Abstract
Introduction.
GeneXpert® MTB/RIF (Xpert) is now widely distributed in high human immunodeficiency virus (HIV)/tuberculosis (TB)-burden countries. Yet, whether the test improves patient-important outcomes within HIV treatment programs in limited resource settings is unknown.
Methods.
To investigate whether use of Xpert for TB screening prior to initiation of antiretroviral treatment (ART) improves patient-important outcomes, in a pragmatic randomized controlled trial we assigned 424 patients to Xpert or fluorescence sputum smear microscopy (FM) at ART initiation. The primary endpoint was a composite of 3-month mortality and ART-associated TB.
Results.
There was no difference in overall TB diagnosis at ART initiation (20% [n = 43] Xpert vs 21% [n = 45] FM; P = .80), with most patients in both groups treated empirically. There was no difference in time to TB treatment initiation {5 days (interquartile range [IQR], 3–13) vs 8 days [IQR, 3–23; P = .26]} or loss to follow-up (32 [15%] vs 38 [18%]; P = 0.38). Although a nonsignificant reduction in mortality occurred in the Xpert group (11 [6%] vs 17 [10%]; 95% CI, −9% to 2%; P = .19), there was no difference in the composite outcome (9% [n = 17] Xpert vs 12% [n = 21] FM; difference −3%; 95% CI, −9% to 4%).
Conclusions.
Among HIV-infected initiating ART, centralized TB screening with Xpert did not reduce the rate of ART-associated TB and mortality, compared with fluorescence microscopy.
Keywords: ART-associated TB, GeneXpert MTB/RIF, HIV, screening, tuberculosis
Ninety-five percent of the estimated 34 million adults infected with human immunodeficiency virus (HIV) live in low- and middle-income countries [1]. Although antiretroviral treatment (ART) has dramatically reduced morbidity and mortality in these settings, the risk of death in the early post-ART period remains high [2]. From 4% to 25% of individuals initiating ART in high HIV/tuberculosis (TB)-burden settings have unrecognized active TB [3], and TB is likely a significant contributor to early mortality.
The World Health Organization (WHO) recommends intensified case finding among individuals infected with HIV. Symptom-based screening is nonspecific and has poor predictive value for identification of individuals with TB. Direct sputum smear microscopy is fast, inexpensive, and specific, but it is poorly sensitive compared with Mycobacterium tuberculosis culture, particularly among persons infected with HIV. Compared with direct microscopy, fluorescence microscopy (FM) has higher sensitivity and similar specificity among patients infected with HIV, it is associated with reduced laboratory technician burden, and it has been facilitated by the roll-out of light-emitting diode technology in low- and middle-income countries [4].
The WHO-endorsed Xpert M tuberculosis (MTB)/rifampin (RIF) (Xpert; Cepheid, Sunnyvale, CA) assay, an automated nucleic acid amplification test, is now widely distributed in low- and middle-income countries [5]. Optimized case finding with Xpert reduces diagnostic delay among TB suspects [6] and substantially increases diagnostic yield among ART program enrollees, relative to FM [7]. However, empiric treatment of TB suspects is common in high-burden settings, and how Xpert might influence clinical decision making is uncertain [8]. We hypothesized that compared with conventional TB screening with FM, intensified screening with Xpert at the time of ART initiation would “miss” fewer cases of prevalent TB, with resulting reduction in subsequent TB diagnoses and/or death during ART [9, 10].
Policymakers are increasingly calling for robust evidence for healthcare interventions that focus on patient-important outcomes in typical populations [11]. Relative to classic randomized controlled trials (RCTs), pragmatic trials aim to assess whether interventions work in conditions more appropriate to routine practice, with an emphasis on external generalizability [12]. We report results of a pragmatic RCT of Xpert conducted at an ART initiation clinic in Harare, Zimbabwe, an urban setting with high HIV and TB prevalence. Some of the results of this study have been previously reported in abstract form [13].
