Abstract
It has been questioned if Candida pneumonia exists as a clinical entity. Only histopathology can establish the definite diagnosis. Less invasive diagnostic strategies lack specificity and have been insufficiently validated. Scarcity of this pathomechanism and nonspecific clinical presentation make validation and the development of a clinical algorithm difficult. In the present study, we analyze whether Candida pneumonia exists in our critical care population. We used a bronchoalveolar lavage (BAL) specimen database that we have built in a structural diagnostic approach to ventilator-associated pneumonia for more than a decade consisting of 832 samples. Microbiological data were linked to clinical information and available autopsy data. We searched for critically ill patients with respiratory failure with no other microbiological or clinical explanation than exclusive presence of Candida species in BAL fluid. Five cases could be identified with Candida as the likely cause of pneumonia.
Keywords: bronchoalveolar lavage, Candida, pneumonia, Zenker's diverticulum
Pneumonia is a frequent diagnosis in patients admitted to the intensive care unit (ICU). In the majority of cases, the pathogenic organism is of bacterial origin. There are known differences in the spectrum of these pathogens, depending on the circumstances at the time the patient acquired pneumonia. When pneumonia is developed at home it is called community-acquired pneumonia (CAP). Community-acquired pneumonia is distinguished from hospital-acquired pneumonia (HAP) because the latter develops when the patient is already admitted to hospital. If the patient lives in a nursing home or comparable institution before admittance, the causative pathogen is more likely within the spectrum of HAP. If pneumonia affects a patient on the respirator it is called ventilator-associated pneumonia (VAP) [1]. The bronchoalveolar lavage (BAL) is a widely accepted diagnostic tool to confirm the diagnosis of pneumonia and to identify the nature of the associated pathogen. BAL is indicated if there is clinical suspicion of pneumonia, ie, a newly developed infiltrate on the chest x-ray, fever, and an increased leukocyte count in the blood. Bronchoalveolar lavage examination showing more than the cutoff value of 2% intracellular organisms (ICO) and BAL culture yielding more than 104 colony-forming units (CFU)/mL is recognized as proof of a bacterial infection [2–4]. There is still debate whether fungal infections play a role in the development of pneumonia. In particular, the role of Candida species remains a diagnostic dilemma. It has been questioned whether Candida pneumonia exists at all. In contrast to the defined culture threshold for bacterial CFU, there is no such standard to distinguish fungal colonization from fungal infections. The question raised in this study is whether we can identify critical ill patients with respiratory failure with Candida growth in BAL specimens as a single possible explanation of pneumonia? To answer that question, we reviewed our database of BAL specimens that was built over more than 10 years and contained 832 BAL samples.
MATERIALS AND METHODS
The study was conducted at the ICU of the University Hospital Maastricht. The ICU is an 18-bed, mixed medical and surgical unit. Patients suspected of pneumonia were included. Clinical criteria were as follows: rectal temperature >38°C or <35.5°C; blood leukocytosis (>10 × 103/mm3) and/or left shift or blood leukopenia (<3 × 103/mm3); more than 10 leukocytes in Gram stain of tracheal aspirate (in high-power field); positive culture of tracheal aspirate; and a new, persistent or progressive infiltrate on chest radiograph. Patients underwent bronchoscopy with BAL conducted by senior fellows or consultant pneumologist. A fiberoptic bronchoscope (Pentax FB-15H/FB-15X; Pentax Medicals, Tokyo, Japan) was introduced and “wedged” into the affected segmental or subsegmental bronchus. Sterile saline (0.9% NaCl, room temperature) was instilled in 4 aliquots of 50 mL, immediately aspirated, and recovered. Bronchoalveolar lavage samples were transported to the laboratory within 15 minutes after collection and processed immediately upon arrival. Bronchoalveolar lavage workup included the following: a total cell count, a differential cell count, microscopic investigation of a Gram-stained preparation, and quantitative bacterial and fungal culture. Depending on clinical suspicion, polymerase chain reaction for viruses and atypical bacteria were included. Bronchoalveolar lavage fluid samples were excluded if (1) the recovered volume was <20 mL, (2) the total cell count was <60 000 cells/mL, (3) more than 1% squamous epithelial cells, or (4) more than 5% bronchial epithelial cells were present [2]. Between January 2000 and December 2010, 832 BAL fluid samples were retrieved from ICU patients and therefore eligible for inclusion. One hundred thirty-one BAL fluid samples were excluded because they fit 1 or more of the exclusion criteria. Therefore, 701 BAL fluid samples were finally included in the present study. Only clinical data collected during the treatment of patients were used in the analysis. No further experimental data were collected. Patients consented to the use of clinical data for scientific analysis according to the admission regulations if they did not opt out. This approach was approved by our local ethics commission.
