Abstract
Chagas disease continues to cause substantial morbidity and mortality in endemic areas in Latin America. Although there have been some well documented successes in halting the transmission of Chagas disease through preventive interventions to decrease vector-borne and blood-transfusion cases, this parasitic infection continues to be transmitted through these routes in some areas as well through perinatal and foodborne routes. In addition, transmission through solid-organ transplantation has been described in nonendemic settings due to the increasing globalization of Chagas disease to the United States of America, Europe, and other areas. Because there has been a concomitant increase in the number of solid-organ transplantations performed in Latin American settings endemic for American trypanosomiasis, there is increasing concern for the potential reactivation of Trypanosoma cruzi in a previously infected recipient and as a result of aggressive immunosuppression; or via transmission from organs donated by a latently infection donor transplanted onto an uninfected recipient. In this study, we report 2 cases of Chagas disease reactivation in 2 solid-organ transplant recipients in Northeastern Colombia, and we discuss the implications for screening as a crucial strategy for preventing transmission in endemic settings.
Keywords: Chagas disease, Colombia, death, reactivation, transplantation
Chagas disease, also known as American trypanosomiasis, is a zoonotic tropical disease caused by the parasite Trypanosoma cruzi [1, 2]. Millions of inhabitants of rural Latin America have been burdened by Chagas disease [2, 3]. As the trend for global migration increases, Chagas disease is expanding from rural to urban areas and from endemic to nonendemic areas and countries [2, 4]. Due to the globalization of this neglected tropical disease, the scope of Chagas disease is already a public health problem in United States of America, Europe and other regions of the World [5–7].
An emerging route of transmission of T cruzi is via solid-organ transplantation which has been described among patients with previously unidentified latent infection who require aggressive regimens of immunosuppressive drugs with subsequent reactivation [4]. Alternatively, an uninfected recipient may become infected by obtaining an organ donated by a latently infected donor. The biological and epidemiological rationale for this transmission relies on the fact that most infected individuals have a prolonged asymptomatic phase of infection with T cruzi and thus their disease remains latent and undiagnosed before it reactivates (directly from the donated organ, or in a previously infected recipient) [4, 8, 9]. This similar mechanism of transmission has been described for other previously undiagnosed parasitic diseases when the donor is latently infection such as it occurs in toxoplasmosis.
Among transplant recipients, reactivation of T cruzi infection may lead to acute myocarditis, acute meningoencephalitis, and dermatologic manifestations associated to the use of high dose corticosteroids or other immunosuppressive agents used during solid-organ transplantation [4]. Furthermore, many cases of reactivation of Chagas disease can be fatal, even in endemic settings such as Colombia [4, 8, 9]. However, these cases are frequently undiagnosed and not frequently reported [10, 11]. In this report, we present 2 cases of fatal reactivation of T cruzi in a case of a heart and in a case of kidney transplant patients in Santander, Northeastern Colombia. Both cases occurred a few years after transplantation and both were unfortunately diagnosed at the time of necropsy.
CASE DISCUSSION
Case 1
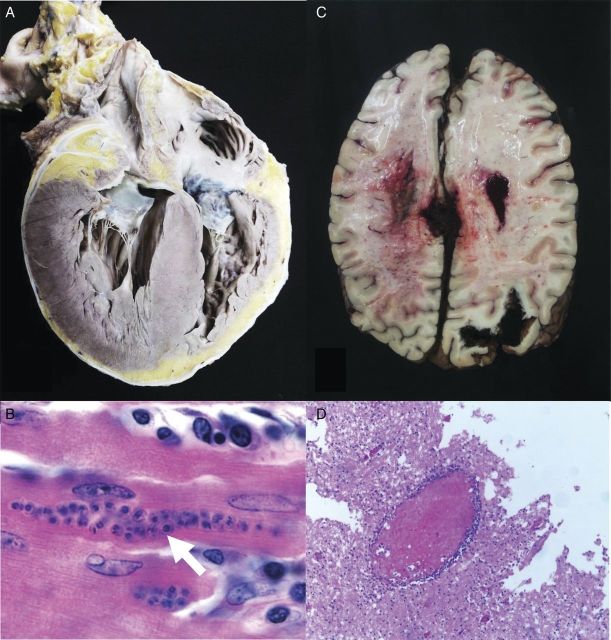
A 59-year-old man from Santander, Northeastern Colombia underwent orthotopic heart transplantation from a cadaveric donor because of end-stage chronic Chagas dilated cardiomyopathy (stage IV from the Brazilian Consensus classification and the classification of the American College of Cardiology/American Heart Association) [12, 13]. He was doing well for ∼7 years when he developed acute onset of asthenia, hyporexia, paraparesis, and dysarthria. He was admitted to our institution for evaluation and management. A contrast-enhanced magnetic resonance imaging demonstrated enhancement of the corpus callosum, periventricular white matter, leukoencephalopathy, and bilateral cerebellar ischemia. The results of a neurological examination revealed absence of pupillary reflexes, oculocephalic reflex, and generalized hypotonia. His condition worsened and required mechanical ventilation. Despite aggressive intensive care support, the patient succumbed after 3 days. Autopsy results revealed brain edema, with necrohemorrhagic findings in left hemisphere and in the brainstem. Marked vascular congestion of the leptomeninges was identified with extensive necrosis at the right hemisphere and the presence of amastigotes associated with perivascular lymphocytic infiltrate. Trypanosoma cruzi DNA was amplified from a tissue sample obtained from the corpus callosum (using the DNA extraction kit from CorpoGen [Bogota, Colombia] and the marker Lambda DNA Hind III [Promega Corporation]). The heart showed dilatation of both right and left ventricles, with concentric left ventricular hypertrophy (Figure 1A), with zones of fibrosis, mononuclear infiltrates with pseudocysts containing amastigotes (Figure 1B). Using the same molecular technique as in brain tissue, T cruzi was also identified in myocardial tissue.