METHODS
Trial Design
This single-center, pragmatic, RCT was conducted in accordance with CONSORT guidelines at Beatrice Road Infectious Diseases Hospital ([BRIDH] Harare, Zimbabwe) between October 2011 through June 2012. Beatrice Road Infectious Diseases Hospital is the largest ART initiation center in Zimbabwe. The objective of the trial was to examine the effect of baseline TB screening with Xpert vs FM on a composite outcome of incident, ART-associated TB, and mortality in the first 3 months post-ART initiation. This trial is registered with Pan-African Clinical Trials Registry, number PACTR201303000505764.
Randomization and Masking
A randomization code was generated by the data manager and supplied to the laboratory manager in sealed opaque envelopes. Sputum specimens were transported daily to a central, external, quality-assured laboratory, with laboratory technicians blinded to patient identifiers. Blinding of patients or clinicians was not feasible in this pragmatic trial because test results were used to guide patient management. The study protocol was approved by the Medical Research Council of Zimbabwe and the institutional review boards of the Biomedical Research and Training Institute, Harare City Council, and the University of Zimbabwe; all patients provided written informed consent.
Patients
The study included consecutive symptomatic and asymptomatic HIV-infected patients initiating ART. Patients were excluded from the trial if they were (1) less than 18 years of age, (2) not ART-naive, or (3) receiving TB treatment. Before ART initiation, patients attended counseling and adherence sessions and were assessed for opportunistic infections including TB. In accordance with 2010 WHO guidelines, patients were initiated on ART at CD4 counts ≤350 cells/µL. Standard first-line ART during the study period included coformulated stavudine and lamivudine and either efavirenz or nevirapine. In accordance with Zimbabwe national treatment guidelines, ART initiation aims to occur within 2 weeks of TB treatment [14]. Three counseling sessions occur before ART initiation, and patients remain in care for 3 months (with monthly clinical reviews at time of medication refill) before referral to a local clinic for continuation of treatment. As in most settings in Southern Africa, isoniazid preventive therapy is not routinely provided within the public sector in Zimbabwe [15].
Patients completed a standardized questionnaire including the WHO symptom screen (current cough, fever, night sweats, and weight loss) and provided 2 spot sputum specimens at least 1 hour apart. If patients were unable to expectorate sputum, attempts were made to induce sputum using nebulized 6% hypertonic saline. Chest radiography performed at baseline was at the discretion of treating clinicians.
Diagnostic test results were provided within 24 h, and TB treatment was initiated at the discretion of care providers. Standardized follow-up assessments at months 1 and 3 included chest radiography, clinical examination, and symptom assessment by a study doctor. Symptomatic patients were referred back to BRIDH, with any further testing performed at the discretion of the clinician on duty according to routine care. Clinic records were reviewed for patients not attending follow-up assessments; home visits by the study team were undertaken for patients not returning to BRIDH for any reason. Patients were considered lost to follow-up if they did not attend the final follow-up assessment, had no available clinic records, and could not be located after 3 phone calls and at least 1 home visit.
Laboratory Assays
Sputum specimens were transported within 2 hours of collection to an accredited centralized laboratory. Samples in the FM group had a direct smear performed on each sample followed by staining with auramine O (Leica, Germany). Xpert MTB/RIF assays were performed on direct sputum according to previously described procedures [6]. In brief, the sample reagent was added in a volume twice that of the untreated sputum and incubated for 15 minutes. Two milliliters of the processed sample was then transferred to the test cartridge (G3) for analysis.
Study Definitions
Tuberculosis diagnosed at enrollment (before ART initiation) was considered prevalent TB. Tuberculosis not detected on initial screening but diagnosed at any time after initiation of ART, regardless of timing of symptom-onset, was defined as “ART-associated TB.” Tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS) was diagnosed on the basis of at least 1 major or 2 minor clinical criteria [16]. Deaths occurring after TB diagnosis were considered “TB deaths.” Patients with at least 1 positive Xpert or FM (for “scanty” samples, both smears needed to be recorded as “scanty”) result were considered “bacteriologically confirmed TB.” Patients initiated on anti-TB therapy based on clinical diagnosis without bacteriologic confirmation were considered “clinical TB.” Time to treatment was defined as the time from the baseline visit until initiation of TB treatment.
