Abstract
Background.
Endothelial activation and dysfunction play a central role in the pathogenesis of sepsis and viral hemorrhagic fevers. Hantaviral disease is a viral hemorrhagic fever and is characterized by capillary dysfunction, although the underlying mechanisms for hantaviral disease are not fully elucidated.
Methods.
The temporal course of endothelial activation and repair were analyzed during Puumala hantavirus infection and associated with disease outcome and a marker for hypoxia, insulin-like growth factor binding protein 1 (IGFBP-1). The following endothelial activation markers were studied: endothelial glycocalyx degradation (syndecan-1) and leukocyte adhesion molecules (soluble vascular cellular adhesion molecule 1, intercellular adhesion molecule 1, and endothelial selectin). Cytokines associated with vascular repair were also analyzed (vascular endothelial growth factor, erythropoietin, angiopoietin, and stromal cell-derived factor 1).
Results.
Most of the markers we studied were highest during the earliest phase of hantaviral disease and associated with clinical and laboratory surrogate markers for disease outcome. In particular, the marker for glycocalyx degradation, syndecan-1, was significantly associated with levels of thrombocytes, albumin, IGFBP-1, decreased blood pressure, and disease severity.
Conclusions.
Hantaviral disease outcome was associated with endothelial dysfunction. Consequently, the endothelium warrants further investigation when designing future medical interventions.
Keywords: endothelial activation, endothelial surface layer, endothelium, hantavirus, hemorrhagic fever with renal syndrome, glycocalyx, Puumala virus, vasculogenesis/angiogenesis
Endothelial involvement has been implicated in sepsis [1] and viral hemorrhagic fevers [2]. Hantaviral disease belongs to the category of viral hemorrhagic fevers and has been divided into hemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS) [2]. Hemorrhagic fever with renal syndrome in Europe is caused by Puumala virus (PUUV), among others. Capillary leakage seems to be central to hantaviral disease pathogenesis, although the exact underlying mechanisms are not fully elucidated. Hantaviruses target endothelial cells lining the microvasculature, although without any observable cytopathic effect [3–5]. Capillary leakage during hantaviral disease could be caused by released soluble mediators from, or direct interaction with, immune cells [6, 7], direct hantaviral endothelial cell infection [8, 9], or most likely a combination of both.
The endothelium is covered by the endothelial surface layer, which constitutes the first barrier to fluid exudation, and endothelial junctions form the second barrier [10]. The endothelial surface layer consists of the endothelial glycocalyx with adsorbed plasma proteins. This layer plays an important role in vascular homeostasis by inhibiting hemostasis, leukocyte and platelet adhesion, and regulating vascular permeability and tone [11–13]. The endothelial glycocalyx consists of core proteoglycans, such as transmembrane proteoglycan syndecan-1, to which glycosaminoglycans are attached [11, 13]. Endothelial glycocalyx degradation is one of the earliest signs of endothelial activation, and syndecan-1 is a recognized marker of glycocalyx degradation [14–16]. The endothelial glycocalyx degradation is followed by up-regulation and shedding of endothelial adhesion molecules, such as endothelial selectin (sE-selectin), vascular cell adhesion molecule (VCAM-1), and intercellular adhesion molecules (ICAM-1) [17]. However, endothelial activation need not be synonymous with endothelial dysfunction, which is defined as a state representing a liability to the host [18]. If endothelial activation leads to dysfunction and damage, the endothelium is regenerated by angiogenesis and vasculogenesis (ie, repair), which are dependent on, among others, soluble signaling factors such as vascular endothelial growth factor (VEGF), erythropoietin (EPO), angiopoietin (Ang-2), and stromal cell-derived factor 1 (SDF-1) [19, 20].
We wanted to study the implication of the first barrier consisting of the endothelial glycocalyx in the course of HFRS and relate these findings to disease outcome. Several in vitro studies [8, 9, 21, 22] have shown that the second endothelial barrier, which consists of intercellular junctions, plays a role in disease outcome. However, so far there are few in vitro models for the endothelial glycocalyx; therefore, in vivo studies are required to assess the involvement of the glycocalyx in pathogenesis [23].
We aimed at assessing the impact of endothelial activation and repair during HFRS, by studying the levels of endothelial glycocalyx degradation and leukocyte adhesion molecules as indicators of endothelial activation and cytokines that are implicated in vascular repair. Furthermore, we assessed whether there was a correlation between excessive endothelial activation, ie, dysfunction, and repair with hantaviral disease outcome.
MATERIALS AND METHODS
Patients
Patients (n = 19) were enrolled in this study after HFRS diagnosis from April 2007 to April 2010. The diagnosis was established by clinical manifestations typical of HFRS followed by detection of immunoglobulin (Ig)G and IgM antibodies to PUUV using an immunofluorescence assay. Typical clinical manifestations indicative of HFRS included fever, thrombocytopenia, malaise, and proteinuria. These patients were observed consecutively with regards to symptoms and routine laboratory tests. Blood samples were obtained at first contact with the clinic, followed by sampling approximately twice a week during the acute phase, and ending with a follow-up sample at least 60 days after disease onset. The study was approved by the Regional Ethical Review Board in Umeå. All participants gave oral and written informed consent.
