Rates of virological suppression after 6 months of ART were high in a cohort of 678 HIV-positive adults managed in Ethiopian health centers, with no significant difference with regard to concomitant tuberculosis at baseline (TB 135; non-TB 543).
Keywords: ART, Ethiopia, health centers, outcome, tuberculosis
Abstract
Background.
Antiretroviral therapy (ART) initiation during treatment for tuberculosis (TB) improves survival in human immunodeficiency virus (HIV)/TB-coinfected patients. We compared virological suppression (VS) rates, mortality, and retention in care in HIV-positive adults receiving care in 5 Ethiopian health centers with regard to TB coinfection.
Methods.
Human immunodeficiency virus-positive ART-naive adults eligible for ART initiation were prospectively recruited. At inclusion, all patients underwent microbiological investigations for TB (sputum smear, liquid culture, and polymerase chain reaction). Virological suppression rates after 6 months of ART (VS; viral load <40 and <400 copies/mL) with regard to TB status was the primary outcome. The impact of HIV/TB coinfection on VS rates was determined by multivariate regression analysis. Mortality and retention in care were analyzed by proportional hazard models.
Results.
Among 812 participants (TB, 158; non-TB, 654), 678 started ART during the follow-up period (TB, 135; non-TB, 543). No difference in retention in care between TB and non-TB patients was observed during follow-up; 25 (3.7%) patients died, and 17 (2.5%) were lost to follow-up (P = .30 and P = .83, respectively). Overall rates of VS at 6 months were 72.1% (<40 copies/mL) and 88.7% (<400 copies/mL), with similar results for subjects with and without TB coinfection (<40 copies/mL: 65 of 92 [70.7%] vs 304 of 420 [72.4%], P = .74; <400 copies/mL: 77 of 92 [83.7%] vs 377 of 420 [89.8%], P = .10, respectively).
Conclusions.
High rates of VS can be achieved in adults receiving ART at health centers, with no significant difference with regard to TB coinfection. These findings demonstrate the feasibility of combined ART and anti-TB treatment in primary healthcare in low-income countries.
Clinical Trials Registration.
Human immunodeficiency virus (HIV) infection is the strongest risk factor known for developing active tuberculosis (TB), and TB remains the most common opportunistic infection globally [1]. Rates of HIV coinfection among TB patients are high in sub-Saharan Africa, exceeding 50% in some countries [1–3]. In many cases, HIV infection is first detected at presentation with TB, and most such persons have advanced immunosuppression at the time of HIV diagnosis. The risk of death among HIV/TB-coinfected people is greatly increased compared with HIV-infected subjects without TB [4–7]. Survival for coinfected persons is significantly improved if antiretroviral therapy (ART) is started during the course of anti-TB treatment (ATT) [8–10]. Consequently, the World Health Organization (WHO) recommends starting ART during ATT for all HIV/TB-coinfected patients, preferably during the first 2 months of ATT [11].
However, ART initiation during ATT may be associated with complications. The risk of immune reconstitution inflammatory syndrome (IRIS) is elevated for patients starting ART during the initial months of ATT; although IRIS is rarely fatal, this condition may be difficult to recognize and manage in low-resource settings [12]. Furthermore, concomitant ATT and ART might compromise adherence and increase the risk of side effects and drug-drug interactions, all of which could adversely impact treatment outcome [12, 13]. More importantly, inadequate suppression of viral replication during suboptimal ART promotes selection of HIV variants with mutations conferring antiretroviral drug resistance [14].
Although most HIV/TB-coinfected subjects live in resource-limited settings, initial studies on the efficacy of concomitant ART and ATT were performed in high- and middle-income regions [15–17]. Recently, Soeters et al [18] presented a systematic review on the effect of ATT on ART outcome that showed virologic suppression rates in patients receiving concomitant ART/ATT comparable to those in subjects only receiving ART. This review included 8 studies conducted in sub-Saharan Africa (5 of 8 from South Africa), which were mainly based in hospitals or specialized ART clinics. To increase treatment coverage, continued decentralization of ART services to primary health centers is needed. This process requires simplification of management due to less qualified staff and limited access to laboratory monitoring; in particular, viral load testing is rarely available. Although comparable outcomes of ART in clinics managed by nurses compared with physician-run facilities have been reported [19, 20], the outcome of ART provided at public health centers for patients with HIV/TB coinfection is not well documented.
