbation from June 1, 2008 to December 31, 2009. Diagnosis was made by chest high-resolution computed tomography (HRCT) (34), bronchoscopy (1), and bronchography (1). Mortality rates among Indigenous Australians with bronchiectasis are high. In our previous study, for example, 34.2% of our cohort died during the study period [14]; therefore, the present study includes only 18 subjects whose clinical details were presented previously [14].
Controls were Indigenous adults admitted during the same period for cardiological investigations (12), trauma (10), general surgery (9), and missed dialysis (3) who had no evidence of LRTIs and no dermatological or neurological symptoms that might be attributable to HTLV-1 infection. Chest x-rays were reviewed for all potential control patients, and those with pulmonary abnormalities were excluded. A research team member (S. J.) who was unaware of the HTLV-1 serostatus was responsible for recruitment. All patients gave written informed consent.
Matching of Controls
Human T-lymphotropic virus type 1 seroprevalence varies with age, gender, and place of residence [1]. Cases were therefore matched to controls 1 : 1 according to age (age of case ±10 years), gender, and primary language, which was determined by 2 Aboriginal Research Officers. Subjects were then matched by another research team member (E. G.). All were unaware of the HTLV-1 serostatus of enrolled patients.
Clinical data were extracted from case notes from date of first admission to date of recruitment using a standardized data-collection form. For cases, data are presented to date of diagnosis. Results for all microbiological tests, full blood examinations, radiology, and other relevant investigations were also extracted. Results were recorded for sputum cultures performed during separate admissions >2 weeks apart. Persistent eosinophilia was defined as an eosinophil count >0.7 × 109/L for >12 months. Strongyloides stercoralis serological status was determined using an in-house enzyme-linked immunosorbent assay based on antigen extracts of Strongyloides ratti, which is highly sensitive and specific. Results were recorded as positive, equivocal, or negative according to predetermined optical density cutoffs.
Pulmonary Injury Scores
High-resolution computed tomography chest images for 33 cases were available, and these were reviewed remotely using the Picture Archiving and Communication System with Inteleviewer software by 2 consultant radiologists (V. A., S. H.) in Adelaide, 1500 km from ASH. Both radiologists were blinded to HTLV-1 serostatus. A “pulmonary injury score” was calculated using a system modified from Bhalla et al [18]. Each lobe was scored independently for bronchiectasis severity (0–3), bronchial wall thickening (0–3), mucus plugging/centrilobular nodules (0,1), sacculation (0,1), parenchymal changes of ground-glass density (0,1), and air-trapping/emphysema (0,1). A maximal score of 10 could therefore be assigned to each lobe. A score of 10 was also assigned to lobes with complete collapse/cicatrisation. The lingular segment of the left upper lobe was regarded as a separate lobe. Chest HRCTs for an additional 8 HTLV-1 carriers without bronchiectasis were similarly scored. These included 2 controls (bronchiectasis previously excluded) and 6 other patients (assessed but excluded as controls due to intercurrent LRTIs).
HTLV-1 Serologic and Molecular Studies
Peripheral blood buffy coats (PBBCs) were transferred to Institut Pasteur, Paris, where analyses were performed blinded to the patient's clinical state. Plasma HTLV-1 antibodies were detected, and titers were determined using a 2-fold endpoint dilution method by particle agglutination (PA) (Serodia HTLV-1; Fujirebio, Tokyo, Japan). All samples for which PA tests were positive or borderline were tested by confirmatory Western blot assay (HTLV-I/II Blot2.4; MP Biomedicals Asia Pacific Pty. Ltd., Singapore).
High molecular weight DNA was extracted and HTLV-1 PVL was determined by SYBR Green real-time polymerase chain reaction (PCR). This assay was performed in 20 μL PCR mixture volume consisting of 2× Quantitect SYBR Green Master Mix (QIAGEN) containing HotstartTaq DNA polymerase, 150 nM of each oligonucleotide primer, and 4 μL of DNA extracted from PBBCs. In parallel, a reference curve was obtained from scalar dilution of cloned PCR product (from 1010 to 0.1 copies of HTLV-1 Px genomic region). Details of HTLV-1 Px gene amplification were as follows: 1 cycle of 10 min at 95°C (hot-start PCR) and 45 cycles in 3 steps each (95°C for 30 s, 56°C for 30 s, 72°C for 30 s). Human T-lymphotropic virus type 1 Px primer sequences designed to specifically amplify HTLV-1c were as follows; PxCfor: 5′ CAC ACC GTC AAG CAC AGA TT 3′ and PxCrev: 5′ TCT CCA TAC ACG TAG ACT GGA T 3′. Amplication using these primers yielded a 184-base pair Px fragment. All standard dilutions and samples from patients were run in duplicate. SYBR Green real-time PCR for a single copy host cell gene (HLA-DQ alpha) was used to normalize the genomic DNA input. Primers used were as follows: GH26; 5′ GTG CTG CAG GTG TAA CTT GTA CCA G 3′ and GH27: 5′ GGA TCC GGT AGC AGC GGT AGA GTT G 3′ [19]. Human T-lymphotropic virus type 1 PVLs are expressed as number of proviral copies per 102 PBLs. The sensitivity of the method used is 1 copy of proviral DNA per 104 PBL. DNA was not available for 1 HTLV-1-seropositive case.