Blood Samples
Peripheral venous blood samples were collected into commercially available vacutainers (Becton Dickinson, Franklin Lakes, NJ) followed by centrifugation and plasma retrieval. The plasma samples were then stored at −80°C until use.
Clinical and Laboratory Routine Analyses of Patients
Samples from patients were assessed according to the clinical routine at an accredited laboratory at the Department of Clinical Chemistry at Umeå University Hospital for thrombocyte counts, d-dimer levels, creatinine, and albumin levels. Furthermore, clinical assessment of patient symptoms was obtained from medical records after inclusion, during acute disease, and during follow-up.
Assays for Endothelial Activation Biomarkers
Plasma samples were analyzed for the concentration levels of the following endothelial activation and repair markers according to the manufacturer's instructions: syndecan-1 (Gen-Probe Diaclone SAS, France); soluble sE-selectin, EPO, and VEGF, and insulin-like growth factor binding protein 1 (IGFBP-1) (Meso Scale Discovery, Gaithersburg, MD); soluble ICAM-1 (sICAM-1), sVCAM-1, Ang-2, and SDF-1 (R&D Systems, Minneapolis, MN).
Disease Grouping of the Patients
The patients were included in the group moderate/severe HFRS based on presence of 2 or more of the following criteria: dialysis, treatment in an intensive care unit, thrombosis-verified radiologically, platelet transfusion, moderate/severe hypotension (systolic blood pressure ≤90 mm Hg and requirement for intravenous fluid administration during hospitalization), and moderate/major hemorrhagic manifestations such as gastrointestinal bleeding, macroscopic hematuria, and epistaxis. If the patient did not display 2 or more of the above criteria, they were placed in the mild disease category [24].
Statistical Analysis
Statistical analyses were performed in SPSS for Windows (version 20; IBM, Armonk, NY, USA). Differences between levels of endothelial activation and repair markers in patient plasma during disease and follow-up were tested using the Wilcoxon signed-rank test. Furthermore, the kinetics of the various markers were analyzed against the follow-up time point using generalized estimating equations (GEEs) assuming an exchangeable correlation structure between repeated measurements within the same patients. The association within the endothelial activation markers and associations between endothelial activation and repair markers were analyzed using GEEs. Potential associations between endothelial activation and repair markers with laboratory and clinical parameters were assessed using GEEs. Possible associations with disease severity were assessed by choosing the maximal level of each investigated marker and associated with disease severity using the Spearman's rank correlation coefficient (two-tailed). The level of significance was set at .05.
RESULTS
Clinical Characteristics of HFRS Patients
Ninteen patients with confirmed HFRS were enrolled in our study. Of these patients, 15 were hospitalized during their disease. There were 2 patients with HFRS who fulfilled criteria for moderate/severe disease scoring, and the remaining 17 patients were categorized as having a mild disease. The main patient demography, clinical characteristics, and laboratory values are shown in Table 1.
Table 1.
The HFRS Study Group Demography, Clinical Symptoms and Laboratory Data
| Characteristics | Patients | Reference Values |
|---|---|---|
| Age (median and IQR) | 62 (53–68) | NA |
| Sex, n female/male (%) | 11/8 (58/42) | NA |
| Hospital care, n (%) | 15 (78.9%) | NA |
| Days of hospital care (median and IQR) | 7 (6–10) | NA |
| Clinical symptoms | ||
| Temperature, maximum (median and IQR) | 39 (38.2–39.7) | NA |
| Headache, n (%) | 17 (89.5) | NA |
| Backache, n (%) | 9 (47%) | NA |
| Nausea/vomiting, n (%) | 14 (73.7) | NA |
| Respiratory symptoms, n (%) | 7 (36.8%) | NA |
| Abdominal pains | 7 (36.8%) | NA |
| Blurred vision | 6 (31.4%) | NA |
| Hemorrhagic manifestations | 5 (26.3%) | NA |
| Laboratory data | ||
| Proteinuria, n (%) | 19 (100%) | 0 |
| Hematuria, n (%) | 17 (89.5%) | 0 |
| Minimum serum albumina, g/L (median and IQR) | 28 (25–31.5) | 36–48 |
| Serum creatinine, median μmol/L (IQR) | 177 (151–265) | 50–100 |
| Creatinine, highest fold differenceb | 2.4 (1.7–3.8) | NA |
| Thrombocyte countc, 109/L | 70 (42–91) | 145–387 |
| Maximum LDH, U/L (median and IQR) | 311.4 (287.4–383.2) | <205.4 |
| Maximum leukocyte, 109/L (mean and SD) | 8.8 (8.2–9.6) | 3.5–8.8 |
| Disease severity: Moderate/severe/mild, n (%) | 2/17 (11/89) | NA |
Abbreviations:HFRS, hemorrhagic fever with renal syndrome; IQR, interquartile range; LDH, lactate dehydrogenase; NA, not applicable; SD, standard deviation.
a Albumin levels were only available for 17 patients.
b The creatinine levels at follow-up at least 60 days after disease onset was set as baseline for each patient, and all other creatinine levels were compared against this value. The value shown is the highest fold difference observed for the patient within 18 days after HFRS disease onset (IQR).
c Thrombocyte count is based on the lowest number of thrombocytes obtained from the patient within 14 days after disease onset. Values are median (IQR).