In Ethiopia, an increasing number of patients receive ART at public health centers throughout the country since decentralization of ART began in 2006. We have recently reported a high prevalence of TB detected through intensified case-finding in a cohort of adults receiving care in 5 health centers in an uptake area of Central Ethiopia [3]. The current study compares the outcome of ART after 6 months with regard to the presence of concomitant TB among participants in this cohort, with particular focus on virological suppression (VS) rates.
MATERIALS AND METHODS
Setting
This study was performed in all public health centers (Adama, Dhera, Geda, Mojo, and Wolenchiti) providing ART in the city of Adama, Oromia Regional State, Ethiopia, and adjacent rural and suburban districts, covering an uptake area with approximately 600 000 inhabitants. Adama is situated on the highway connecting Addis Ababa and Djibouti, which is considered a high-risk corridor for HIV infection in Ethiopia. In 2005, the estimated HIV prevalence in Adama was 9%, compared with the average national prevalence of 3.5% [21].
Non-physician clinicians with 3–4 years of academic training provide care at Ethiopian public health centers. Apart from HIV services, health centers consist of several different sections, such as outpatient department, antenatal clinic, delivery service, and TB clinic. Clinicians attend the different sections according to rotating schedules; although assigned to ART clinics, they are fully responsible for care, treatment initiation, and follow-up of HIV-positive patients. In the 2008 Ethiopian National ART guidelines, ART initiation was recommended for all patients with CD4 cell counts <200 cells/µL, patients with WHO clinical stage 3 and CD4 cell counts <350 cells/µL, and patients with WHO clinical stage 4 irrespective of CD4 cell counts [22]. Since 2012, the Ethiopian National ART guidelines recommend ART initiation for all patients with CD4 cell counts <350 cells/µL or WHO clinical stage 4 [23]. The first-line treatment regimen for TB consists of an intensive phase with rifampicin, isoniazid, pyrazinamide, and ethambutol for 8 weeks, followed by a 4-month continuation phase with isoniazid and rifampin [23].
Study Participants
Human immunodeficiency virus-positive patients presenting to the study health centers between October 3, 2011 and March 1, 2013 were eligible for inclusion if the following criteria were met: age 18 years or greater, eligibility to start ART (defined as a documented CD4 cell count <350 cells/µL and/or WHO stage 4 disease, in accordance with the 2012 Ethiopian National ART guidelines [23]), and residency in the catchment area of any of the study sites. Patients with current or previous ART, as well as patients on ATT for more than 2 weeks before inclusion, were excluded. This cohort has since been continuously followed. Patients who have started ART since inclusion constitute the study population for the current study, with follow-up data collected until data abstraction on December 31, 2013.
Methods
At inclusion, detailed demographic and clinical data, including physical examination details, were collected using structured questionnaires. All patient-related study procedures were performed by health center staff who had been trained and certified to provide ART according to the national Ethiopian guidelines [22]. Blood for CD4 cell and complete blood counts was obtained, with storage of plasma for viral load measurements. In association with inclusion, all patients underwent bacteriological investigations for intensified TB case-finding. For this purpose, participants were asked to provide 2 paired morning sputa, expectorated on consecutive days. In case of peripheral lymphadenopathy, fine-needle aspirates (FNA) were obtained. Blood and bacteriological results were communicated to the responsible clinicians who decided if and when to start ART or ATT. In accordance with Ethiopian national TB guidelines, patients could also be diagnosed and treated for TB based on clinical and radiological criteria [23].
Participants were observed until ART initiation, and at months 1, 2, 3, and 6 thereafter. At each visit, structured investigations were repeated, covering disease symptoms, adherence, and medications used, as well as physical examination. Blood tests were repeated until ART initiation (at months 3, 6, 12, and 18) and at months 1, 3, and 6 after ART initiation. After 6 months of ART, viral load testing was performed. Upon clinical suspicion of TB at any time during follow-up, the responsible clinician was encouraged to repeat TB diagnostics according to the study protocol. Tracing was recommended for patients more than 1 day late for scheduled visits.
To ensure adherence to the study protocol, the investigators (A. R., T. T. B., P. B.) or members of the research team conducted weekly visits to each health center and monitored all study data. Trained data clerks continuously entered study data into databases, with cross-checking of all entries after digitalization.