RESULTS
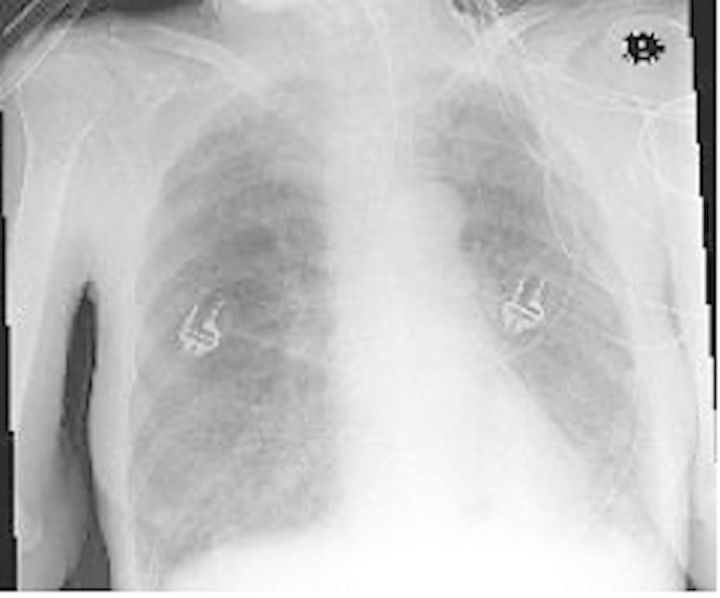
Case 1 patient was an elderly woman who was admitted to hospital in a state of malnourishment. She suffered from loss of appetite, with significant weight loss, diarrhoea, vomiting, and dehydration. At presentation, she had shortness of breath and decreased consciousness. An x-ray examination showed bilateral micronodular infiltrations (Figure 1). Community-acquired pneumonia was suspected. After resuscitation, intubation and ventilation BAL was carried out. Bronchoalveolar lavage analysis revealed 360 000 cells/mL, no squamous epithelium, many leucocytes, but no bacteria on Gram staining. Further analysis showed 6.2% intracellular yeasts and no intracellular bacteria (Figure 2). Differentiation showed 97.4% polymorphonuclear cells, 2.2% lymphocytes, and 0.2% macrophages. Thoracic computed tomography (CT) scanning revealed bullae, interstitial abnormalities with fibrosis, and nodules as well as a cystic mediastinal abnormality (Figure 3). Further diagnostic evaluation by gastroscopy elucidated the presence of a Zenker's diverticulum in the upper respiratory tract. Sputum cultures, BAL, and cultures from the Zenker's diverticulum revealed colonization with a high load of Candida glabrata. We suspect that recurrent silent aspiration of food remnants colonized with C glabrata out of the Zenker's diverticulum finally caused pneumonia. The development of pneumonia was promoted by a reduced immunological defence due to serious malnourishment and cachexia. After surgical treatment of the Zenker's diverticulum and administration of antifungal medication, she recovered from respiratory insufficiency and was eventually discharged from hospital.
Figure 1.
Chest x-ray of case 1 patient showing bilateral micronodular infiltrations.