Endothelial Activation and Repair During HFRS Compared to Follow-up
Paired plasma samples from 19 patients with HFRS were analyzed for markers of endothelial activation (syndecan-1, sE-selectin, sICAM-1, and sVCAM-1) and repair (VEGF, EPO, Ang-2, and SDF-1) during disease (within 18 days after disease onset) and follow-up. All samples tested were significantly higher during HFRS than follow-up (Table 2).
Table 2.
Endothelial Activation and Repair Markers in PUUV-Infected Patients During Disease Compared to Follow-up
| Diseasea |
Follow-upb |
||||
|---|---|---|---|---|---|
| Median | IQRc | Median | IQRc | P Valued | |
| Endothelial Activation | |||||
| Endothelial glycocalyx degradation | |||||
| Syndecan 1 (ng/mL) | 235.5 | 129.4–281.7 | 32.9 | 23.2–50.5 | <.001 |
| Endothelial adhesion molecules | |||||
| sE-selectin (ng/mL) | 24.9 | 19.5–35.5 | 14.4 | 11.2–16.6 | .004 |
| sICAM-1 (ng/mL) | 424 | 341.8–576.7 | 219.9 | 180–263 | <.001 |
| sVCAM-1 (ng/mL) | 2572.5 | 1996.9–3176.3 | 621.7 | 491.3–795.2 | <.001 |
| Endothelial repair | |||||
| VEGF (pg/mL) | 74.4 | 51.6–120.4 | 36.1 | 27.7–46.5 | .002 |
| EPO (mIU/mL) | 11.9 | 6.5–17 | 7.5 | 5.9–8.7 | .005 |
| Angiopoietin-2 (pg/mL) | 473.1 | 369.7–866.9 | 295.6 | 252.5–657.8 | .005 |
| SDF-1e (pg/mL) | 102.9 | 30–135.3 | 30 | 30–30 | .001 |
Abbreviations:Ang-2, angiopoietin; EPO, erythropoietin; IQR, interquartile range; PUUV, Puumala virus; SDF-1, stromal cell-derived factor 1; sE-selectin, endothelial selectin; sICAM-1, soluble intercellular adhesion molecules; sVCAM-1, soluble vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
a First sample drawn from patient (0–18 days after disease onset).
b Sample minimum 60 days after disease onset.
c Interquartile range (25%–75%).
d Wilcoxon signed-rank test for related samples.
e Here values were below limit of detection, the value for half the limit of detection was inserted.
Time Course of Endothelial Activation and Repair
The average levels of syndecan-1, sE-selectin, sICAM-1, and sVCAM-1 were highest during the first week after onset of disease, and for syndecan-1 also the second week, followed by a decline (Figure 1A–D). However, VEGF remained high during HFRS and peaked at 13–18 days after disease onset (Figure 1E). Angiopoietin-2 levels were highest during the first week, dropping to lowest levels during 13–18 days after disease onset, and then increased at follow-up (Figure 1F). The levels of EPO were increased during HFRS compared to follow-up (Figure 1G). Stromal cell-derived factor 1 levels were highest during early HFRS followed by a decline (Figure 1H).
Figure 1.
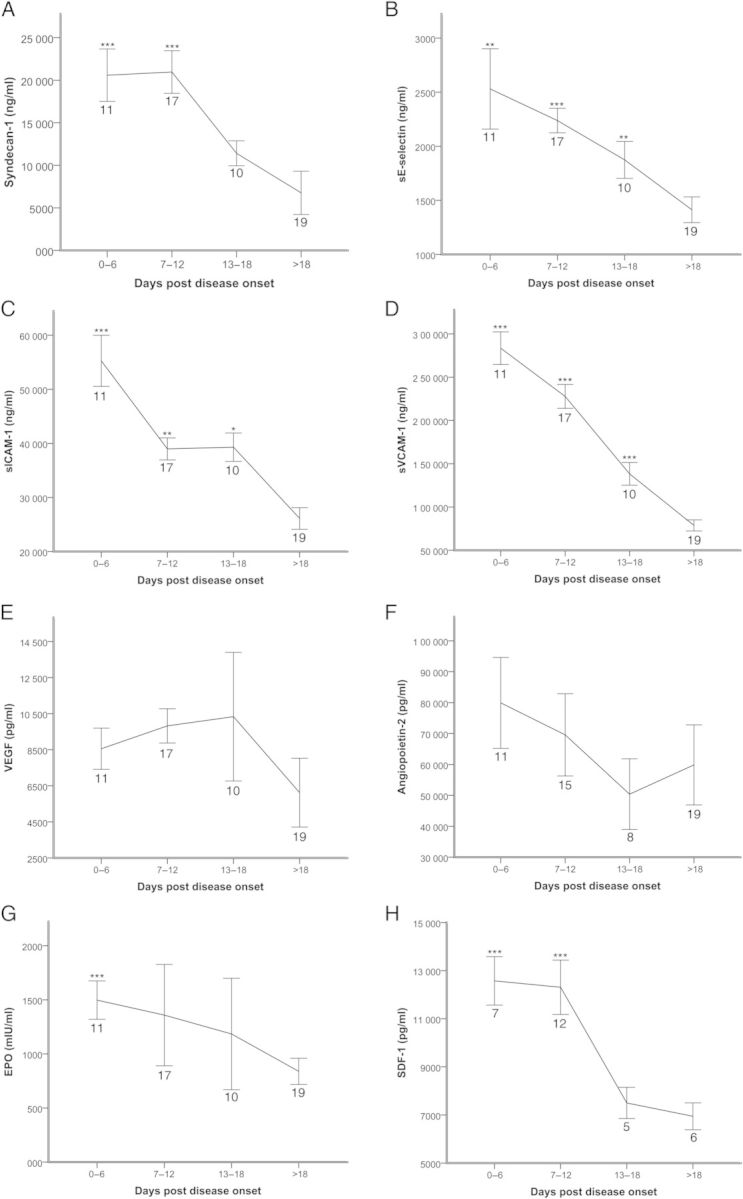
Time line kinetics of syndecan-1 (A), endothelial selectin (sE-selectin) (B), soluble intercellular adhesion molecules (sICAM-1) (C), soluble vascular cell adhesion molecule (sVCAM-1) (D), vascular endothelial growth factor (VEGF) (E), angiopoietin (Ang-2) (F), erythropoietin (EPO) (G), and stromal cell-derived factor 1 (SDF-1) (H) in patients with hemorrhagic fever with renal syndrome. The markers are depicted as mean values ± standard error of the mean. Samples were obtained from 19 patients (syndecan-1, sICAM-1, sVCAM-1: 93 samples; sE-selectin and VEGF: 92 samples; EPO: 91 samples) and 17 patients (SDF-1: 44 samples). Asterisks indicate where there is a significant difference between the time points vs the follow-up (>18 days after disease onset) using generalized estimating equations GEEs (***P < .001; **P < .01; *P < .05). The number of patients included in each time point is displayed below the graph point in the respective figures.