Laboratory Analyses
All laboratory analyses, except TB cultures, were performed at Adama Regional Laboratory. CD4 cell counts were analyzed using BD FACSCalibur cytometer (Becton Dickinson, San Jose, CA). Sputum and FNA samples were analyzed with direct smear microscopy using Ziehl-Neelsen staining and Xpert MTB/RIF (Cepheid, Sunnyvale, CA) for polymerase chain reaction. Liquid cultures for TB were performed at International Clinical Laboratories, Addis Ababa, using a BACTEC MGIT 960 (BD Diagnostics, Franklin Lakes, NJ).
Plasma HIV-RNA levels were determined using Abbott RealTime HIV-1 assay (Abbott Molecular Inc., Des Plaines, IL) with a detection limit of 40 copies/mL. External quality assurance of the regional laboratory is regularly performed by the Center for Disease Control and Prevention (Atlanta, GA).
Statistical Analysis
The primary study outcome was the rate of VS after 6 months of ART in patients with prevalent TB at inclusion, compared with those without TB. Retention in care and mortality during the first 6 months after starting ART and CD4 cell count evolution during this period were secondary outcomes. Human immunodeficiency virus/TB cases were defined as subjects with either bacteriologically confirmed or clinically diagnosed TB at inclusion, or patients who started ATT within 3 months after inclusion (based on either bacteriological or clinical criteria). The 3-month follow-up period for the definition of prevalent TB was used to reduce the risk of misclassification of patients with unrecognized active TB at baseline; subjects with TB presenting during the first 3 months of follow-up were hence considered to represent cases of prevalent TB. All other subjects were defined as HIV-only cases. Participants who received a diagnosis of TB after 3 months of follow-up were considered to represent cases of incident TB, and they were excluded from analysis.
Patients more than 3 months late for a scheduled follow-up visit were considered as lost to follow-up; in case such individuals returned to care, their follow-up was resumed. For blood test results at ART initiation, the results closest in time were chosen, allowing samples collected 6 months before and 1 week after ART initiation; similar criteria were applied for 6-month blood results, allowing samples collected between 3 and 9 months after ART initiation. The time distribution of patient outcomes (mortality and lost to follow-up) was analyzed using Kaplan-Meier curves. Time of follow-up started at ART initiation and ended at 6 months of ART, last study visit before reaching an outcome (death, lost to follow-up, transferred out, or declined further follow-up), or December 31, 2013, whichever came first. Cox proportional hazard models were used to compare HIV/TB with HIV-only, adjusting for gender, age, and CD4 cell counts at ART initiation.
Virological suppression was defined as a viral load <40 copies/mL; a separate analysis for viral load <400 copies/mL was also performed. For sensitivity analysis, patients with missing 6-month viral loads, or not in active care at the 6-month follow-up, were defined as nonvirologically suppressed.
Between groups comparisons of patient characteristics were performed using Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. Variables associated with VS were analyzed by logistic regression. Variables with a P value < .20 were entered in the multivariate analysis. Two-sided hypotheses and tests were used for all statistical inferences. P values < .05 were considered statistically significant. All analyses were performed using SPSS, version 21 (IBM Corp, Armonk, NY).
Ethical Approval
Ethical approval was obtained from the national Research Ethics Review Committee at the Ministry of Science and Technology of Ethiopia and the Regional Ethical Review Board of Lund University, Sweden. All study participants provided written informed consent. An impartial witness confirmed consent received from illiterate study participants.