Study Outcomes
The primary endpoint was the proportion of patients who were diagnosed with ART-associated TB or who died within 3 months of randomization.
Statistical Analysis
The primary purpose of the data analysis was to determine whether a 3-month composite endpoint of ART-associated TB and mortality differed significantly between patients who underwent baseline TB screening using Xpert and those who underwent baseline TB screening using FM. We calculated a sample size of 429 with 80% power to detect a 10% absolute difference in the composite outcome assuming a probability of ART-associated TB and of death of 12% [17, 18] and 6% [2, 19] respectively, in the FM arm, decreasing to 5% [7] and 2%, respectively, in the Xpert arm; 20% and 15% overlap in ART-associated TB and death in the FM and Xpert arm, respectively (prevalent TB diagnoses “missed” by Xpert were considered less likely to die); [2, 20] and attrition due to loss to follow up of 10% in both arms. Categorical variables, including the primary endpoint and its components, were compared using χ2 tests. Continuous variables were compared using a Wilcoxon rank-sum test. To achieve an unbiased estimate for the magnitude of treatment difference in mortality, an adjusted analysis was also performed using a generalized linear model with a log link and robust standard errors to generate relative risk (RR) estimates; a priori-specified covariates (sex, weight, CD4+ T-lymphocyte count, and TB diagnosis) were those prognostic for mortality in prior literature. All analyses were 2-sided at α = 0.05 and were performed using Stata 11 (Stata Corporation, College Station, TX) and R, version 2.13.2 (R Project for Statistical Computing, http://cran.r-project.org).
RESULTS
Patient Characteristics
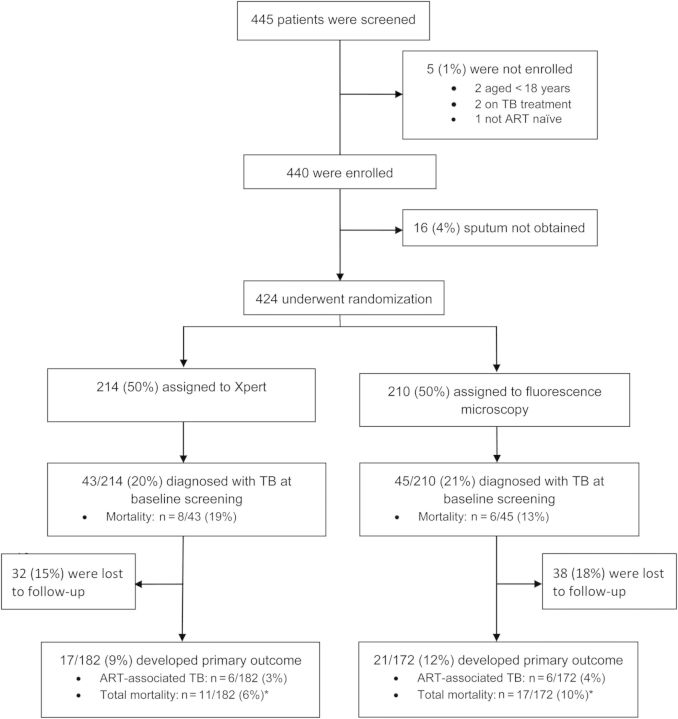
From October 2011 and March 2012, 445 persons were assessed for eligibility. Of these, 424 (95%) were randomized, regardless of symptoms, to either Xpert (n = 214) or FM (n = 210) for TB screening before ART initiation (Figure 1). Baseline demographic and clinical characteristics are shown in Table 1. The median baseline CD4+ T-cell count was 164 cells/µL (interquartile range [IQR], 63–256). Three-hundred fifty-six patients (84%) initiated ART at a median interval of 8 days (IQR, 4–22). By the end of the study period, 32 (15%) patients in the Xpert arm and 38 (18%) patients in the FM arm had been lost to follow-up (LTFU) (P = .38). Persons LTFU were more likely to be women; other demographic and clinical characteristics were similar to those of analyzed cases (Supplementary Table).