Figure 1.
Pathological findings of the 2 fatal reactivation cases of Chagas disease demonstrating acute myocarditis and acute meningoencephalitis. A, case 1, heart showing dilatation of both right and left ventricles, with concentric left ventricular hypertrophy. B, case 1, myocardium tissue with zones of fibrosis, mononuclear infiltrates with pseudocysts containing amastigotes (arrow) (hematoxylin and eosin stain [H&E] stain, ×600 original magnification). C, case 2, Cerebral edema, necrohemorrhagic destruction associated with Trypanosoma cruzi was found in the left hemisphere. D, case 2, marked congestion of the choroid plexus, with marked congestion of the leptomeninges, demonstrative extensive parenchymal necrosis associated with T cruzi amastigotes, and perivascular lymphocytic infiltrates with pseudocysts (H&E stain, ×400 original magnification).
Case 2
A 40-year-old man, also from rural Santander, Northeastern Colombia, underwent orthotropic renal transplantation from a deceased donor due to end-stage renal disease. He remained well after transplantation and was complaint to his immunosuppressive drug regimen. However, ∼6 years after transplantation, the patient presented with malaise, fever, night sweats, asthenia, diarrhea, and acute kidney injury. He was diagnosed as cytomegalovirus (CMV) reactivation, due to positive anti-CMV immunoglobulin M-antibodies, and was treated with valganciclovir. Despite this intervention, his clinical condition continued to deteriorate over the next 3 days with persistent fever, weight loss, abdominal pain, nausea, and vomiting requiring hospitalization. The patient was admitted to the intensive care unit requiring mechanical ventilation and hemodynamic support with vasopressors. Unfortunately, the patient died within 24 hours after his admission to the intensive care unit. Autopsy revealed an edematous and congested transplanted kidney. Histopathological examination revealed generalized glomerular sclerosis, fibrosis, and interstitial lymphocytic infiltrates. His heart demonstrated dilatation of both right and left ventricles, with concentric left ventricular hypertrophy, areas of fibrosis, and lymphocytic infiltrates with pseudocysts containing amastigotes. It is interesting to note that brain tissue demonstrated necrohemorrhagic findings in the left hemisphere and the presence of amastigotes with associated perivascular lymphocytic infiltrates with pseudocysts containing amastigotes (Figure 1C and D). Molecular amplification of T cruzi DNA was found in brain tissue obtained from this patient (using the DNA extraction kit from CorpoGen and the marker Lambda DNA Hind III from Promega Corporation). In addition, tissue samples from heart and transplanted kidney were also positive for T cruzi.
DISCUSSION
Our cases illustrate the growing epidemiological and clinical concern for T cruzi reactivation through solid-organ transplantation in endemic areas for Chagas disease transmission [7]. We are particularly concerned for this association given the growing number of institutions performing solid-organ and bone marrow transplantations in endemic settings. As it has been described in nonendemic areas, the number of reported cases of Chagas disease associated with transplantation also continues to increase in these areas [4, 11]. Therefore, we believe that our report and others suggest that there is an urgent need for basic intervention to reduce the occurrence of this potential complication associated with transplantation. Screening of recipients and donors seems to be the most feasible intervention. However, logistic issues associated with urgent transplantation and also cost of screening may pose some limitations. Alternatively, treatment of those identified as infected may reduce the risk of reactivation with potential life-threatening consequences [4, 11]. In this regard, 1 of our cases demonstrates that even when a patient had previously received effective antiparasitic treatment, there is still a life-long risk of reactivation or even of potential reinfection. We were unable to make this clinical distinction in our patient, but we do believe that reinfection is feasible because he resided in a highly endemic area and he had previously received antiparasitic treatment.