RESULTS
Patient Characteristics
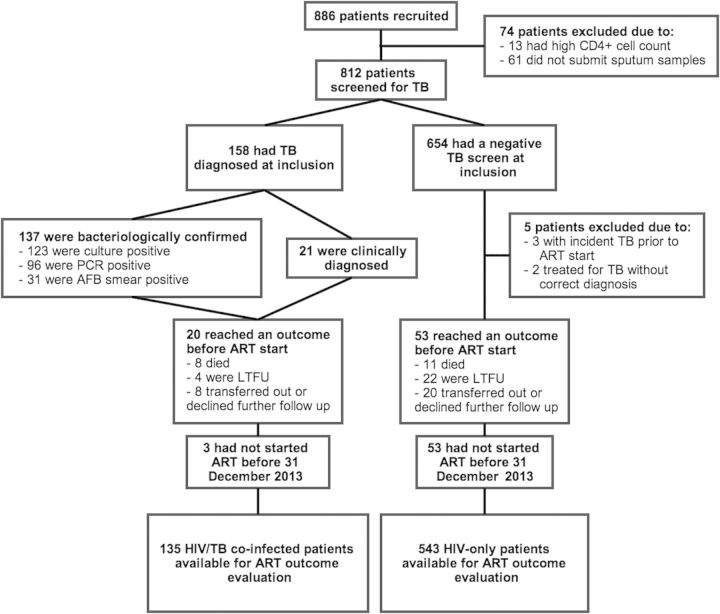
During the inclusion period, 886 patients were screened for eligibility; 812 participants (59% female) completed TB investigations (Figure 1). Characteristics of TB among these patients have been described in detail [3]. Tuberculosis was diagnosed in 158 of these subjects (137 bacteriologically confirmed, 8 clinically diagnosed at inclusion, 13 clinically diagnosed within 3 months of baseline).
Figure 1.
Flowchart of the study participants.
Seventy-three participants discontinued study follow-up after completion of baseline investigations and before having initiated ART (Figure 1). At the time of data abstraction, the proportion of HIV/TB cases that died before starting ART was higher than that among HIV-only (8 of 158 [5.1%] vs 11 of 649 [1.7%]; P = .04). The median time from study inclusion until ATT initiation was 14 days (interquartile range [IQR], 6–41). Three HIV/TB cases who initiated ART did not start ATT during follow-up; 1 died, 1 was transferred out, and 1 started ATT >6 months after ART initiation (due to long delay for TB culture result delivery).
Table 1 shows the characteristics of 678 participants who were included for analysis of ART outcomes. Five participants starting ART were excluded from this analysis (2 subjects initiated ATT due to erroneously reported positive TB results; 3 patients were diagnosed with incident TB before starting ART [Figure 1]). Antiretroviral therapy was initiated at a median of 33 days (IQR, 15–116) after study inclusion, and blood samples were obtained at a median of 25 days (IQR, 8–43) before ART initiation. Most HIV/TB patients were male, in contrast to HIV-only cases. Persons with HIV/TB had lower (1) body mass index (BMI), (2) mid-upper arm circumference (MUAC), and (3) hemoglobin levels. CD4 cell counts showed a trend of being lower among HIV/TB cases, but the distribution of CD4 cell count strata was similar between the groups. Efavirenz was the most commonly used nonnucleoside reverse-transcriptase inhibitor irrespective of TB status. Among HIV/TB patients, 6 of 135 (4%) started ART within 2 weeks, 51 of 135 (38%) started ART between 2 and 8 weeks, and 43 of 135 (32%) started ART after 8 weeks of ATT initiation; ART was initiated before ATT in 35 of 135 (26%).
Table 1.
Characteristics of 678 Participants Initiating ART*
| HIV/TB (n = 135) | HIV Only (n = 543) | P Valuea | |
|---|---|---|---|
| Male sex, n (%) | 74 (54.8) | 209 (38.5) | <.01 |
| Age, years | 34 (28–42) | 32 (28–40) | .25 |
| BMI, kg/m2 | 19 (17–20) | 19 (18–21) | <.01 |
| MUAC, cm | 21.5 (20.0–23.0) | 23.0 (21.0–25.0) | <.01 |
| Karnofsky performance score, n (%) | .14 | ||
| <70 | 6 (4.5) | 24 (4.5) | |
| 70 or 80 | 72 (54.5) | 242 (45.2) | |
| 90 or 100 | 54 (40.9) | 269 (50.3) | |
| On co-trimoxazole at study inclusion, n (%) | 98 (73.1) | 420 (77.6) | .27 |
| Newly enrolled in HIV care, n (%) | 51 (37.8) | 166 (30.7) | .12 |
| CD4 cell count, cells/µLb | 161 (98–243) | 184 (118–256) | .05 |
| CD4 cell strata, n (%), cells/µL | .11 | ||
| <100 | 34 (25.8) | 103 (19.3) | |
| 100–200 | 53 (40.2) | 203 (37.9) | |
| 201–350 | 31 (23.5) | 180 (33.6) | |
| >350 | 14 (10.6) | 49 (9.2) | |
| CD4 cell percentage, % | 11.0 (7.8–15.0) | 11.0 (7.0–15.0) | .91 |
| Hemoglobin, g/dLc | 10.4 (9.2–12.0) | 11.6 (10.5–12.7) | <.01 |
| White blood cell count, ×109 cells/L | 4.7 (3.7–6.2) | 4.2 (3.4–5.4) | <.01 |
| Total lymphocyte count, ×109 cells/L | 1.3 (1.0–1.8) | 1.4 (1.1–1.8) | .30 |
| ART regimen – n (%)d | <.01 | ||
| Nevirapine-based | 10 (7.4) | 113 (20.8) | |
| Efavirenz-based | 125 (92.6) | 430 (79.2) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; MUAC, mid-upper arm circumference; TB, tuberculosis.