The study was approved by the Central Australian Human Research Ethics Committee.
Statistical Analysis
Categorical variables were compared using a χ2 test or Fisher's Exact test, as appropriate. Continuous variables were assessed for significant departures from normality. Normally distributed variables were summarized using mean and standard deviation and compared using a t test. Skewed variables were summarized using median and interquartile range (IQR) and compared using a Wilcoxon rank-sum test.
Predictors of bronchiectasis and death were analyzed using unadjusted and adjusted Poisson regression with robust standard errors [20]. Direct modeling of relative risk (RR) using a Poisson approach was preferred over RR estimation via odds ratios from logistic modeling due to the relative frequency of both outcome variables (50% of the sample recorded the bronchiectasis event, and 36% recorded a mortality event). Candidate predictors for inclusion in adjusted modeling were identified by (1) their clinical and biological importance and (2) predictor performance on unadjusted modeling. Colinearity and interactions between model covariates were examined using a likelihood ratio test. For the modeling of both outcome variables, a link test was used to determine specification error, whereas overall goodness of fit was assessed using both visual examination of residuals with a likelihood-ratio test and a Pearson goodness-of-fit test. For each outcome variable, we used an Akaike Information Criterion to assist in identifying which particular combination of explanatory variables in any 1 multivariate model returned estimates that best fit the observed data. Mortality events were obtained from the hospital patient management system until 30th June 2012. For all analyses, P < .05 was considered significant. All analyses were undertaken using Stata, version 12 (StataCorp, College Station, TX).
RESULTS
A total of 47 patients with bronchiectasis (HTLV-1 seropositive, 33; 70.0%) met the inclusion criteria and 36 (76.6%) were recruited to the study. Eleven patients (men, 8; women, 3) with a mean age of 47 ± 9 years were discharged before consent could be obtained in their primary languages. This was due to our inability to find an appropriate interpreter in 9 cases and self-discharge in 2 cases. Human T-lymphotropic virus type 1 serology was performed for each patient who was not recruited; 8 (72.7%) were HTLV-1 seropositive. The characteristics of patients who were not recruited were therefore similar to those who consented to study participation (Table 1). Cases and controls were well matched with regards to age and gender. In adulthood, cases were more likely to dwell in the Alice Springs township (Table 1), less likely to require admission for harmful alcohol consumption, but more likely to have respiratory failure (Table 1) and echocardiographic findings consistent with pulmonary disease (Table 1).
Table 1.
Patient Characteristics and Echocardiographic Findings for Indigenous Adults With Bronchiectasis and Their Matched Controlsa
| Characteristic | Cases (n = 36) | Controls (n = 36) | P Value |
|---|---|---|---|
| Sex (male/female) (n) | 25/11 | 25/11 | 1.000 |
| Age at recruitment (years) (mean ± SE) | 43.5 (11.9) | 46.1 (12.6) | .384 |
| Residence in childhoodb (n, %) | |||
| Urban | 1 (4.4) | 4 (21.1) | .096 |
| Remote | 22 (99.7) | 15 (78.9) | |
| Residence in adulthood (n, %) | |||
| Urban | 17 (47.2) | 8 (22.2) | .026 |
| Remote | 19 (52.8) | 28 (77.8) | |
| Comorbidities (n, %) | |||
| Diabetes | 9 (25.0) | 16 (44.4) | .083 |
| Tobacco | 14 (38.9) | 20 (55.6) | .157 |
| Alcohol | 20 (55.6) | 28 (77.8) | .046 |
| Asthma | 6 (16.7) | 2 (5.6) | .260 |
| COPD | 4 (11.1) | 1 (2.8) | .357 |
| Ischemic heart disease (n, %) | 4 (11.1) | 11 (30.6) | .079 |
| Congestive cardiac failure | 2 (5.6) | 4 (11.1) | .674 |
| Chronic liver disease | 4 (11.1) | 3 (8.3) | 1.000 |
| Respiratory failure | 14 (38.9) | 0 (0.0) | <.001 |
| Right heart failure | 5 (13.9) | 0 (0.0) | .054 |
| Echocardiography (n, %) | 32 (88.9) | 21 (58.3) | |
| Dilated right ventricle | 13 (40.6) | 0 (0.0) | .001 |
| Tricuspid regurgitation | 10 (31.3) | 1 (4.8) | .035 |
| Pulmonary hypertension | 13 (43.3) | 0 (0.0) | <.001 |
Abbreviations: Alcohol, harmful alcohol consumption; COPD, chronic obstructive pulmonary disease; SE, standard error; Tobacco, tobacco smoker.
a Continuous variables are summarized using mean and SE.
b Place of residence in childhood could be ascertained for 23 cases and 19 controls.