Association Between Endothelial Activation and Repair
The associations between the endothelial markers were assessed, and all associated significantly with each other apart from syndecan-1 and sICAM-1 (Supplementary Table 1).
The associations between endothelial activation and repair markers were also assessed (Supplementary Table 2). Stromal cell-derived factor 1 was significantly associated with syndecan-1 and sVCAM-1 levels, and EPO associated significantly with levels of sICAM-1 and sE-selectin. Angiopoietin was significantly associated with sVCAM-1. Vascular endothelial growth factor was not significantly associated with any of the endothelial activation markers.
Correlation of Endothelial Activation Markers With Parameters for Disease Outcome
We chose thrombocytes [25, 26], creatinine [24], and blood pressure [25] as surrogate markers because these have been implicated in hantaviral disease outcome. Low plasma protein levels correlated with leakage of plasma proteins due to endothelial activation in dengue patients; therefore, we also chose albumin [27]. Insulin-like growth factor binding protein-1 was used as a marker for hypoxia [28].
We investigated whether endothelial activation was associated with the mentioned disease outcome markers (Table 3). Endothelial glycocalyx degradation (syndecan-1) was significantly associated with levels of thrombocytes, albumin, systolic and diastolic blood pressure, and the marker for hypoxia, IGFBP-1. The adhesion molecule sVCAM-1 was significantly associated with the levels of thrombocytes, albumin, systolic blood pressure, and hypoxia (IGFBP-1). Soluble ICAM-1 was significantly associated with the levels of creatinine and the hypoxia marker IGFBP-1, whereas sE-selectin was only significantly associated with albumin out of the tested parameters for disease outcome.
Table 3.
Association Between Endothelial Activation and Clinical Markers for Hantaviral Disease Outcomea
| Syndecan-1 (ng/mL) | sVCAM-1 (ng/mL) | sICAM-1 (ng/mL) | sE-selectin (ng/mL) | |
|---|---|---|---|---|
| Clinical laboratory values | ||||
| Thrombocytes, 109/L |
β= −0.487 P < .001 |
β= −0.071 P < .001 |
β = −0.311 P = .086 |
β = -3.668 P = 0.103 |
| Creatinine, μmol/L |
β = 0.24 P = .09 |
β= 0.026 P = .323 |
β= −0.256 P = .008 |
β= −3.032 P = .371 |
| Albumin, g/L |
β= −0.014 P < .001 |
β= −0.002 P = .001 |
β= −0.006 P = .237 |
β= −0.173 P = .03 |
| Blood pressure | ||||
| Systolic, mmHg |
β= −0.045 P < .001 |
β= −0.003 P = .009 |
β = −0.042 P= .052 |
β= −0.388 P = .1 |
| Diastolic, mmHg |
β= −0.015 P = .006 |
β= −0.003 P = .055 |
β= −0.017 P = .238 |
β= −0.244 P = .118 |
| Hypoxia | ||||
| IGFBP-1 |
β= 40.283 P < .001 |
β= 6.151 P = .001 |
β= 32.754 P = .038 |
β= 659.573 P = .323 |
| Disease severityb |
ρ = 0.47 P = .042 |
ρ = 0.094 P = .702 |
ρ = −0.188 P = .441 |
ρ = −0.407 P = .084 |
Abbreviations: IGFBP-1, insulin-like growth factor binding protein 1; sE-selectin, endothelial selectin; sICAM-1, soluble intercellular adhesion molecules; sVCAM-1, soluble vascular cell adhesion molecule.
a The estimated β-coefficients from the generalized estimating equations analysis is given along with the P value. This result corresponds to the change in endothelial activation marker levels for 1 unit increase for continuous covariates (slope). Significant associations are shown highlighted in bold.
b The maximal values for each marker is associated with disease severity (mild vs moderate/severe disease groups) using Spearman's rank correlation coefficient analysis.