* Variables presented as median (IQR), if not stated otherwise. P values of < .05 have been indicated in bold.
a The P values were calculated using Mann-Whitney U or χ2 test, as appropriate.
b CD4 cell counts were available for 667 patients.
c Blood cell counts were available for 639 patients.
d Nucleoside/nucleotide components: lamivudine 100%; stavudine 2%; zidovudine 12%; tenofovir 86%.
ART Outcome in Participants With and Without Concomitant TB
Survival and Retention in Care
During the 6-month follow-up after ART initiation, 25 (3.7%) died, 17 (2.5%) were lost to follow-up, and 75 (11.1%) transferred out or declined further follow-up, leaving 561 (82.7%) patients in active care (Table 2). Kaplan-Meier estimates showed no differences in the time distribution of events (death, lost to follow-up, or both) between HIV/TB cases and HIV-only during ART (Supplementary Figure 1). Unadjusted hazard ratios (HRs) were nonsignificant for death (HR, 1.57; 95% confidence interval [CI], .66–3.77), loss to follow-up (HR, 0.87; 95% CI, .25–3.04), and death and loss to follow-up combined (HR, 1.27; 95% CI, .62–2.58). The HRs remained nonsignificant after adjustment for age, gender, and CD4 cell count at ART initiation (Table 3).
Table 2.
Patient Survival and Retention in Care During 6 Months Follow-up After ART Initiation*
| HIV/TB (n = 135) | HIV Only (n = 543) | P Valuea | |
|---|---|---|---|
| Patient Outcome | .38 | ||
| Active in care | 108 (80.0) | 453 (83.4) | |
| Died | 7 (5.2) | 18 (3.3) | |
| Lost to follow-up | 3 (2.2) | 14 (2.6) | |
| Transferred out | 7 (5.2) | 11 (2.0) | |
| Declined further follow-up | 2 (1.5) | 12 (2.2) | |
| Data abstraction before 6-month follow-up | 8 (5.9) | 35 (6.4) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
* Variables presented as no. (%).
a The P value was calculated using χ2 test.
Table 3.
Hazard Ratios for Mortality, Lost to Follow-up, and Combined Outcome Mortality or Lost to Follow-up Among Patients Initiating ART
| Hazard Ratios HIV/TB Coinfected versus HIV-Only |
||||
|---|---|---|---|---|
| Unadjusted (95% CI) | P Value | Adjusteda (95% CI) | P Value | |
| Died | 1.57 (.66–3.77) | .31 | 1.35 (.53–3.48) | .53 |
| Lost to follow-up | 0.87 (.25–3.04) | .83 | 0.89 (.25–3.15) | .85 |
| Died or lost to follow-up | 1.27 (.62–2.58) | .51 | 1.15 (.54–2.45) | .71 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; TB, tuberculosis.
a Adjusted for age, sex, and CD4 cell count at ART initiation.
Virologic Suppression
Six-month follow-up blood samples were provided a median of 25 weeks (IQR, 24–27) after ART initiation, with available viral load results for 512 of 561 (91.3%) patients. The presence of TB coinfection showed no association with the rate of VS in uni- or multivariate analysis (Supplementary Table 1). In total, 369 of 512 (72.1%) achieved a viral load <40 copies/mL; 65 of 92 (70.7%) and 304 of 420 (72.4%) for HIV/TB cases and HIV-only, respectively (P = .74; Table 4). Using the higher threshold (<400 copies/mL), 454 of 512 (88.7%) achieved VS; 77 of 92 (83.7%) and 377 of 420 (89.8%) for HIV/TB cases and HIV-only, respectively (P = .10).