Figure 1.
Screening, enrollment, and follow-up of study patients. *All-cause mortality (mortality related to baseline TB diagnosis, ART-associated TB, or death from any cause during 3-month follow-up).
Table 1.
Baseline Characteristics
| Characteristic | Xpert | FM | All Patients | |
|---|---|---|---|---|
| n = 214 | n = 210 | n = 424 | P Values | |
| Female no. (%) | 110 (51) | 122 (58) | 232 (55) | .17 |
| Age at enrollment, yr | ||||
| Median | 37 | 37 | 37 | .86 |
| IQR | 31–43 | 32–43 | 31–43 | |
| Weight at enrollment, kg | ||||
| Median | 58.2 | 58.2 | 58.2 | .33 |
| IQR | 50–65 | 52–67 | 51–66 | |
| CD4+ T cell count/µL | ||||
| Median | 163 | 160 | 164 | .45 |
| IQR | 77–258 | 60–255 | 63–256 | |
| CD4 < 100 cells/µL | 69 (33) | 79 (38) | 148 (35) | .62 |
| CD4 100–199 cells/µL | 53 (25) | 46 (22) | 99 (24) | |
| CD4 200–349 cells/µL | 80 (38) | 78 (37) | 158 (38) | |
| CD4 ≥ 350 cells/µL | 9 (4) | 6 (3) | 15 (3) | |
| Provided 2 sputum samples for analysisa | 162 (76) | 173 (82) | 335 (79) | |
| TB symptoms, no. (%) | ||||
| Cough >2 weeks | 51 (24) | 59 (28) | 110 (26) | .79 |
| Fever | 122 (57) | 132 (63) | 254 (60) | .22 |
| Night sweats | 105 (49) | 121 (58) | 226 (53) | .08 |
| Weight loss | 149 (70) | 138 (66) | 287 (68) | .39 |
| WHO symptom screen positiveb | 195 (91) | 193 (92) | 388 (92) | .77 |
| TB contact, no. (%) | 49 (23) | 39 (19) | 88 (21) | .27 |
| Prevalent TB, no. (%)c | 43 (20) | 45 (21) | 88 (88) | .94 |
| Time to diagnosis- median days (IQR)c | 2 (1–13) | 6 (1–25) | 4 (1–16) | .07 |
| Time to TB treatment initiation- median days (IQR)c | 5 (3–13) | 8 (3–23) | 6 (3–16) | .25 |
| Other OI, no. (%) | ||||
| Kaposi sarcoma | 1 (1) | 1 (1) | 2 (0.5) | – |
| Oral thrush | 28 (13) | 19 (9) | 47 (11) | .19 |
| Genital warts | 9 (4) | 15 (7) | 24 (6) | .19 |
Abbreviations: FM, fluorescence microscopy; IQR, interquartile range; OI, opportunistic infections; TB, tuberculosis; WHO, World Health Organization.
a All randomized patients provided at least 1 sputum sample for analysis.
b WHO symptom screen includes any 1 of current cough, fever, night sweats, or weight loss.
c TB diagnosed at enrollment.
Prevalent Tuberculosis
Tuberculosis was diagnosed by bacteriological or clinical criteria at baseline in 43 (20%) and 45 (21%) patients randomized to Xpert and FM, respectively; there was no statistical difference in diagnostic yield between Xpert (20 [9% of total]) and FM (14 [7% of total; P = .29]). A second Xpert and FM increased bacteriologic confirmation (ie, positive when the first test was negative) by 2 cases and 1 case, respectively. Empiric TB treatment was initiated based on clinical or radiographic criteria in an additional 23 (of 43 total [54%]) patients in the Xpert arm and 31 (of 45 total [69%]; P = .12) patients in the FM arm. The median time from clinical presentation to TB treatment initiation was 5 days (IQR, 3–13) and 8 days (IQR, 3–23; P = .26) in the Xpert and FM arms, respectively. Among patients with prevalent TB, ART was initiated a median of 17 days (IQR, 22–37) and 26 days (IQR, 20–38; P = .60) in the Xpert and FM arms, respectively. Eleven (5%) of Xpert results were initially reported as indeterminate (results reported as invalid or error), all of which were negative on repeat testing of a second sputum sample. Rifampin resistance was not detected by any Xpert test.