Correlation of Markers for Endothelial Repair With Parameters for Disease Outcome
We assessed the association between markers for endothelial repair with the above-mentioned clinical outcome markers using GEEs (Table 4). Stromal cell-derived factor 1, EPO, and Ang-2 were significantly associated with levels of thrombocytes, albumin, and IGFBP-1. Furthermore, EPO and Ang-2 were significantly associated with systolic blood pressure. Vascular endothelial growth factor was only associated with albumin levels.
Table 4.
Association Between Endothelial Repair and Clinical Markers for Hantaviral Disease Outcomea
| SDF-1 (pg/mL) | EPO (mIU/mL) | Ang-2 (pg/mL) | VEGF (pg/mL) | |
|---|---|---|---|---|
| Clinical laboratory values | ||||
| Thrombocytes, 109/L |
β= −0.971 P = .026 |
β= -1.314 P = .012 |
β= −0.053 P = .004 |
β= 0.228 P = .136 |
| Creatinine, μmol/L |
β= 0.426 P = .31 |
β= −0.525 P = .052 |
β= 0.03 P = .167 |
β= 0.32 P = .319 |
| Albumin, g/L |
β= −0.027 P = .015 |
β= −0.218 P = .017 |
β= −0.001 P < .001 |
β= −0.007 P = .02 |
| Blood pressure | ||||
| Systolic, mmHg |
β= −0.148 P = .096 |
β= −0.074 P = .042 |
β= −0.008 P < .001 |
β= 0.011 P = .718 |
| Diastolic, mmHg |
β= −0.032 P = .391 |
β= 0.013 P = .592 |
β= −0.001 P = .407 |
β= −0.012 P = .466 |
| Hypoxia | ||||
| IGFBP-1 |
β= 96.009 P = .016 |
β= 257.36 P = .000 |
β= 4.968 P = .050 |
β = 16.039 P = .35 |
| Disease severityb |
ρ = 0.454 P = .089 |
ρ = 0.031 P = .899 |
ρ = 0.344 P = .149 |
ρ = −0.063 P = .799 |
Abbreviations: Ang-2, angiopoietin; EPO, erythropoietin; IGFBP-1, insulin-like growth factor binding protein 1; SDF-1, stromal cell-derived factor 1; VEGF, vascular endothelial growth factor.
a The estimated β-coefficients from the generalized estimating equations analysis is given along with the P value. This result corresponds to the change in endothelial activation marker levels for 1 unit increase for continuous covariates (slope). Significant associations are shown highlighted in bold.
b The maximal values for each marker is associated with disease severity (mild vs moderate/severe disease groups) using Spearman's rank correlation coefficient analysis.
HFRS Disease Severity and Association With Endothelial Activation and Repair
The maximal level for each endothelial activation and repair marker (n = 19; apart from SDF-1, n = 15) was investigated for association with disease severity using Spearman's rank correlation coefficient. Only the maximal level of syndecan-1 correlated with disease severity out of all the tested markers (Tables 3 and 4).
DISCUSSION
In our study of acutely ill HFRS patients, we show significant endothelial activation and repair during acute disease compared to follow-up. The level of endothelial activation was mirrored by the level of endothelial repair. Endothelial activation markers correlated with surrogate markers for disease outcome, indicating a role of endothelial dysfunction in HFRS pathogenesis. Endothelial dysfunction was even more pronounced with regards to endothelial glycocalyx degradation, which was significantly associated with disease severity.
We showed a significant up-regulation of the endothelial activation markers syndecan-1, sVCAM-1, sICAM-1, and sE-selectin during disease compared to follow-up. The adhesion molecules (sVCAM-1, sICAM-1, and sE-selectin) were shown to be up-regulated in hantavirus-infected patients in previous studies [25, 29, 30] and also to correlate with sites of leukocyte infiltration in biopsies from kidney [31] and lung [7]. In addition, sVCAM-1 was shown to correlate with severity of HFRS disease caused by Hantaan virus [29]; however, we did not observe this effect in our PUUV-infected patients. It is possible that sVCAM-1 did not correlate with disease severity in our study because we had fewer patients and a milder form of HFRS. Hantaan virus causes a more severe form of HFRS compared with PUUV [3], and the disease categorization into mild and moderate/severe differs between previous studies and our study [29]. We observed a highly significant negative association between endothelial activation (syndecan-1 and sVCAM-1) and thrombocyte levels during the acute phase of HFRS. The endothelial glycocalyx harbors antithrombotic molecules, which are released upon glycocalyx degradation resulting in a prothrombotic and adhesive environment [11, 13, 32], indicating a possible mechanism for the observed thrombocytopenia and coagulopathy in our HFRS patients. In a recent study, we observed a strong association between HFRS and acute myocardial infarction and stroke within the first 3 weeks after disease onset [33].