Risk Factors for Bronchiectasis
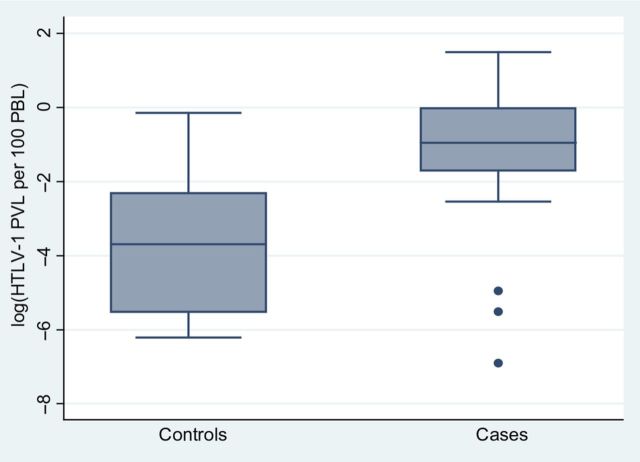
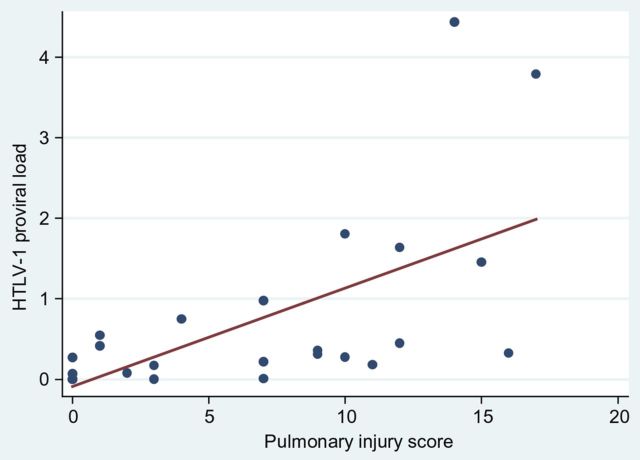
Human T-lymphotropic virus type 1 infection was more common among cases (25 of 36; 69.4%) than controls (15 of 36; 41.7%) (P = .018) (Table 2), and this was predictive of bronchiectasis in a multivariable model (adjusted risk ratio [aRR], 1.84; 95% confidence interval [CI], 1.19–2.84; P = .006) (Table 3). The median HTLV-1c PVL (IQR) for cases was >100-fold higher than that of controls (cases, 0.319 [0.007, 0.749]; controls, 0.003 [0.000, 0.051] per 100 PBL; P = .007) (Table 2) (Figure 1). Each unit increase in HTLV-1c PVL was associated with 1.21 times the risk of bronchiectasis (Table 3). Higher pulmonary injury scores were significantly correlated with higher HTLV-1c PVL (Figure 2). Higher HTLV-1 antibody titers, a persistent eosinophilia, positive or equivocal Strongyloides serology, and skin infections were also associated with bronchiectasis on univariable analysis. Positive or equivocal Strongyloides serology and childhood skin infections remained predictive of bronchiectasis in a multivariable model (Table 3).
Table 2.
Results of Infection-Related Investigations for Indigenous Adults With Bronchiectasis and Their Matched Controlsa
| Cases (n = 36) | Controls (n = 36) | P Value | |
|---|---|---|---|
| HTLV-1 Studies | |||
| WB positive (n, %) | 25 (69.4) | 15 (41.7) | .018 |
| PVL (per 100 PBL) (median, IQR) | 0.319 (0.007, 0.749) | 0.003 (0.000, 0.051) | .007 |
| Ab titer (log units) (mean ± SE) | 8.53 (6.93, 9.92) | 5.55 (4.85, 7.62) | .0035 |
| Strongyloides Serologyb (n, %) | |||
| Positive | 15 (41.7) | 4 (16.0) | <.001 |
| Negative | 11 (30.6) | 20 (80.0) | |
| Equivocal | 10 (27.8) | 1 (4.0) | |
| Sputum Cultures (median, IQR) | 3 (2, 6)d | 3 (2,7) | .814 |
| Haemophilus influenzae | 1 (0, 3)d | 0 (0, 1) | .058 |
| Number of pathogens | 2.7 (3.9)d | 1.2 (2.3) | .023 |
| Eosinophiliac (n, %) | 22 (61.1) | 10 (27.8) | .004 |
Abbreviations: Ab, HTLV-1 antibody titer; HTLV-1, human T-lymphotropic virus type 1; IQR, interquartile range; PBL, peripheral blood lymphocytes; PVL, HTLV-1c proviral load; SE, standard error; WB, Western blot.
a Continuous variables are summarized using mean and SE or median and IQR as appropriate.
b Strongyloides serology performed for 36 cases and 25 controls.
c Eosinophils >0.7 × 109/L for >12 months.
d Pathogens isolated from sputum specimens cultured prior to diagnosis with bronchiectasis.
Table 3.