Figure 2.
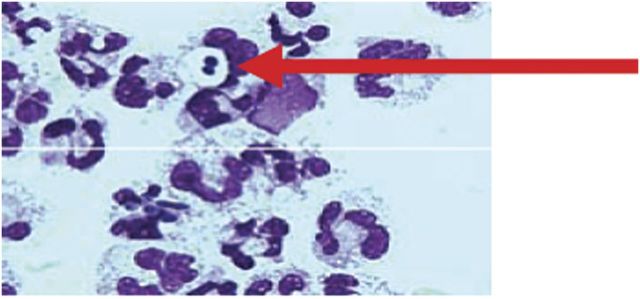
May-Grünwald-Giemsa stain (1000-fold) of case 1 patient bronchoalveolar lavage fluid showing intracellular microorganisms (arrow).
Figure 3.
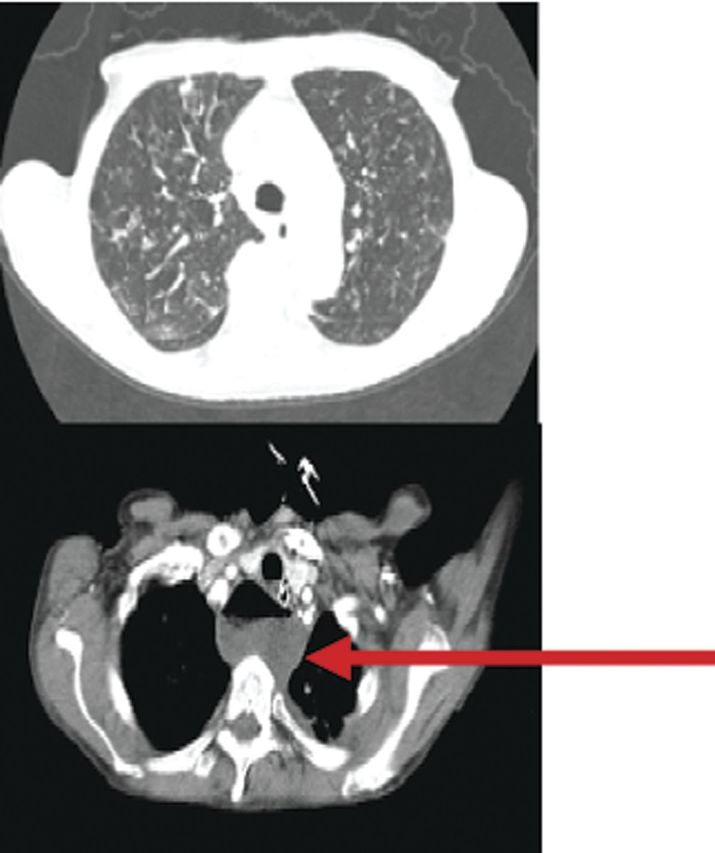
Thoracic computed tomography scan of case 1 patient showing bullae, interstitial abnormalities, fibrosis, and a cystic mediastinal abnormality (arrow).
Case 2 concerns a 55-year-old male patient who was immunocompromised by prednisolone and cyclosporine medication after stem cell transplantation due to myelodysplastic syndrome. The treatment was complicated by graft-versus-host disease. The patient was admitted to ICU in state of general malaise with serious respiratory failure. Chest x-ray revealed infiltrative consolidations in the right lung and pleural effusions. Under suspicion of HAP, a BAL targeting the affected site was done and yielded 55% intracellular yeasts and more than 105 CFU/mL Candida albicans. Despite maximal treatment efforts, the condition of the patient deteriorated and he subsequently died from respiratory failure. We obtained permission for an autopsy. Lung tissue culture yielded Candida species. Therefore, we consider this a proven Candida pneumonia.