Primary Endpoint
There was no significant difference in the composite outcome of ART-associated TB and mortality comparing patients in the Xpert arm (9%; n = 17) versus the FM arm (12%; n = 21; difference −3%; 95% CI, −9% to 4%; P = .39; Table 2). After ART initiation, 6 (3%) patients in the Xpert arm were diagnosed with incident clinical TB and begun on anti-TB treatment compared with 6 (4%) patients in the FM arm (P = .92). Two patients (both in the FM group) diagnosed with incident TB also died; in the composite outcome, these patients were counted only as deaths. Two patients (both in the Xpert group) with radiographic abnormalities at the first month follow-up visit were referred back to BRIDH and begun on anti-TB treatment; all other incident TB diagnoses were initiated within routine clinical care services. Median time to incident TB diagnosis after ART initiation was 22 days (IQR, 18–57) for Xpert and 27 days (IQR, 20–53; P = .81) for FM. One patient was considered to have paradoxical IRIS on the basis of a clinical course consistent with a new inflammatory process and radiographic worsening.
Table 2.
Outcomes According to Study Group
| Xpert MTB/RIF | Fluorescence Microscopy | Relative Risk | 95% CI | P Value | |
|---|---|---|---|---|---|
| Outcome | n = 182 | n = 172 | |||
| Compositea | 17 (9.3) | 21 (12.2) | 0.77 | .42–1.40 | .39 |
| Early mortality | 11 (6.0) | 17 (9.9) | 0.61 | .29–1.27 | .19 |
| ART-associated TBb | 6 (3.3) | 6 (3.5) | 0.95 | .31–2.89 | .92 |
Abbreviations: ART, antiretroviral treatment; CI, confidence interval; MTB, Mycobacterium tuberculosis; RIF, rifampin; TB, tuberculosis.
a The primary endpoint was a composite of mortality and ART-associated tuberculosis.
b Two individuals with ART-associated TB also died; these patients were counted only as deaths for purposes of the composite outcome.
At 3-month follow-up, 11 (6%) patients in the Xpert arm had died, compared with 17 (10%) in the FM arm (−4%; 95% CI, −9 to 2%; P = .19). The median time to death from enrollment was 50 days (IQR, 36–73) and 27 days (IQR, 24–65) for Xpert and FM, respectively (P = .29). One-half of all deaths (n = 14 of 28) occurred among those diagnosed with TB at baseline, and 43% of deaths (n = 12 of 28) occurred among patients empirically diagnosed with TB. Allocation group also did not independently predict mortality in a multivariable analysis adjusted for baseline characteristics (RR, .48; 95% CI, .21–1.08) (Table 3).
Table 3.
Results of Multivariate Analysis of Baseline Characteristics Prognostic for Mortality
| Variable | Relative Risk (95% confidence interval) | P Value |
|---|---|---|
| Weight, per 1 kg increase | 0.96 (.91–1.02) | .17 |
| Gender | ||
| Male | 1 | |
| Female | 0.38 (.16–.91) | .03 |
| CD4 count | ||
| CD4 ≥100 | 1 | |
| CD4 < 100 | 2.51 (1.12–5.64) | .03 |
| Tuberculosis diagnosisa | ||
| No | 1 | |
| Yes | 2.30 (1.06–4.96) | .03 |
| Diagnostic group | ||
| Fluorescence microscopy | 1 | |
| Xpert | 0.48 (.21–1.08) | .08 |
a Prevalent tuberculosis diagnosed at enrollment.