Univariable and Multivariable Predictors of Bronchiectasisa
| Univariable Predictors |
Multivariable Predictors |
|||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P Value | RR | 95% CI | P Value | |
| HTLV-1 Studies | ||||||
| WB | 3.79 | 2.23–6.42 | .000 | 1.84 | 1.19–2.84 | .006 |
| Ab titerb | 1.18 | 1.05–1.32 | .006 | … | … | … |
| PVLc | 1.21 | 1.08–1.36 | .001 | … | … | … |
| Eosinophiliad | 1.96 | 1.21–3.19 | .006 | … | … | … |
| Strongyloidese | ||||||
| Positive | 2.23 | 1.31–3.79 | .003 | 1.69 | 1.13–2.53 | .011 |
| Equivocal | 2.56 | 1.53–4.28 | .000 | 1.83 | 1.18–2.82 | .006 |
| Skin Infections | ||||||
| Childhoodf | 3.77 | 2.36–6.03 | .000 | 1.62 | 1.07–2.44 | .022 |
| Adulthoodf | 1.86 | 1.24–2.78 | .003 | … | … | … |
| Scabies | 1.74 | 1.04–2.92 | .036 | … | … | … |
| IDH | 2.09 | 1.63–2.68 | .000 | … | … | … |
| HTLV-1-associated conditionsg | 2.24 | 1.71–2.94 | .000 | … | … | … |
| Haemophilus influenzaeh | 1.09 | 1.03–1.14 | .001 | … | … | … |
| Alcoholi | 0.63 | 0.40–0.97 | .037 | … | … | … |
Abbreviations: Ab, HTLV-1 antibody titer; CI, confidence interval; HTLV-1, human T-lymphotropic virus type 1; IDH, HTLV-1-associated infective dermatitis; RR, relative risk; PVL, proviral load; WB, Western blot.
a Multivariable predictors were adjusted for the covariates listed in the table.
b Increased risk per 1 log increase in antibody titer.
c Increased risk of bronchiectasis per unit increase in HTLV-1c proviral load.
d Eosinophils > 0.7 × 109/L for at least 12 months.
e Strongyloides serology result.
f Admission specifically for the treatment of a skin infection.
g Infective (infective dermatitis, 3; complicated strongyloidiasis, 2) and inflammatory (uveitis, 1; pericarditis, 1) conditions likely to be HTLV-1 associated.
h Haemophilus influenzae isolated from sputum prior to a formal diagnosis of bronchiectasis.
i Harmful alcohol consumption.
Figure 1.
Human T-lymphotropic virus type 1 (HTLV-1) subtype C proviral loads for cases and controls. Box plot depicting median (interquartile range [IQR]) HTLV-1 proviral loads for 24 bronchiectasis cases and 15 controls. Human T-lymphotropic virus type 1 subtype C proviral load expressed as proviral copies per 100 peripheral blood lymphocytes. Data are logarithmically transformed for clarity of presentation.
Figure 2.
Pulmonary injury scores versus human T-lymphotropic virus type 1 (HTLV-1) subtype C proviral load. Correlation between HTLV-1c proviral loads (proviral copies per 100 peripheral blood lymphocytes) and pulmonary injury scores, which were calculated by applying a predetermined scoring system to chest high-resolution computed tomography for 29 HTLV-1-seropositive patients (bronchiectasis, 21; no bronchiectasis, 8). Spearman's rho = 0.7457; Prob > |t| = 0.0000.
Cases were more likely to have been admitted previously with probable HTLV-1-related conditions (Table 4). These conditions included infective dermatitis (3), complicated strongyloidiasis unresponsive to treatment with thiabendazole (2), inflammatory pericarditis for which no other cause was identified (1), and recurrent uveitis (1). One patient with complicated strongyloidiasis was also admitted with infective dermatitis. Patients with pericarditis and uveitis were both ataxic; however, clinical criteria for HAM/TSP were not met.
Table 4.
Details of Previous Admissions for Indigenous Adults With Bronchiectasis and Their Matched Controlsa
| Cases (n = 36) | Controls (n = 36) | P Value | |
|---|---|---|---|
| Skin Conditions (n, %) | |||
| Any admission | 9 (25.0) | 0 (0.0) | .002 |
| Childhood admissionb | 6 (30.0) | 0 (0.0) | .022 |
| HTLV-1-associated conditionsc (n, %) | 6 (16.7) | 0 (0.0) | .025 |
| Childhood | |||
| Any admission (n, %) | 20 (55.6) | 17 (47.2) | .638 |
| Age, first admissiond (mean ± SE) | 1.72 (3.02) | 1.60 (3.29) | .476 |
| ICU admissions (mean ± SE) | 0.03 (0.17)e | 0 (0.0) | .317 |
| Respiratory admissions (mean ± SE) | 0.97 (2.13)e | 0.78 (1.33) | .889 |
| Respiratory pathogens (mean ± SE) | 0.08 (0.28)f | 0.08 (0.28) | 1.000 |
| Adulthood | |||
| Age, first admissiong (mean ± SE) | 15.1 (13.6) | 26.9 (9.4) | .001 |
| Age, first respiratory (mean ± SE) | 19.3 (17.2)e | 36.9 (10.1) | <.001 |
| ICU admissions (mean ± SE) | 0.2 (0.6)e | 0.1 (0.3) | .694 |
| Respiratory admissions (mean ± SE) | 3.1 (6.0) | 2.3 (2.9) | .889 |
Abbreviations: HTLV-1, human T-lymphotropic virus type 1; ICU, intensive care unit; SE, standard error.
a Continuous variables are summarized using mean and SE as appropriate.
b Twenty cases and 17 controls were admitted in childhood.
c Infective (infective dermatitis, 3; complicated strongyloidiasis, 2) and inflammatory (uveitis, 1; pericarditis, 1) conditions likely to be HTLV-1 associated.
d Age of Admission with any condition in childhood.
e Admission with a respiratory condition prior to diagnosis with bronchiectasis.
f Number of respiratory pathogens isolated in childhood prior to diagnosis with bronchiectasis.
g Age of admission with any condition after the age of 14 years.