Table 4.
Viral Suppression and CD4 Cell Distribution at 6 Months After ART Initiation*
| HIV/TB (n = 108) | HIV-Only (n = 453) | P Valuea | |
|---|---|---|---|
| Viral load, n (%), copies/mLb | |||
| <40 | 65 (70.7) | 304 (72.4) | .74 |
| <400 | 77 (83.7) | 377 (89.8) | .10 |
| CD4 cell count, cells/µLc | 247 (190–349) | 300 (205–393) | .04 |
| CD4 cell count, n (%), cells/µL | .29 | ||
| <100 | 6 (6.5) | 27 (6.8) | |
| 100–200 | 20 (21.7) | 67 (16.8) | |
| 201–350 | 43 (46.7) | 165 (41.5) | |
| >350 | 23 (25.0) | 139 (34.9) | |
| CD4 cell percentage, % (IQR) | 15 (12–22) | 17 (12–22) | .27 |
| CD4 cell increase, cells/µLd | 87 (26–178) | 103 (38–173) | .49 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
* Variables presented as median (IQR), if not stated otherwise. P values of < .05 have been indicated in bold.
a The P values were calculated using Mann-Whitney U or χ2 test, as appropriate.
b Viral loads were available for 512 patients.
c CD4 cell counts were available for 490 patients.
d CD4 cell increase data were available for 488 patients.
In univariate analysis, using either <40 copies/mL or <400 copies/mL as definition for VS, female gender, higher BMI, and MUAC, as well as higher CD4 cell count and percentage at ART initiation were associated with higher odds of viral suppression. In multivariate analysis, female gender retained significant association with VS (P < .01) for viral load <40 copies/mL, in addition to higher MUAC for viral load <400 copies/mL (Supplementary Table 1). In the sensitivity analysis, defining patients with missing 6-month viral loads or not in active care at the 6-month follow-up as nonvirologically suppressed, the proportion achieving VS (<40 copies/mL) was 369 of 641 (57.6%); 65 of 128 (50.8%) and 304 of 513 (59.3%) for HIV/TB cases and HIV-only, respectively (P = .08). In multivariate analysis, female gender remained significantly associated with VS (Supplementary Table 2).
CD4 Cell Evolution
At baseline, median CD4 cell counts were slightly lower in HIV/TB-coinfected subjects (Table 1). This difference remained and was statistically significant at 6 months: median CD4 cell counts 247 and 300 cells/μL (P = .04) for HIV/TB and HIV-only, respectively (Table 4). However, the median increase of CD4 cell counts during ART did not show significant difference with regard to TB status: median CD4 cell counts increase 87 and 103 cells/μL (P = .49) for HIV/TB and HIV-only, respectively.
DISCUSSION
In this prospective cohort study performed at Ethiopian health centers, no difference in the rates of VS at 6 months after ART initiation was detected with regard to the presence of concomitant TB and ATT. Furthermore, TB coinfection did not affect rates of mortality and retention in care during ART. This finding provides support for current recommendations to initiate ART during the course of ATT for subjects with HIV/TB coinfection, and it demonstrates the feasibility of this strategy even at primary healthcare level in resource-limited settings.
Our results on ART outcome in HIV/TB-coinfected subjects are in agreement with a recent review [18]. Viral suppression (using the definition of viral load <400 copies/mL) was achieved by 89% of participants, with no difference with regard to TB coinfection. There was no difference between the 2 groups when using a stricter definition of VS (<40 copies/mL), although suppression was less pronounced (72%). In multivariate analysis, male gender was the only variable found to be associated with a lower likelihood of achieving VS.
The rates of VS found in this cohort are comparable with those reported elsewhere [13, 16, 24]. However, most previous studies on the outcome of ART in TB patients have been based in hospitals or specialized ART clinics. Our study provides confirmatory data from an Ethiopian primary healthcare setting, typical for low-income countries in sub-Saharan Africa where great proportions of people living with HIV and TB are managed.
For comparison of VS rates, we included subjects for whom a viral load result obtained at 6 months after ART initiation was available. In an attempt to assess whether retention in care or availability of samples for viral load testing could have affected our results, we performed a sensitivity analysis, defining patients with missing viral load results, or not in active care at the 6-month follow-up, as non-VS; yet, TB coinfection remained nonsignificantly associated with VS (P = .08).