DISCUSSION
In this local investigator-initiated RCT from a high HIV/TB burden setting, intensive screening of ART initiators with Xpert MTB/RIF resulted in no statistical improvement in clinical endpoints relative to conventional fluorescence sputum smear microscopy. Differences between the 2 intervention arms were potentially minimized by a higher than expected yield of fluorescence microscopy and high rates of empiric treatment.
Globally, some 10 million people are registered in HIV treatment programs, the great majority of whom reside in sub-Saharan Africa [1]. The WHO recommends implementation of baseline and repeated intensified TB case finding among individuals infected with HIV as a key response to the HIV/TB syndemic, and optimizing TB screening of ART initiators remains critical for early diagnosis of clinical and subclinical disease, as well as implementation of isoniazid preventive therapy. The effectiveness of intensified case finding depends on the epidemiologic setting, regional TB prevalence, and screening strategy used [3]. Tuberculosis prevalence, disease stage at ART initiation [7, 17, 21] loss to follow-up [18, 19, 22], and time to treatment initiation [20, 23] in our study were similar to comparable settings in Southern Africa.
Despite known limitations, Xpert MTB/RIF is the most significant advance in TB diagnostics in modern history, and it is now widely distributed in resource-limited settings at substantial donor and health sector cost [24]. Although the population-level impact of Xpert continues to be debated [25–27], empiric controlled data focused on clinical endpoints remain limited. A multicenter RCT (TB NEAT) demonstrated no difference in TB-related morbidity among individuals with presumptive TB [28], and a “pre-post” implementation study of hospitalized patients in Kampala, Uganda, noted no difference in 2-month mortality [29]. More recently, a cluster randomized trial carried out in 20 districts in South Africa (XTEND) showed no benefit in time to treatment initiation or 6-month mortality [23]. Similar to our study, substantial empiric treatment occurred in the course of these investigations [30]. Consistency of our overall results with these studies supports the generalizability of our findings under current programmatic conditions.
Clinicians in high-burden settings have correctly interpreted the “rule-out” value of Xpert as suboptimal among persons with HIV-infection, and empiric treatment regardless of bacteriologic confirmation is common [28, 31, 32]. Likewise, 63% of patients initiating TB treatment in our study were Xpert- or FM-negative. Given a population of 1000 ART initiators and an overall Xpert sensitivity of 79% [33], clinicians in our setting could expect 42 of 200 “true” TB cases to be Xpert-negative. Given this uncertainty and the risk of TB mortality (in particular among those with CD4+ T-cell counts <50, regardless of symptoms), empiric treatment may be life-saving and is currently being evaluated in a multicenter randomized trial [34]. Thus, protocolization of TB treatment based only on Xpert results would be ethically dubious, and it was not consistent with the pragmatic nature of our study. Further inquiry into the influence of Xpert on TB treatment thresholds in limited resource settings deserves attention.
Ultimately, in addition to effectiveness data, improving patient-important outcomes on a population level will require consideration of implementation setting and resource limitations [35]. Xpert cost-effectiveness studies in high TB-burden settings have been favorable [36], although to date they have been based on accuracy rather than clinical outcome data, and the effect of empiric TB treatment may be underappreciated [37]. Although patient outcomes were similar in our study, material costs associated with Xpert ($19.96 per patient [at $9.98/cartridge per subsidized prices]) were higher than for FM ($2.40 per patient); in a country where health spending per capita is approximately $8.00 [38], such a difference in up-front costs are considerable and warrant examination if transition of financial responsibility from donor-funded programs to governments is to be successful.
A common critique of trials powered to a composite outcome is that the overall effect is often driven by the less important component [39]. In our study, events occurred with greater frequency within the mortality component, and differences in ART-associated TB were in part minimized by a greater than expected yield of FM. Assuming that our best estimate for mortality (a 4% absolute risk reduction in the Xpert arm) represents a true effect, a trial of approximately 720 individuals in each arm would be required to confirm this with 80% power. Although such post hoc power calculations are methodologically problematic [40], it cannot be ruled out that Xpert conveys an important benefit to a subset of patients.