Similar to what occurs in African trypanosomiasis, where patients who have been treated with melarsoprol may develop acute hemorrhagic leukoencephalopathy, in reactivated Chagas disease, a form of granulomatous or multifocal necrotizing encephalitis with abundant amastigote parasites is found [14]. Finally, if all preventive efforts fail, it is crucial that clinicians who treat patients undergoing solid-organ or even bone marrow transplantation have a low threshold for clinically suspecting reactivation or new infection with T cruzi when patients present with a severe illness resembling severe sepsis or septic shock with negative work up for frequent bacterial causes and residing in endemic areas for Chagas disease [4, 5, 9, 11]. In these cases, molecular diagnosis, peripheral blood smears, tissue sampling for histopathologic analysis, or serologic evaluation may be helpful for establishing the diagnosis of T cruzi infection [4].
We also recommend that these strategies need to be used when treating patients who emigrated from endemic areas and are now living in nonendemic settings before, during, or after solid-organ transplantation.
Notes
Acknowledgments. We thank the critical review of an anonymous colleague.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pinto Dias JC. The treatment of Chagas disease (South American trypanosomiasis) Ann Intern Med. 2006;144:772–4. doi: 10.7326/0003-4819-144-10-200605160-00012. [DOI] [PubMed] [Google Scholar]
- 2.Franco-Paredes C, Von A, Hidron A, et al. Chagas disease: an impediment in achieving the Millennium Development Goals in Latin America. BMC Int Health Hum Rights. 2007;7:7. doi: 10.1186/1472-698X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aufderheide AC, Salo W, Madden M, et al. A 9,000-year record of Chagas’ disease. Proc Natl Acad Sci U S A. 2004;101:2034–9. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco-Paredes C, Jacob JT, Hidron A, et al. Transplantation and tropical infectious diseases. Int J Infect Dis. 2010;14:e189–96. doi: 10.1016/j.ijid.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales AJ, Benitez JA, Tellez I, et al. Chagas disease screening among Latin American immigrants in non-endemic settings. Travel Med Infect Dis. 2008;6:162–3. doi: 10.1016/j.tmaid.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Morales AJ, Silvestre J, Cazorla-Perfetti DJ. Chagas disease in Barcelona, Spain. Acta Trop. 2009;112:86–7. doi: 10.1016/j.actatropica.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Coura JR. Chagas disease: control, elimination and eradication. Is it possible? Mem Inst Oswaldo Cruz. 2013;108:962–7. doi: 10.1590/0074-0276130565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Prevention (CRC) Chagas disease after organ transplantation--United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:210–2. [PubMed] [Google Scholar]
- 9.Centers for Disease Prevention (CRC) Chagas disease after organ transplantation--Los Angeles, California, 2006. MMWR Morb Mortal Wkly Rep. 2006;55:798–800. [PubMed] [Google Scholar]
- 10.Gallerano V, Consigli J, Pereyra S, et al. Chagas’ disease reactivation with skin symptoms in a patient with kidney transplant. Int J Dermatol. 2007;46:607–10. doi: 10.1111/j.1365-4632.2007.03127.x. [DOI] [PubMed] [Google Scholar]
- 11.Jackson Y, Dang T, Schnetzler B, et al. Trypanosoma cruzi fatal reactivation in a heart transplant recipient in Switzerland. J Heart Lung Transplant. 2011;30:484–5. doi: 10.1016/j.healun.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Ministerio da Saude. Secretaria de Vigilancia em S . [Brazilian Consensus on Chagas disease] Revista da Sociedade Brasileira de Medicina Tropical. 2005;38(Suppl 3):7–29. [PubMed] [Google Scholar]
- 13.Acquatella H. Echocardiography in Chagas heart disease. Circulation. 2007;115:1124–31. doi: 10.1161/CIRCULATIONAHA.106.627323. [DOI] [PubMed] [Google Scholar]
- 14.Ellison D, Love S, Chimelli L, et al. A Reference Text of CNS Pathology: Neuropathology. New York: Elsevier; 2013. [Google Scholar]