There was a negative association between endothelial activation (syndecan-1, sVCAM-1, and sE-selectin) and plasma albumin levels. The loss of the endothelial glycocalyx enables passage of albumin across the endothelium with concomitant fluid exudation [11]. Consequently, the degradation of endothelial glycocalyx manifests systemically as increased systemic microvascular permeability and albuminuria [34]. We observed proteinuria in all of our included HFRS patients, and a previous study showed increased pulmonary vascular leakage in HFRS patients [35]. A similar observation was made for dengue patients where glycocalyx degradation was linked to decreased plasma protein levels and proteinuria [27].
In our study, we observed a negative association between excessive endothelial activation (syndecan-1 and sVCAM-1) with low blood pressure. These findings are supported by a previous study correlating plasma levels of the stable metabolites of nitrogen oxide with hypotension in PUUV-infected patients [36]. Furthermore, severe cases of HFRS are characterized by decreased blood pressure with some entering shock [3]. It could be speculated that endothelial activation leading to increased vascular permeability and vasodilatation may play a key role in the observed decreased blood pressure during hantaviral disease.
The capillary leakage resulting in tissue edema is central to hantaviral disease pathogenesis [3]. The endothelial glycocalyx represents the first barrier to fluid exudation, with endothelial junctions forming the second barrier [34]. Several in vitro studies [8, 9, 21, 22] showed that the second barrier consisting of endothelial intercellular junctions plays a role disease outcome. However, to our knowledge, this is the first study to highlight the importance of the endothelial glycocalyx in hantaviral disease pathogenesis. Removal of the endothelial glycocalyx results in tissue edema formation [10, 34, 37], which causes tissue hypoxia [38]. Hypoxia leads to transcription of many target genes such as IGFBP-1 [28]. All of the endothelial activation markers (except sE-selectin) were positively associated with IGFBP-1. In addition, the reduction of gas diffusion capacity in PUUV-infected patients was correlated to vascular pulmonary leakage [35]. Speculatively, the endothelial activation with glycocalyx degradation could cause tissue hypoxia by tissue edema formation [38] and obstruction of blood flow due to leukocyte adhesion and thrombi formation [39]. This result would consequently be reflected in HFRS disease severity, and we indeed observed a significant association between syndecan-1 levels and disease severity. In previous studies, the level of glycocalyx degradation has been linked to sepsis severity and mortality [15], Crimean-Congo hemorrhagic fever disease severity [40], mortality of trauma patients [14], and ischemia during surgery [16]. Several factors could contribute to glycocalyx degradation and thereby potentially exacerbate HFRS disease. Proteases released from infected or activated endothelial cells, as shown for dengue-infected endothelial cells [41], or infected or activated leukocytes, such as hantavirus-infected dendritic cells [6], could be some of the factors that cause glycocalyx degradation [11]. Further studies are needed to clarify the mechanisms underlying endothelial glycocalyx degradation. The study of endothelial glycocalyx is complicated by the fact that there are few in vitro endothelial glycocalyx models [23] and no good animal models for HFRS [3].
We highlight the role of the endothelial glycocalyx in hantaviral disease outcome in our study; therefore, it would be interesting to speculate on medical interventions targeting this structure. Corticosteroids were shown to protect the endothelial glycocalyx in an in vivo model [37]. Unfortunately, a recent study indicated that corticosteroids did not prove beneficial in clinical outcome of HCPS [42]. Other medical intervention strategies targeting the endothelial surface layer warrant further notice [12]. For example, in a heart transplantation model, the level of glycocalyx degradation and tissue damage was decreased by incubating the heart in an albumin-augmented solution [43]. In a study of dengue patients, the infusion of a low dextran dose was hypothesized to temporarily restore the normal function of the endothelial surface layer [44]. The supposed mechanism was that dextrans adsorbed to the endothelial glycocalyx causing a similar protection as observed for the albumin in the above-mentioned heart transplant model. Whether this process would be applicable for hantaviral disease and other viral hemorrhagic fevers is unknown.
Activation of endothelial cells determines the amplitude of inflammation and thereby also tissue damage [1]. In our study, we used VEGF, EPO, SDF-1, and Ang-2 as markers for endothelial repair [19]. These markers were higher during acute disease compared to follow-up. In general, the levels of these cytokines were higher in the first stage of disease, apart from VEGF, which had another time course peaking during the later stage of disease, which has also been observed in 2 other studies [45, 46]. The delayed presence of VEGF in plasma in patients with HCPS and HFRS were interpreted as an indicator of vascular remodeling and repair [45, 46], and we observed a similar time line kinetic in our patients. In general, the level of endothelial activation and the surrogate markers for disease outcome were mirrored by the level of repair markers. Of all the cytokines associated with endothelial repair, only VEGF did not associate with endothelial activation. The endothelial repair markers studied here have all been shown to play a role in mobilizing endothelial progenitor cells [19, 20]. A recent study showed increased circulating endothelial progenitor cells in patients with HFRS compared with healthy volunteers, which were correlated to levels of C-reactive protein, thrombocytes, and albumin [46].