Admissions for LRTIs
A total of 1161 admissions were reviewed, including 122 admissions in childhood (cases, 57; controls, 65) and 1039 in adulthood (cases, 285; controls, 754). Twelve cases and 13 controls were admitted in childhood with 35 and 28 LRTI episodes, respectively; however, only 1 case required an intensive care admission. Six cases were diagnosed with bronchiectasis in childhood. The age of first admission and the numbers of total admissions, LRTI admissions, and LRTI admissions requiring intensive care did not differ between groups (Table 4).
In adulthood, 21 cases and 25 controls were admitted with 110 and 82 LRTIs, respectively. These resulted in an intensive care admission for 5 cases and 4 controls. Cases were significantly younger when first admitted with an adult LRTI (Table 4). There were no significant differences in the numbers of adult LRTI admissions or the number of associated intensive care admissions between cases and controls (Table 4).
Respiratory Pathogens
Respiratory pathogens were not more likely to be cultured from children who were subsequently diagnosed with bronchiectasis (Table 4). Including pathogens isolated in adulthood, 53 and 45 respiratory pathogens were cultured from the sputum of 15 cases prior to diagnosis with bronchiectasis and from 14 controls, respectively. Among subjects whose sputum cultures yielded a respiratory pathogen, these were more often isolated from cases (Table 2). Pathogens isolated from HTLV-1-seropositive patients included Mycobacterium tuberculosis (case, 1; control, 1) and Mycobacterium avium/intracellulare complex (case, 1). Severe pneumonia due to Nocardia sp preceded the development of bronchiectasis in 1 HTLV-1-uninfected case with a history of harmful alcohol consumption.
Mortality
Twenty cases (55.5%) and 7 controls (19.4%) died during 208.18 years of follow-up (P = .028). Although cases died at a younger age, this difference was not significant (cases, 48.4 ± 12; controls, 58.9 ± 10.7; P = .052). Among patients whose cause of death could be ascertained, respiratory complications were responsible for the deaths of 16 of 17 bronchiectasis cases and 1 of 7 controls (P = .205). Bronchiectasis was strongly associated with death in a multivariable model (aRR, 2.71; 95% CI, 1.36–5.39; P = .004) (Table 5).
Table 5.
Univariable and Multivariable Predictors of Deatha
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P Value | RR | 95% CI | P Value | |
| Ageb | 1.16 | 1.04–1.30 | .011 | 1.20 | 1.07–1.35 | .002 |
| Bronchiectasis | 2.86 | 1.38–5.94 | .005 | 2.71 | 1.36–5.39 | .004 |
| Right heart failure | 2.33 | 1.34–4.05 | .003 | … | … | … |
| Respiratory failure | 2.85 | 1.72–4.71 | .000 | … | … | … |
| Eosinophiliac | 2.13 | 1.13–4.00 | .019 | 1.63 | 0.90–2.96 | .106 |
| Strongyloidesd | 1.80 | 0.94–3.41 | .074 | … | … | … |
| Uveitis/pericarditis | 2.80 | 2.04–3.84 | .000 | … | … | … |
Abbreviations: CI, confidence interval; RR, relative risk.
a Multivariable predictors were adjusted for the covariates listed in the table.
b For each 5 years increase in age.
c Eosinophils >0.7 × 109/L for >12 months.
d Strongyloides serology positive.
DISCUSSION
The adult Indigenous population of central Australia has the highest reported prevalence of bronchiectasis worldwide [14], yet identifying the responsible etiological conditions has proven difficult. Measles and tuberculosis, which are frequent causes of bronchiectasis in developing countries, are rare; cystic fibrosis has not been described; and other causes, such as immunoglobulin deficiency, are uncommon [14, 21]. Therefore, previous pneumonia is thought to be the cause of bronchiectasis in >90% of childhood cases [21]. In contrast, the development of bronchiectasis in our adult cohort was seldom preceded by an identifiable precipitant. A diagnosis of bronchiectasis was not made until adulthood in >80% of cases and no respiratory pathogens were isolated from nearly 60% of cases prior to diagnosis. Risk of LRTIs was high in both groups; however, there were no differences between cases and controls in the number of admissions with LRTIs or their requirement for intensive care. Higher HTLV-1 seropositivity rates and HTLV-1 PVL among cases suggest that an HTLV-1-mediated inflammatory process might contribute to pulmonary disease in our study population.