Case 3 describes a 64-year-old male patient admitted to hospital with bacterial meningitis. He was treated with ceftriaxone and high-dose dexamethasone. In hospital he developed peritonitis due to perforated diverticulitis. After laparotomy he was admitted to ICU with septicaemia and respiratory failure. Chest x-ray revealed bilateral both upper quadrant infiltrative consolidations. Hospital-acquired pneumonia was suspected. Bronchoalveolar lavage fluid contained a low percentage of intracellular yeast (0.4%) but yielded 4 × 103 CFU/mL C albicans and 5 × 102 CFU/mL C glabrata. Peritoneal fluid and sputum specimen cultures also revealed Candida species. The patient received fluconazole treatment and finally recovered. Specific risk factors that colonization with Candida results in infection were the immunosuppression with dexamethasone and gastrointestinal perforation. We think that the pneumonia in this case was likely caused by Candida species in absence of other microorganisms.
Case 4 refers to a 55-year-old male patient with a history of alcohol abuse. He was treated in the department of neurosurgery for brain metastasis due to adenocarcinoma of unknown origin. The histology was most compatible with lung carcinoma. To reduce oedema, he was treated with dexamethasone after surgery. Later, he developed acute pancreatitis and was admitted to ICU in septic shock with respiratory failure. He was intubated and ventilated. Thorax CT revealed infiltrative consolidations in all quadrants. We performed BAL and demonstrated the presence of 7.4% intracellular yeasts and yielded 5.6 × 105 CFU/mL C albicans. Sputum culture from a tracheal aspirate also contained C albicans. The origin of a Streptococcus mitis found in a blood culture was not evident. Streptococcus mitis are commensal bacteria that colonize hard surfaces in the oral cavity as well as mucous membranes. These Gram-positive bacteria are not usually pathogenic but can cause bacterial endocarditis. There were no signs of endocarditis or central venous line infections in this patient. Despite antimycotic and antibacterial treatment and maximal efforts, the patient subsequently died from respiratory failure. Permission for autopsy could not be obtained. We recognize a number of risk factors in this patient that could explain that Candida colonization probably resulted in pulmonary infection. The patient was immunocompromised due to steroid treatment after neurosurgery. He had an active malignant process, a history of alcohol abuse, and developed an acute pancreatitis with septic shock.
Case 5 concerns a 70-year-old male patient with coronary artery disease who was admitted to hospital with acute myocardial infarction. Before successful intubation and ventilation, he aspirated a significant amount of gastric contents. Subsequently, he developed VAP with bilateral infiltrations on chest x-ray. We performed a BAL and started with broad-spectrum antibiotics. Bronchoalveolar lavage revealed a small amount of intracellular yeasts and yielded C glabrata in culture. The patient finally died from circulatory failure. There was no permission for an autopsy to further confirm pulmonary infection. In absence of other causative microorganisms, Candida species most likely played a role in the development of pneumonia. Although Candida species may have been innocent bystander in an otherwise chemical damage of the lungs by acidic gastric fluid aspiration, we would support the choice for antifungal treatment in a deteriorating clinical situation.
Table 1.