Despite having a higher frequency of negative prognostic characteristics (such as lower BMI, MUAC, and hemoglobin levels), HIV/TB cases did not manifest increased mortality during ART. Furthermore, a greater proportion of these persons were of male gender, which has also been associated with higher mortality [25]. Although some studies have reported an increased risk of death in TB-coinfected subjects starting ART [5], a recent meta-analysis did not identify any such difference for short-term mortality [26]; however, the risk of death was elevated at 1 year after ART initiation in HIV/TB-coinfected patients. For the current analysis, we restricted follow-up to 6 months after ART, because we mainly wished to exclude a potential negative impact of concomitant ATT on ART outcome. The observation that long-term outcome may be compromised in TB-coinfected patients suggests that other factors are involved [27, 28]; such as delayed immune recovery among patients with HIV/TB coinfection [29, 30]. In our cohort, TB cases tended to have lower CD4 cell levels than subjects without TB at inclusion. Despite similar increases in CD4 cell counts, HIV/TB-coinfected persons had significantly lower median CD4 cell counts after 6 months of ART, with >25% having levels <200 cells/μL.
Our study participants consisted of HIV-positive adults who fulfilled criteria for starting ART; at inclusion, all patients underwent microbiological investigation for TB. These investigations led to detection of active TB in 137 patients (16.9%), among whom only 13 had been diagnosed with TB previously [3]. It is likely that this active case-finding approach contributed to satisfactory ART outcomes. Incident TB has been associated with poor ART outcome [31], and by screening all patients several cases were probably detected at less advanced stages of TB disease. Yet, 5.1% of HIV/TB-coinfected patients died before ART initiation compared with 1.7% among those with negative TB screening. This result illustrates the need for early ART initiation during ATT, especially for individuals with advanced immunosuppression [8–10].
Our study has some limitations. It was conducted in a limited uptake area; however, we consider the participating health centers to be representative of primary healthcare in sub-Saharan Africa, and except for the intensified TB screening at study inclusion, no special interventions were introduced that might have improved treatment outcomes. We did not analyze adherence data or rates of adverse events (including IRIS) in the 2 groups. Furthermore, we did not analyze viral load at baseline. Tuberculosis coinfection is associated with significantly increased HIV replication [32], yet rates of viral suppression at 6 months of ART were similar in subjects with and without TB, suggesting that this factor has not obscured a potential disadvantage in coinfected patients. Despite the use of intensified TB case-finding at baseline, it is possible that some cases of extrapulmonary TB were missed, which could have led to misclassification. To reduce this risk, we used a follow-up period of 3 months after inclusion for the definition of prevalent TB. However, the occurrence of TB after inclusion was low.
In conclusion, rates of VS were high; however, no significant difference was found with regard to the presence of concomitant TB in these HIV-positive adults receiving care at Ethiopian health centers managed by non-physician clinicians. These findings demonstrate the feasibility of combined ART and ATT at primary healthcare level in low-income countries.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Notes
Acknowledgments. We extend our gratitude to the patients who participated in the study as well as to the staff members at the health centers and the Adama Regional laboratory for their work with this study. We also acknowledge our data management team led by Gadissa Merga, who contributed greatly to this study. We are also grateful for the excellent collaboration with the Oromia Regional Health Bureau.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Swedish Civil Contingency Agency, the Swedish International Development Cooperation Agency, the Swedish Medical Society, and the Crafoord Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Geneva: World Health Organization; 2013. Global tuberculosis report 2013. [Google Scholar]
- 2.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balcha TT, Sturegård E, Winqvist N, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLoS One. 2014;9:e85478. doi: 10.1371/journal.pone.0085478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siika AM, Yiannoutsos CT, Wools-Kaloustian KK, et al. Active tuberculosis is associated with worse clinical outcomes in HIV-infected African patients on antiretroviral therapy. PLoS One. 2013;8:e53022. doi: 10.1371/journal.pone.0053022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Kranzer K, Edwards DJ, et al Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24:1323–8. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong EB, Omar T, Setlhako GJ, et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLoS One. 2012;7:e47542. doi: 10.1371/journal.pone.0047542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–81. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) Stop TB Partnership. Geneva: World Health Organization (WHO) Stop TB Partnership; 2012. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. [PubMed] [Google Scholar]