Our study has potential limitations. First, as in many high-burden countries, culture is not routinely performed in our setting, and we are unable to comment on potential misclassification of TB cases. However, therapeutic outcomes (rather than surrogate endpoints such as test accuracy) are of primary interest in diagnostic RCTs [41], and lack of intensive bacteriologic confirmation is consistent with the pragmatic nature of our trial [12] and reality in most low-income settings. Second, neither fluorescence smear microscopy nor Xpert were performed onsite with same-day results. However, the intended sites for Xpert rollout include district or sub-district laboratories similar to our study [42], and substantial implementation barriers exist for use of Xpert at more peripheral sites [43]. Third, ART-associated TB was included in our composite outcome because it may represent missed cases of prevalent TB. However, in a high TB-burden setting incident TB might also occur within the 3-month follow-up, a circumstance that would bias results toward the null hypothesis. Fourth, because of a lower likelihood of empiric treatment initiation and higher likelihood of death, individuals with rifampin resistance detected by Xpert may be especially likely to show benefit in patient-important outcomes. However, no rifampin resistance was detected in our study and we are unable to comment on this important group. Finally, clinicians and patients were not blinded as to diagnostic assignment group, and we cannot be certain that a differential bias did not occur with respect to patient expectation or clinician diagnosis during study follow-up.
In conclusion, intensified case finding with Xpert MTB/RIF among ART-initiators in a high-burden setting did not decrease a composite outcome of ART-associated TB or 3-month mortality relative to fluorescence sputum smear microscopy. Highly sensitive, rapid diagnostic tests not performed at point-of-care should not be anticipated to improve patient-important outcomes in Southern Africa in the absence of significant health system strengthening.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Notes
Acknowledgments. We wish to thank study patients and research staff, without whom this work would not have been possible. We are also grateful to the staff at Beatrice Road Infectious Disease Hospital and Harare City Health Department for their kind assistance and cooperation in conducting this study.
Author contributions. L. M. and R. M. designed the study; B. M. and T. S. acquired the data and performed laboratory analysis; L. M., M. C., J. Z. M., and R. M. analyzed the data and drafted the manuscript; P. M., J. Z. M., and S. Z. provided critical input from data collection to interpretation.
Financial support. This work was supported by Southern Africa Consortium for Research Excellence (SACORE), and in part by the National Institutes of Health (K23 AI094251 and Fogarty D43TW009539).
References
- 1.UN Joint Programme on HIV/AIDS, Treatment 2015. 2013. July. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/JC2484_treatment-2015_en.pdf . Accessed 13 August 2013.
- 2.Lawn SD, Harries AD, Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranzer K, Houben RM, Glynn JR, et al. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steingart KR, Henry M, Ng V, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–81. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 5.FIND. WHO monitoring of Xpert MTB/RIF roll-out. Available at: http://www.stoptb.org/wg/gli/assets/documents/map/1/atlas.html . Accessed 20 August 2013.
- 6.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauker SG, Kassirer JP. The threshold approach to clinical decision making. New Engl J Med. 1980;302:1109–17. doi: 10.1056/NEJM198005153022003. [DOI] [PubMed] [Google Scholar]
- 9.Lawn SD, Myer L, Edwards D, et al. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–25. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn SD, Mwaba P, Bates M, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–61. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann PJ, Tunis SR. Medicare and medical technology--the growing demand for relevant outcomes. New Engl J Med. 2010;362:377–9. doi: 10.1056/NEJMp0912062. [DOI] [PubMed] [Google Scholar]
- 12.Bratton DJ, Nunn AJ. Alternative approaches to tuberculosis treatment evaluation: the role of pragmatic trials. Int J Tuberc Lung Dis. 2011;15:440–6. doi: 10.5588/ijtld.10.0732. [DOI] [PubMed] [Google Scholar]
- 13.Mupfumi L, Mason P, Zinyowera S, Mtetwa R. The value of universal TB screening with GeneXpert MTB/RIF in pre-ART patients in Harare.. XIX International AIDS Conference; Washington DC. 2012. [Google Scholar]
- 14.National TB Program. Zimbabwe National TB Guidelines. 4th ed. 2010. p. 116.