Characteristics of Patients With Presumed Candida Pneumonia, Medical History, and Results of Examinations
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age | 84 | 58 | 74 | 55 | 70 |
| Sex | Female | Male | Male | Male | Male |
| Admission | Respiratory insufficiency, general malaise, watery diarrhea, loss of appetite, vomiting |
General malaise | Bacterial meningitis | Respiratory insufficiency | Myocardial infarction |
| Medical history | Radical abdominal hysterectomy for endometrial carcinoma | Myelodysplastic syndrome, allogenic stem cell transplantation |
Nonactive tuberculosis, urothelial carcinoma |
Resection of brain metastasis, poorly differentiated adenocarcinoma |
Coronary artery disease |
| Complicating factors | Zenker's diverticel, malnourished, nicotine abuse |
Graft-versus-host disease, prednisolone and cyclosporine use |
Perforated diverticulitis, dexamethasone medication |
Necrotizing pancreatitis, dexamethasone medication |
Aspiration |
| Radiology | X-ray: bilateral micronodular infiltrates | X-ray: infiltrate in right lung, pleural effusions | X-ray: bilateral upper quadrant infiltrates | CT thorax: bilateral infiltrative consolidations | X-ray: bilateral infiltrative consolidations |
| Microbiology | BAL: 6.2% intracellular yeasts 8×103 CFU/mL, Candida glabrata Sputum: C glabrata Zenkeŕs diverticel culture: C glabrata Blood: sterile Urine: sterile |
BAL: 55% intracellular yeasts >105 CFU/mL Candida albicans Sputum: C albicans, Citrobacter freundii Blood: sterile Urine: sterile Central venous line: sterile |
BAL: 0.4% intracellular yeasts 4×103 CFU/mL, C albicans 5 × 102 CFU/mL, C glabrata Sputum: C albicans Blood: sterile Urine: sterile Peritoneal fluid: C albicans, C glabrata Liquor PCR: Neisseria meningitidis |
BAL: 7.4% intracellular yeasts 5.6 × 104 CFU/mL, C albicans Sputum: C albicans Blood: Streptococcus mitis Urine: sterile Abdominal fluid: sterile Central venous line: sterile |
BAL: 0.6% intracellular yeasts 104 CFU/mL, C glabrata Sputum: pharyngeal flora Blood: sterile Urine: sterile |
| Antibiotics | Moxifloxacin, fluconazole |
Piperacillin/tazobactam amphotericin B |
Ceftriaxone, fluconazole |
Levofloxacin amoxicillin/clavulanic acid fluconazol |
Amoxicillin/clavulanic acid |
| Autopsy | None, patient survived | Lung tissue: Candida species, Enterobacter sakazakii | None, patient survived | Not permitted | Not permitted |
Abbreviations: BAL, bronchoalveolar lavage; CT, computed tomography; PCR, polymerase chain reaction.
DISCUSSION
After reviewing all 701 included BAL specimens and corresponding clinical cases, we were able to identify only 5 cases (0.7%) of presumed Candida pneumonia. In case 1, Zenker's diverticulum represented a source of substantial fungal growth and presumably microaspirations. In state of chronic malnourishment, the elderly patient developed pneumonia. Diagnostic specimens all yielded fungi, and the patient responded well to surgical removal of the source of infection and additional antimycotic therapy. Case 2 patient's specific risk factor was serious immunosuppression by hematological malignancy and subsequent treatment. Diagnostic specimens resulted in a high number of ICO and significant fungal growth. After death, the diagnosis of Candida pneumonia could be established by autopsy. In case 3, the patient was also immunocompromised by steroid treatment. There was a substantial growth of fungi in BAL fluid and peritoneal fluid. In absence of other pathogens, pneumonia was presumably caused by fungi. Case 4 patient was also immunocompromised by steroid treatment and malignancy. Bronchoalveolar lavage fluid analysis revealed a substantial number of ICO and fungal growth. Case 5 patient had a witnessed aspiration. Later, there was substantial growth of fungi in BAL fluid. Although the signs of pneumonia could be caused by chemical damage after aspiration, the treatment with antimycotics can probably be justified in a clinically life-threatening situation.