As with any descriptive study, it is not possible to indicate causality in any of the markers in our study. Therefore, we do not know whether there is a direct correlation between any of the studied markers or whether there is a common underlying factor that is the cause of association. To study direct causality further, in vitro and in vivo studies are needed. We measured indirect markers for endothelial dysfunction; however, these markers may not accurately reflect the level of endothelial activation nor does it indicate the site of release. Therefore, the sources of the activation markers cannot be determined in the present study; however, it seems plausible that they originate from the endothelium because sE-selectin is significantly associated with the other activation markers.
In this study of HFRS patients, we observe an association between endothelial dysfunction with clinical markers for disease outcome and disease severity. However, more studies are needed to further clarify the mechanisms and sequence of events.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Notes
Acknowledgments. We thank all the patients, attending doctors, the study nurse Maria Casserdahl, and laboratory personnel at the Clinic for Infectious Diseases, Umeå University Hospital. Maj Bylund assisted with the detection of the markers in the plasma samples. We also thank Marie Eriksson for assistance with choosing statistical methods.
Financial Support. This work was supported by the Medical Faculty of Umeå University and the County Council of Västerbotten (Grant nos. 216851, 238461, 321411); and the County Councils of Northern Sweden (Grant no. 296301).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Ye X, Ding J, Zhou X, et al. Divergent roles of endothelial NF-κB in multiple organ injury and bacterial clearance in mouse models of sepsis. J Exp Med. 2008;205:1303–15. doi: 10.1084/jem.20071393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnittler HJ, Feldmann H. Viral hemorrhagic fever—a vascular disease? Thromb Haemost. 2003;89:967–72. [PubMed] [Google Scholar]
- 3.Vaheri A, Strandin T, Hepojoki J, et al. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol. 2013;11:539–50. doi: 10.1038/nrmicro3066. [DOI] [PubMed] [Google Scholar]
- 4.Rasmuson J, Andersson C, Norrman E, et al. Time to revise the paradigm of hantavirus syndromes? Hantavirus pulmonary syndrome caused by European hantavirus. Eur J Clin Microbiol Infect Dis. 2011;30:685–90. doi: 10.1007/s10096-010-1141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorkstrom NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsac D, Garcia S, Fournet A, et al. Infection of human monocyte-derived dendritic cells by ANDES Hantavirus enhances pro-inflammatory state, the secretion of active MMP-9 and indirectly enhances endothelial permeability. Virol J. 2011;8:223. doi: 10.1186/1743-422X-8-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmuson J, Pourazar J, Linderholm M, et al. Presence of activated airway T lymphocytes in human Puumala hantavirus disease. Chest. 2011;140:715–22. doi: 10.1378/chest.10-2791. [DOI] [PubMed] [Google Scholar]
- 8.Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J Virol. 2010;84:11227–34. doi: 10.1128/JVI.01405-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krautkramer E, Grouls S, Stein N, et al. Pathogenic old world hantaviruses infect renal glomerular and tubular cells and induce disassembling of cell-to-cell contacts. J Virol. 2011;85:9811–23. doi: 10.1128/JVI.00568-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Bas Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 11.van Golen RF, van Gulik TM, Heger M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med. 2012;52:1382–402. doi: 10.1016/j.freeradbiomed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Becker BF, Chappell D, Bruegger D, et al. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300–10. doi: 10.1093/cvr/cvq137. [DOI] [PubMed] [Google Scholar]
- 13.Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson PI, Stensballe J, Rasmussen LS, et al. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 15.Nelson A, Berkestedt I, Schmidtchen A, et al. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008;30:623–7. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- 16.Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 17.Rabelink TJ, de Boer HC, van Zonneveld AJ. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat Rev Nephrol. 2010;6:404–14. doi: 10.1038/nrneph.2010.65. [DOI] [PubMed] [Google Scholar]
- 18.Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–43. [PubMed] [Google Scholar]
- 19.George AL, Bangalore-Prakash P, Rajoria S, et al. Endothelial progenitor cell biology in disease and tissue regeneration. J Hematol Oncol. 2011;4:24. doi: 10.1186/1756-8722-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leone AM, Valgimigli M, Giannico MB, et al. From bone marrow to the arterial wall: the ongoing tale of endothelial progenitor cells. Eur Heart J. 2009;30:890–9. doi: 10.1093/eurheartj/ehp078. [DOI] [PubMed] [Google Scholar]