Pulmonary involvement has been frequently described among HTLV-1 carriers elsewhere. Radiological abnormalities are found in one-half of Japanese patients with HAM/TSP [22] and 30% of asymptomatic HTLV-1 carriers [10]. These include alveolitis [8], interstitial pneumonia [8, 23], chronic bronchiolitis/bronchitis [9, 11], and bronchiectasis [10, 11]. Therefore, all lung compartments may be involved; however, airway inflammation seems to be particularly frequent [10, 11]. Consistent with these radiological findings, histopathological studies reveal infiltration of bronchiole walls by lymphocytes [24], which may result in their partial occlusion [25]. Small airway inflammation might contribute to a higher incidence of self-reported asthma among HTLV-1 carriers in the United States [13], yet significant pulmonary disease is an uncommon feature of HTLV-1 infection in developed countries [12, 13]. In contrast, HTLV-1 infection nearly doubled the risk of presenting with bronchiectasis among Indigenous Australians in the present study. In our previous retrospective study, bronchiectasis was more extensive, ground glass opacities were more often present at chest HRCT, and a pathogen was less likely to be isolated from sputum during an acute exacerbation of bronchiectasis among HTLV-1 carriers. These observations suggest that a diffuse inflammatory process that is associated with HTLV-1 infection might contribute to the development of bronchiectasis in our patient population [14].
The risk of inflammatory disorders, such as HAM/TSP [5], is associated with high HTLV-1 PVL in PBLs [5]. The pathophysiological processes that result in HAM/TSP have not been fully elucidated, but they may include the release of proinflammatory cytokines from HTLV-1-infected cells [26] and injury resulting from the immune response to a high HTLV-1 antigen load that is predominantly derived from infected lymphocytes [15]. Likewise, a marked inflammatory response follows the infiltration of HTLV-1-infected lymphocytes into the pulmonary parenchyma. Increased recovery of infected lymphocytes from bronchoalveolar lavage (BAL) fluid has been reported in patients with HAM/TSP [27–29] and in otherwise healthy carriers [30]. The high HTLV-1 PVL [30] of these cells was correlated with that of peripheral blood mononuclear cells [30]. The presence of HTLV-1-infected lymphocytes in BAL fluid is associated with increased HTLV-1 tax/rex mRNA expression in vivo [31, 32], a proinflammatory milieu [32], and an HTLV-1-specific cytotoxic T cell-mediated inflammatory response among patients with HTLV-1-associated interstitial pneumonitis [33].
In the present study, the median HTLV-1 PVL among cases was more than 100-fold greater than controls, and the HTLV-1 PVL was correlated with the extent of radiologically defined pulmonary injury. These findings suggest that an HTLV-1-mediated inflammatory process contributes to the development of bronchiectasis in our study population. Cases were also more likely to have been admitted with other HTLV-1-associated conditions including infective dermatitis and were more likely to be Strongyloides seropositive. Childhood infective dermatitis is associated with a high HTLV-1 PVL and with childhood bronchiectasis in Jamaica [34]. Although an association between HTLV-1 infection and Strongyloides seropositivity has not been consistently shown [35, 36], the risk of symptomatic strongyloidiasis is increased among subjects with high HTLV-1 PVL [37]. The clinical markers of HTLV-1-associated bronchiectasis described here are therefore those HTLV-1-related diseases that are associated with high HTLV-1 PVL in other populations. Nevertheless, the primary clinical manifestation of HTLV-1 infection in our cohort was severe pulmonary disease, and this is uncommon among HTLV-1 carriers in other developed countries [12, 13]. In our socially disadvantaged study population, progressive lung disease might result from repeated LRTI in HTLV-1 carriers with preexisting HTLV-1-associated pulmonary injury.
A number of limitations should be noted. First, recruitment occurred over a relatively short period and was confined to patients admitted with bronchiectasis for >48 h. Therefore, our findings may not be relevant to patients with less severe disease who are primarily managed in the community. We were also unable to enroll all patients in the ASH bronchiectasis cohort, raising the possibility of selection bias. However, team members were blinded to HTLV-1 serostatus, and there was no difference in HTLV-1 seropositivity rates between eligible patients and those who were recruited to the study. A further limitation lies in the retrospective collection of data relating to previous admissions. The diagnostic confirmation of clinical conditions, such as infective dermatitis, that had resolved prior to recruitment was therefore not possible. Finally, some patients with limited bronchiectasis may have been recruited as controls because chest x-rays, which have a low sensitivity for detecting early bronchiectasis, were used to exclude pulmonary disease in this group. Although this might contribute to the high HTLV-1 seropositivity rate in our control group (42%), this most likely reflects the cohort's age and the matching of controls to cases by ethno-geographic origin [1].
Among the Indigenous population of central Australia, HTLV-1 infection is associated with multilobar bronchiectasis, which carries a poor prognosis [14]. The present study suggests that an HTLV-1-mediated inflammatory process is etiologically associated with this life-threatening condition. Human T-lymphotropic virus type 1 carriers with bronchiectasis had higher HTLV-1 PVLs, which were correlated with the extent of pulmonary injury, and other manifestations of HTLV-1 infection were only found among cases. In contrast to other developed countries, pulmonary disease was the predominant clinical manifestation of HTLV-1 infection in our socially disadvantaged study population. In this setting, progression to clinically significant bronchiectasis might result from further pulmonary injury after recurrent LRTIs. Further studies are required to determine whether HTLV-1 infection contributes to the development of bronchiectasis in other socially disadvantaged settings.
Notes
Acknowledgments. We acknowledge the assistance of Lena McCormack and Magdalene Lynch (Northern Territory Rural Clinical School) for determining the primary languages for patients recruited to this study; the staff at the Pathology service (Alice Springs Hospital) for preparing the clinical material; and Cathy Magann for ensuring that this material was safely shipped to Paris, France.