Candida species are frequently found in tracheal aspirate specimens even in healthy patients. After 48 hours of intubation and ventilation, up to 20% of patients are colonized with Candida species at the tracheobronchial site. This proportion increases with the duration of ventilation [5]. Wood et al [6] analyzed BAL cultures from critically ill patients over 3 years. They found 8% positivity for Candida. Nine-seven percent of positive findings were thought to be colonization or inconclusive and only 3% were classified as VAP by the treating physician. Even though antifungal treatment was not initiated, no patient developed systemic candidiasis. Candida risk score was conceptualized and validated to identify patients with a high risk to develop invasive candidiasis. In a multicenter approach, enough patients with invasive candidiasis could be included to develop a clinical score that consists of the components severe sepsis, septic shock, total parenteral nutrition, surgery, and multifocal Candida colonization. In patients with a score >3, the risk of developing invasive candidiasis was significantly increased. Those patients would benefit from antifungal therapy [7, 8]. Candida pneumonia is an even scarcer pathomechanism. This finding makes the development of a comparable diagnostic algorithm difficult. Treatment of all patients with BAL fluid analysis positive for Candida would result in excessive use of antifungal agents with risk of rapid development of drug resistance. Sheer presence of Candida in the BAL obviously does not prove a pathogenetic role of this microorganism in the development of pneumonia. Kontoyiannis et al [9] found in their study of cancer patients a poor association among tracheal aspiration specimens, BAL specimens, and autopsy studies for fungal infections. The radiological morphology of Candida lesions is diverse. Bronchopneumonia, abscesses, granulomas, and intracavitary membranous exudates have all been described as x-ray findings [10]. Moreover, there is no specific appearance on high-resolution chest tomography. Random nodules are commonly seen in patients with candidiasis. The real incidence of Candida pneumonia is thus notoriously difficult to determine. The most reliable method would be lung histology and proof of an association between Candida lung invasion and local inflammation. Needle biopsy of a suspected lesion is a diagnostic option only if the infiltrate is safely accessible. The patient's clinical condition, high oxygen dependency, and thrombocytopenia, which are commonly present, all exclude the possibility of pulmonary biopsies. For this reason, there have been no studies so far to validate biopsy strategies to prove Candida pulmonary infections. Therefore, most reports on Candida pneumonia are based on isolation of Candida from sputum aspirates or BAL in the absence of other causative pathogens. A few autopsy studies in cancer patients claim to have identified Candida species as causing pathogens in pneumonia. Wakayama et al [11] found 9 cases of Candida pneumonia in a series of 149 autopsies in patients who had undergone hematopoetic stem cell transplantation. Haron et al [12] reviewed fatal cancer patients from a period of 20 years and could only find 55 cases with unequivocal evidence of primary candidiasis. el-Ebiary et al [13] undertook a small prospective study to assess the incidence and significance of isolation of Candida species from various diagnostic sites in critically ill, ventilated, and nonneutropenic patients. Quantitative cultures from tracheal aspirate and BAL could not discriminate presence from absence of Candida pneumonia established by autopsy findings. The general incidence of Candida in biopsy findings was found to be as high as 40%, but the definite incidence of Candida-associated pneumonia was found to be only 8% [13]. However, an elaborately designed study by Meersseman et al [14] provided no evidence for the existence for such clinical entity at all. The group was able to perform autopsies in a large number of patients who died in ICU. The routine tracheal surveillance cultures were used to classify the patients into 1 group having Candida species in their respiratory tract and 1 group who had not. In the post-mortal examination, the presence of Candida pneumonia was established using histological criteria that included pseudohyphae and budding yeasts in an area with various signs of acute inflammation. Cases of Candida pneumonia could neither be found in 232 autopsied patients nor in 77 patients with pre-mortem positive tracheal aspirates. Taking into account the high number of patients colonized with Candida species in the tracheal tract, it seems a convincing conclusion that colonization alone does not lead to pulmonary infection [14]. We concede, by the present data, that it is convincingly proven that Candida species are at most a very rare cause of pneumonia. However, do these findings rule out the existence of Candida pneumonia as a clinical entity at all? The next question would be whether these Candida species colonizing the tracheobronchial tree are merely innocent bystander? It has recently been shown that mechanically ventilated patients colonized with Candida species were more at risk to develop Pseudomonas aeruginosa VAP. Those patients who received antifungal treatment had a reduction in P aeruginosa VAP. Furthermore, it could be demonstrated that colonization with Candida species is an independent risk factor for an increased morbidity and mortality in ICU patients. However, it could not be established convincingly whether colonization with Candida had a causative role or was merely a marker for poor outcome [15–17].