- 12.Cohen K, Meintjes G. Management of individuals requiring antiretroviral therapy and TB treatment. Curr Opin HIV AIDS. 2010;5:61–9. doi: 10.1097/COH.0b013e3283339309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shipton LK, Wester CW, Stock S, et al. Safety and efficacy of nevirapine- and efavirenz-based antiretroviral treatment in adults treated for TB-HIV co-infection in Botswana. Int J Tuberc Lung Dis. 2009;13:360–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Delaugerre C, Gallien S, Flandre P, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One. 2012;7:e36673. doi: 10.1371/journal.pone.0036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–9. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 16.Breen RA, Miller RF, Gorsuch T, et al. Virological response to highly active antiretroviral therapy is unaffected by antituberculosis therapy. J Infect Dis. 2006;193:1437–40. doi: 10.1086/503437. [DOI] [PubMed] [Google Scholar]
- 17.Hung CC, Chen MY, Hsiao CF, et al. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–22. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 18.Soeters HM, Napravnik S, Patel MR, et al. The effect of tuberculosis treatment on virologic and CD4+ cell count response to combination antiretroviral therapy: a systematic review. AIDS. 2014;28:245–55. doi: 10.1097/01.aids.0000434936.57880.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen R, Lynch S, Bygrave H, et al. Antiretroviral treatment outcomes from a nurse-driven, community-supported HIV/AIDS treatment programme in rural Lesotho: observational cohort assessment at two years. J Int AIDS Soc. 2009;12:23. doi: 10.1186/1758-2652-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–98. doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Federal Ministry of Health. Addis Ababa: Federal Ministry of Health; 2006. AIDS in Ethiopia. Available at: http://www.etharc.org/aidsineth/publications/aidsineth6th_en.pdf . Accessed 13 June 2014. [Google Scholar]
- 22.Ministry of Health of Ethiopia. Addis Ababa: Ministry of Health of Ethiopia; 2008. Guidelines for Management of Opportunistic Infections and Antiretroviral Treatment in Adolescents and Adults in Ethiopia. Available at: http://www.who.int/hiv/pub/guidelines/ethiopia_art.pdf . Accessed 13 June 2014. [Google Scholar]
- 23.Federal Ministry of Health. Addis Ababa: Federal Ministry of Health; 2012. Guidelines for clinical and programmatic management of TB, leprosy and TB/HIV in Ethiopia. Available at: http://www.etharc.org/ . Accessed 13 June 2014. [Google Scholar]
- 24.Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Nadkarni G, Yang WT, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One. 2011;6:e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soeters HM, Poole C, Patel MR, Van Rie A. The effect of tuberculosis treatment at combination antiretroviral therapy initiation on subsequent mortality: a systematic review and meta-analysis. PLoS One. 2013;8:e78073. doi: 10.1371/journal.pone.0078073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deribew A, Tesfaye M, Hailmichael Y, et al. Common mental disorders in TB/HIV co-infected patients in Ethiopia. BMC Infect Dis. 2010;10:201. doi: 10.1186/1471-2334-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datiko DG, Lindtjørn B. Mortality in successfully treated tuberculosis patients in southern Ethiopia: retrospective follow-up study. Int J Tuberc Lung Dis. 2010;14:866–71. [PubMed] [Google Scholar]
- 29.Skogmar S, Schön T, Balcha TT, et al. CD4 cell levels during treatment for tuberculosis (TB) in Ethiopian adults and clinical markers associated with CD4 lymphocytopenia. PLoS One. 2013;8:e83270. doi: 10.1371/journal.pone.0083270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cingolani A, Cozzi Lepri A, Castagna A, et al. Impaired CD4 T-cell count response to combined antiretroviral therapy in antiretroviral-naive HIV-infected patients presenting with tuberculosis as AIDS-defining condition. Clin Infect Dis. 2012;54:853–61. doi: 10.1093/cid/cir900. [DOI] [PubMed] [Google Scholar]
- 31.Gupta-Wright A, Wood R, Bekker L, Lawn SD. Temporal association between incident tuberculosis and poor virological outcomes in a South African antiretroviral treatment service. J Acquir Immune Defic Syndr. 2013;64:261–70. doi: 10.1097/QAI.0b013e3182a23e9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.