Disclaimer. No funding source was involved in any aspect of study design, data collection, data analyses, or manuscript preparation.
Financial support. This study was supported by the National Health and Medical Research Council (project grant 1012945), the Northern Territory Rural Clinical School, which is an initiative of the Australian Department of Health and Ageing, and with grants from the Association pour la Recherche sur le Cancer (ARC), the Cancéropole/Ile de France, the CNRS (UMR 3569), and the Institut Pasteur, Paris, France, and through the Investissement d′Avenir as part of a Laboratoire d′Excellence (LabEx) French research program: Integrative Biology of Emerging Infectious Diseases (IBEID).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastian I, Hinuma Y, Doherty RR. HTLV-1 among Northern Territory Aborigines. Med J Aust. 1993;159:12–6. doi: 10.5694/j.1326-5377.1993.tb137694.x. [DOI] [PubMed] [Google Scholar]
- 3.Bastian I. Darwin: University of Sydney; 1996. HTLV-1 studies in the Northern Territory. PhD Thesis. [Google Scholar]
- 4.Einsiedel L, Cassar O, Bardy P, et al. Variant human T-cell lymphotropic virus type 1c and adult T-cell leukemia, Australia. Emerg Infect Dis. 2013;19:1639–41. doi: 10.3201/eid1910.130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdonck K, Gonzalez E, Van Dooren S, et al. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–81. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 6.Gotuzzo E, Terashima A, Alvarez H, et al. Strongyloides stercoralis hyperinfection associated with human T cell lymphotropic virus type I in Peru. Am J Trop Med Hyg. 1999;60:146–9. doi: 10.4269/ajtmh.1999.60.146. [DOI] [PubMed] [Google Scholar]
- 7.Einsiedel L, Cassar O, Gordon L, et al. Human T-lymphotropic virus type 1 infective dermatitis in central Australia. J Clin Virol. 2013;57:370–3. doi: 10.1016/j.jcv.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto M, Nakashima H, Watanabe S, et al. T-lymphocyte alveolitis in HTLV-1 associated myelopathy. Lancet. 1987;ii:1220. doi: 10.1016/s0140-6736(87)91362-6. [DOI] [PubMed] [Google Scholar]
- 9.Kadota J, Mukae H, Fujii T, et al. Clinical similarities and differences between human T-cell lymphotropic virus type 1 associated bronchiolitis and diffuse panbronchiolitis. Chest. 2004;125:1239–47. doi: 10.1378/chest.125.4.1239. [DOI] [PubMed] [Google Scholar]
- 10.Okada F, Ando Y, Yoshitake S, et al. Pulmonary CT findings in 320 carriers of human T-lymphotropic virus type 1. Radiology. 2006;240:559–64. doi: 10.1148/radiol.2402050886. [DOI] [PubMed] [Google Scholar]
- 11.Yamashiro T, Kamiya H, Miyara T, et al. CT scans of the chest in carriers of human T-cell lymphotropic virus type 1: presence of interstitial pneumonia. Acad Radiol. 2012;19:952–7. doi: 10.1016/j.acra.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Stuver S, Tachibana N, Okayama A, et al. Evaluation of morbidity among human T lymphotropic virus type 1 carriers in Miyazaki, Japan. J Infect Dis. 1996;173:584–91. doi: 10.1093/infdis/173.3.584. [DOI] [PubMed] [Google Scholar]
- 13.Murphy E, Wang B, Sacher RA, et al. Respiratory and urinary tract infections, arthritis and asthma associated with HTLV-I and HTLV-II infection. Emerg Infect Dis. 2004;10:109–16. doi: 10.3201/eid1001.020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Einsiedel L, Fernandes L, Spelman T, et al. Bronchiectasis is associated with Human T-Lymphotropic Virus 1 infection in an Indigenous Australian population. Clin Infect Dis. 2012;54:43–50. doi: 10.1093/cid/cir766. [DOI] [PubMed] [Google Scholar]
- 15.Tattermusch S, Bangham CRM. HTLV-1 infection: what determines the risk of inflammatory disease. Trends Microbiol. 2012;20:494–500. doi: 10.1016/j.tim.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Cassar O, Einsiedel L, Afonso PV, et al. HTLV type 1 subtype C molecular variants among Indigenous Australians: new insights on HTLV-1 molecular epidemiology in Australo-Melanesia. PLoS Negl Trop Dis. 2013;7:e2418. doi: 10.1371/journal.pntd.0002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Einsiedel L, van Iersel E, Macnamara R, et al. Self-discharge by adult Aboriginal patients at Alice Springs Hospital, Central Australia: insights from a prospective cohort study. Aust Health Rev. 2013;3:239–45. doi: 10.1071/AH11087. [DOI] [PubMed] [Google Scholar]
- 18.Bhalla M, Turcios N, Aponte V, et al. Cystic fibrosis: scoring system with thin section CT. Radiology. 1991;179:783–8. doi: 10.1148/radiology.179.3.2027992. [DOI] [PubMed] [Google Scholar]