We hypothesize that under certain clinical circumstances, Candida pneumonia can indeed affect patients. However, the clinical presentation is neither specific nor characterized by the presence of sepsis and marked respiratory insufficiency. The presumed route of infection could be either a primary infection that occurs after oropharyngeal aspiration or a secondary hematogenous spread to the lungs in candidiasis. In a primary Candida pneumonia, concomitant Candida esophagitis and colonization of the upper respiratory tract can be frequently found. The presumed mechanism of pulmonary infection is aspiration of esophagopharyngeal contents. It is supposed that under certain circumstances, Candida organisms reach and invade distal air spaces. In this way, there is spread in the airspaces but no vascular invasion. In secondary pulmonary Candida infection, the inoculation with the microorganisms takes place by spread in the bloodstream from any distant site. This can be dissemination from the skin, translocation from the gastrointestinal tract, or spread from extensive mucositis. In this case, vascular invasion including small arteriols and capillaries around the pulmonary tissue can be found. Taking into account the scarcity of Candida pneumonia, a certain special clinical condition is needed to develop it. Diabetes mellitus has been associated with 20% increase in colonization by Candida species. Nicotine and alcohol abuse as well as use of steroids and prior antibiotic use have been linked to increased colonization with Candida species at various anatomical sites [18, 19]. An increasing number of immunocompromised patients due to immunosuppressive therapy (transplant recipients, immunomodulation in rheumatoid arthritis), malignancies, and infections (human immunodeficiency virus, granulomatous diseases) are treated in the ICU. Klapholz et al [20] described a patient with acquired immune deficiency syndrome who developed Candida pneumonia secondary to a tracheobronchial fistula.
CONCLUSIONS
In our institution, BAL has been used as a routine diagnostic tool for more than 10 years. By reviewing all 701 included BAL specimens and subsequently linking it to the clinical cases, we could identify 5 patients (0.7%) with Candida pneumonia. We concede that Candida pneumonia is a rare entity. However, we have gathered evidence that the condition can occur under certain clinical circumstances: (1) immunosuppression by cancer, sepsis, drugs, and malnutrition; (2) risk factors for increased Candida load as diabetes mellitus, nicotine and alcohol abuse, aspiration of gastric fluids, and diverticulum of the esophagus; and (3) broad-spectrum antibiotic treatment. Specific cutoff values for fungi in BALF are lacking. We emphasize that we do not want to promote overuse of antimycotic agents. We strongly believe in a meticulous diagnostic approach in pneumonia and tailored use of antimicrobial drugs. On the other hand, we should not withhold an effective treatment if the evidence is strong by declaring a clinical entity as nonexistent. In cases of serious respiratory failure, radiographic and laboratory evidence for pneumonia, and no other growth than a Candida in the BAL fluid, Candida pneumonia should be considered and subsequently treated.
Notes
Author Contributions. All authors made substantial contributions to conception of the study, acquisitions, analysis and interpretation of data. C. Linssen provided the microbiological test results. R. Schnabel wrote the manuscript and all authors revised and approved the version to be published.