- 21.Gorbunova E, Gavrilovskaya IN, Mackow ER. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J Virol. 2010;84:7405–11. doi: 10.1128/JVI.00576-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor SL, Wahl-Jensen V, Copeland AM, et al. Endothelial cell permeability during hantavirus infection involves factor XII-dependent increased activation of the kallikrein-kinin system. PLoS Pathog. 2013;9:e1003470. doi: 10.1371/journal.ppat.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res. 2009;104:1318–25. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundberg E, Hultdin J, Nilsson S, et al. Evidence of disseminated intravascular coagulation in a hemorrhagic Fever with renal syndrome-scoring models and severe illness. PLoS One. 2011;6:e21134. doi: 10.1371/journal.pone.0021134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takala A, Lahdevirta J, Jansson SE, et al. Systemic inflammation in hemorrhagic fever with renal syndrome correlates with hypotension and thrombocytopenia but not with renal injury. J Infect Dis. 2000;181:1964–70. doi: 10.1086/315522. [DOI] [PubMed] [Google Scholar]
- 26.Rasche FM, Uhel B, Kruger DH, et al. Thrombocytopenia and acute renal failure in Puumala hantavirus infections. Emerg Infect Dis. 2004;10:1420–5. doi: 10.3201/eid1008.031069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills BA, Oragui EE, Dung NM, et al. Size and charge characteristics of the protein leak in dengue shock syndrome. J Infect Dis. 2004;190:810–8. doi: 10.1086/422754. [DOI] [PubMed] [Google Scholar]
- 28.Custodio RJ, do Carmo Custodio VI, Scrideli CA, et al. Impact of hypoxia on IGF-I, IGF-II, IGFBP-3, ALS and IGFBP-1 regulation and on IGF1R gene expression in children. Growth Horm IGF Res. 2012;22:186–91. doi: 10.1016/j.ghir.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Qi BT, Wang P, Li J, et al. Levels of soluble vascular cell adhesion molecule-1 and soluble intercellular adhesion molecule-2 in plasma of patients with hemorrhagic fever with renal syndrome, and significance of the changes in level. Viral Immunol. 2006;19:565–9. doi: 10.1089/vim.2006.19.565. [DOI] [PubMed] [Google Scholar]
- 30.Han Q, Zhang L, Liu Z, et al. Elevated sICAM-1 levels in patients with hemorrhagic fever with renal syndrome caused by Hantaan virus. Eur J Clin Microbiol Infect Dis. 2010;29:1507–11. doi: 10.1007/s10096-010-1032-x. [DOI] [PubMed] [Google Scholar]
- 31.Temonen M, Mustonen J, Helin H, et al. Cytokines, adhesion molecules, and cellular infiltration in nephropathia epidemica kidneys: an immunohistochemical study. Clin Immunol Immunopathol. 1996;78:47–55. doi: 10.1006/clin.1996.0007. [DOI] [PubMed] [Google Scholar]
- 32.Chappell D, Heindl B, Jacob M, et al. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology. 2011;115:483–91. doi: 10.1097/ALN.0b013e3182289988. [DOI] [PubMed] [Google Scholar]
- 33.Connolly-Andersen AM, Hammargren E, et al. Increased risk of acute myocardial infarction and stroke during hemorrhagic fever with renal syndrome: a self-controlled case series study. Circulation. 2014;129:1295–302. doi: 10.1161/CIRCULATIONAHA.113.001870. [DOI] [PubMed] [Google Scholar]
- 34.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. 2012;226:562–74. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 35.Rasmuson J, Lindqvist P, Sorensen K, et al. Cardiopulmonary involvement in Puumala hantavirus infection. BMC Infect Dis. 2013;13:501. doi: 10.1186/1471-2334-13-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linderholm M, Groeneveld PH, Tarnvik A. Increased production of nitric oxide in patients with hemorrhagic fever with renal syndrome—relation to arterial hypotension and tumor necrosis factor. Infection. 1996;24:337–40. doi: 10.1007/BF01716075. [DOI] [PubMed] [Google Scholar]
- 37.Chappell D, Jacob M, Hofmann-Kiefer K, et al. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107:776–84. doi: 10.1097/01.anes.0000286984.39328.96. [DOI] [PubMed] [Google Scholar]
- 38.Chappell D, Westphal M, Jacob M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Curr Opin Anaesthesiol. 2009;22:155–62. doi: 10.1097/ACO.0b013e328328d1b6. [DOI] [PubMed] [Google Scholar]
- 39.De Backer D, Donadello K, Taccone FS, et al. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011;1:27. doi: 10.1186/2110-5820-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozturk B, Kuscu F, Tutuncu E, et al. Evaluation of the association of serum levels of hyaluronic acid, sICAM-1, sVCAM-1, and VEGF-A with mortality and prognosis in patients with Crimean-Congo hemorrhagic fever. J Clin Virol. 2010;47:115–9. doi: 10.1016/j.jcv.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Luplertlop N, Misse D. MMP cellular responses to dengue virus infection-induced vascular leakage. Jpn J Infect Dis. 2008;61:298–301. [PubMed] [Google Scholar]
- 42.Vial PA, Valdivieso F, Ferres M, et al. High-dose intravenous methylprednisolone for hantavirus cardiopulmonary syndrome in Chile: a double-blind, randomized controlled clinical trial. Clin Infect Dis. 2013;57:943–51. doi: 10.1093/cid/cit394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacob M, Paul O, Mehringer L, et al. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation. 2009;87:956–65. doi: 10.1097/TP.0b013e31819c83b5. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen-Pouplin J, Pouplin T, Van TP, et al. Dextran fractional clearance studies in acute dengue infection. PLoS Negl Trop Dis. 2011;5:e1282. doi: 10.1371/journal.pntd.0001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gavrilovskaya I, Gorbunova E, Koster F, et al. Elevated VEGF levels in pulmonary edema fluid and PBMCs from patients with acute hantavirus pulmonary syndrome. Adv Virol. 2012;2012:674360. doi: 10.1155/2012/674360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krautkramer E, Grouls S, Hettwer D, et al. Mobilization of circulating endothelial progenitor cells (cEPCs) correlates with the clinical course of hantavirus disease. J Virol. 2014;88:483–9. doi: 10.1128/JVI.02063-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