- 19.Lee T, Chafets DM, Busch MP, et al. Quantitation of HTLV-I and II proviral load using real-time quantitative PCR with SYBR Green chemistry. J Clin Virol. 2004;3:275–82. doi: 10.1016/j.jcv.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Valery P, Torzillo PJ, Mulholland K, et al. Hospital-based case-control study of bronchiectasis in Indigenous Children in Central Australia. Pediat Infect Dis J. 2004;23:902–8. doi: 10.1097/01.inf.0000142508.33623.2f. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa M, Izumo S, Kubota H, et al. HTLV-1-associated myelopathy: analysis of 213 patients based on clinical features and laboratory findings. J Neurovirol. 1995;1:50–61. doi: 10.3109/13550289509111010. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto M, Mita S, Tokunaga M, et al. Pulmonary involvement in human T-cell lymphotropic virus type I uveitis: T-lymphocytosis and high proviral DNA load in bronchoalveolar lavage fluid. Eur Respir J. 1993;6:938–43. [PubMed] [Google Scholar]
- 24.Sugisaki K, Tsuda T, Kunamoto T, et al. Clinicopathologic characteristics of lungs of patients with human T-cell lymphotropic virus type 1 associated myelopathy. Am J Trop Med Hyg. 1998;58:721–5. doi: 10.4269/ajtmh.1998.58.721. [DOI] [PubMed] [Google Scholar]
- 25.Tateishi U, Nishihara H, Miyasaka K. HTLV-1-associated bronchopneumonopathy (HAB): CT-pathological correlation. Clin Radiol. 2001;56:664–6. doi: 10.1053/crad.2001.0677. [DOI] [PubMed] [Google Scholar]
- 26.Araujo A, Silva M. The HTLV-1 neurological complex. Lancet Infect Dis. 2006;5:1068–76. doi: 10.1016/S1474-4422(06)70628-7. [DOI] [PubMed] [Google Scholar]
- 27.Couderc L, Caubarrere I, Venet A, et al. Bronchoalveolar lymphocytosis in patients with tropical spastic paraparesis associated with human T-cell lymphotropic virus type I (HTLV-I) Ann Intern Med. 1988;109:625–8. doi: 10.7326/0003-4819-109-8-625. [DOI] [PubMed] [Google Scholar]
- 28.Desgranges C, Bechet JM, Couderc LJ, et al. Detection of HTLV-1 DNA by polymerase chain reaction in alveolar lymphocytes of patients with tropical spastic paraparesis. J Infect Dis. 1989;160:162–3. doi: 10.1093/infdis/160.1.162. [DOI] [PubMed] [Google Scholar]
- 29.Setoguchi Y, Takahashi S, Nukiwa T, et al. Detection of human T-cell lymphotropic virus type-1 related antibodies in patients with lymphocytic interstitial pneumonia. Am Rev Resp Dis. 1991;144:1361–5. doi: 10.1164/ajrccm/144.6.1361. [DOI] [PubMed] [Google Scholar]
- 30.Mori S, Mizoguchi A, Kawabata M, et al. Bronchoalveolar lavage lymphocytes correlate with HTLV-1 proviral load in HTLV-1 carriers. Thorax. 2005;60:138–43. doi: 10.1136/thx.2004.021667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seki M, Higashiyama Y, Mizokami A, et al. Up-regulation of human T lymphotropic virus type 1 (HTLV-1) tax/rex mRNA in infected lung tissues. Clin Exp Immunol. 2000;120:488–98. doi: 10.1046/j.1365-2249.2000.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamazato Y, Miyazato A, Kawakami K, et al. High expression of p40tax and proinflammatory cytokines and chemokines in the lungs of human T-lymphotropic virus type-1-related bronchopulmonary disorder. Chest. 2003;124:2283–92. doi: 10.1378/chest.124.6.2283. [DOI] [PubMed] [Google Scholar]
- 33.Kawabata T, Higashimoto I, Takashima H, et al. Human T-lymphotropic virus type I (HTLV-I)-specific CD8+ cells accumulate in the lungs of patients infected with HTLV-I with pulmonary involvement. J Med Virol. 2012;84:1120–7. doi: 10.1002/jmv.23307. [DOI] [PubMed] [Google Scholar]
- 34.La Grenade L. HTLV-1 associated infective dermatitis: past, present and future. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:S46–9. doi: 10.1097/00042560-199600001-00009. [DOI] [PubMed] [Google Scholar]
- 35.Courouble G, Candolfi E, Strobel M, et al. Human T-cell lymphotropic virus type I association with Strongyloides stercoralis: a case control study among Carribean blood donors from Guadeloupe (French West Indies) J Clin Microbiol. 2000;38:3903–4. doi: 10.1128/jcm.38.10.3903-3904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson R, Lindo JF, Neva FA, et al. Immunoepidemiologic studies of Strongyloides stercoralis and human T lymphotropic virus type I infections in Jamaica. J Infect Dis. 1994;169:692–6. doi: 10.1093/infdis/169.3.692. [DOI] [PubMed] [Google Scholar]
- 37.Gillet NA, Cook L, Laydon DJ, et al. Strongyloidiasis and infective dermatitis alter human T lymphotropic virus-1 clonality in vivo. PLoS Pathog. 2013;9:e1003263. doi: 10.1371/journal.ppat.1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]