Potential conflicts of interest. R. M. S. received a research grant from the Dutch association of intensive care medicine in cooperation with MSD company for work on noninvasive diagnosis of pneumonia.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Torres A, Ewig S, Lode H, et al. Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med. 2009;35:9–29. doi: 10.1007/s00134-008-1336-9. [DOI] [PubMed] [Google Scholar]
- 2.Linssen CF, Bekers O, Drent M, et al. C-reactive protein and procalcitonin concentrations in bronchoalveolar lavage fluid as a predictor of ventilator-associated pneumonia. Ann Clin Biochem. 2008;45(Pt 3):293–8. doi: 10.1258/acb.2007.007133. [DOI] [PubMed] [Google Scholar]
- 3.Baselski V. Microbiologic diagnosis of ventilator-associated pneumonia. Infect Dis Clin North Am. 1993;7:331–57. [PubMed] [Google Scholar]
- 4.Allaouchiche B, Jaumain H, Dumontet C, et al. Early diagnosis of ventilator-associated pneumonia. Is it possible to define a cutoff value of infected cells in BAL fluid? Chest. 1996;110:1558–65. doi: 10.1378/chest.110.6.1558. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay E, Timsit JF, Tafflet M, et al. Candida colonization of the respiratory tract and subsequent Pseudomonas ventilator-associated pneumonia. Chest. 2006;129:110–7. doi: 10.1378/chest.129.1.110. [DOI] [PubMed] [Google Scholar]
- 6.Wood GC, Mueller EW, Croce MA, et al. Candida sp. isolated from bronchoalveolar lavage: clinical significance in critically ill trauma patients. Intensive Care Med. 2006;32:599–603. doi: 10.1007/s00134-005-0065-6. [DOI] [PubMed] [Google Scholar]
- 7.Leon C, Ruiz-Santana S, Saavedra P, et al. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37:1624–33. doi: 10.1097/CCM.0b013e31819daa14. [DOI] [PubMed] [Google Scholar]
- 8.Leroy G, Lambiotte F, Thevenin D, et al. Evaluation of “Candida score” in critically ill patients: a prospective, multicenter, observational, cohort study. Ann Intensive Care. 2011;1:50. doi: 10.1186/2110-5820-1-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kontoyiannis DP, Reddy BT, Torres HA, et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis. 2002;34:400–3. doi: 10.1086/338404. [DOI] [PubMed] [Google Scholar]
- 10.Masur H, Rosen PP, Armstrong D. Pulmonary disease caused by Candida species. Am J Med. 1977;63:914–25. doi: 10.1016/0002-9343(77)90546-0. [DOI] [PubMed] [Google Scholar]
- 11.Wakayama M, Shibuya K, Ando T, et al. Deep-seated mycosis as a complication in bone marrow transplantation patients. Mycoses. 2002;45:146–51. doi: 10.1046/j.1439-0507.2002.00753.x. [DOI] [PubMed] [Google Scholar]
- 12.Haron E, Vartivarian S, Anaissie E, et al. Primary Candida pneumonia. Experience at a large cancer center and review of the literature. Medicine (Baltimore) 1993;72:137–42. [PubMed] [Google Scholar]
- 13.el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2 Pt 1):583–90. doi: 10.1164/ajrccm.156.2.9612023. [DOI] [PubMed] [Google Scholar]
- 14.Meersseman W, Lagrou K, Spriet I, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med. 2009;35:1526–31. doi: 10.1007/s00134-009-1482-8. [DOI] [PubMed] [Google Scholar]
- 15.Nseir S, Jozefowicz E, Cavestri B, et al. Impact of antifungal treatment on Candida-Pseudomonas interaction: a preliminary retrospective case-control study. Intensive Care Med. 2007;33:137–42. doi: 10.1007/s00134-006-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nseir S, Ader F. Pseudomonas aeruginosa and Candida albicans: do they really need to stick together? Crit Care Med. 2009;37:1164–6. doi: 10.1097/CCM.0b013e3181987b13. [DOI] [PubMed] [Google Scholar]
- 17.Delisle MS, Williamson DR, Perreault MM, et al. The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J Crit Care. 2008;23:11–7. doi: 10.1016/j.jcrc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Tapper-Jones LM, Aldred MJ, Walker DM, et al. Candidal infections and populations of Candida albicans in mouths of diabetics. J Clin Pathol. 1981;34:706–11. doi: 10.1136/jcp.34.7.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasqualotto AC. Candida and the paediatric lung. Paediatr Respir Rev. 2009;10:186–91. doi: 10.1016/j.prrv.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Klapholz A, Wasser L, Stein S, et al. Candida pneumonia secondary to an acquired tracheoesophageal fistula in a patient with AIDS. N Y State J Med. 1988;88:279–80. [PubMed] [Google Scholar]