- 15.Rangaka MX, Wilkinson RJ. Isoniazid prevention of HIV-associated tuberculosis. Lancet Infect Dis. 2013;13:825–7. doi: 10.1016/S1473-3099(13)70218-4. [DOI] [PubMed] [Google Scholar]
- 16.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassett IV, Wang B, Chetty S, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;51:823–9. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Wood R, Kaplan R, et al. Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PloS One. 2013;8:e55824. doi: 10.1371/journal.pone.0055824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Kerkhoff AD, Vogt M, et al. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54:1071–9. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawn SD, Kranzer K, Edwards DJ, et al. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24:1323–8. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xpert MTB/RIF vs microscopy as the first line TB test in South Africa: mortality, yield, initial loss to follow up and proportion treated. The XTEND study. Available at: http://www.stoptb.org/wg/gli/assets/documents/M6/Churchyard%20-%20XTEND%20study.pdf . Accessed 21 May 2014.
- 24.Treatment Action Group. Tuberculosis Research and Development: 2013 Report on Tuberculosis Research Funding Trends, 2005–2012. 2013. November. Available at: http://www.treatmentactiongroup.org/sites/g/files/g450272/f/201310/TAG_TB_2013_8.5.pdf . Accessed 20 May 2014.
- 25.Trebucq A, Enarson DA, Chiang CY, et al. Xpert(R) MTB/RIF for national tuberculosis programmes in low-income countries: when, where and how? Int J Tuberc Lung Dis. 2011;15:1567–72. doi: 10.5588/ijtld.11.0392. [DOI] [PubMed] [Google Scholar]
- 26.Singh JA, Bhan A. The ethics of national tuberculosis programmes in low-income countries not rolling out Xpert (R) MTB/RIF. Int J Tuberc Lung Dis. 2011;15:1563. doi: 10.5588/ijtld.11.0728. [DOI] [PubMed] [Google Scholar]
- 27.Dowdy DW, Davis JL, den Boon S, et al. Population-level impact of same-day microscopy and Xpert MTB/RIF for tuberculosis diagnosis in Africa. PloS One. 2013;8:e70485. doi: 10.1371/journal.pone.0070485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2013;383:424–35. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 29.Yoon C, Cattamanchi A, Davis JL, et al. Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PloS One. 2012;7:e48599. doi: 10.1371/journal.pone.0048599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theron G, Peter J, Dowdy D, et al. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014 doi: 10.1016/S1473-3099(13)70360-8. doi:10.1016/S1473-3099(13)70360-8. [DOI] [PubMed] [Google Scholar]
- 31.Zar HJ, Workman L, Isaacs W, et al. Rapid diagnosis of pulmonary tuberculosis in African children in a primary care setting by use of Xpert MTB/RIF on respiratory specimens: a prospective study. Lancet Glob Health. 2013;1:e97–104. doi: 10.1016/S2214-109X(13)70036-6. [DOI] [PubMed] [Google Scholar]
- 32.Hanrahan CF, Selibas K, Deery CB, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care Xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PloS One. 2013;8:e65421. doi: 10.1371/journal.pone.0065421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steingart KR, Schiller I, Horne DJ, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database System Rev. 2014;1 doi: 10.1002/14651858.CD009593.pub3. CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.REMEMBER: Reducing Early Mortality & Morbidity by Empiric Tuberculosis (TB) Treatment. AIDS Clinical Trials Group, National Institute of Allergy and Infectious Diseases. Available at: http://clinicaltrials.gov/show/NCT01380080%20 . Accessed 26 November 2013.
- 35.Luoto J, Maglione MA, Johnsen B, et al. A comparison of frameworks evaluating evidence for global health interventions. PLoS Med. 2013;10:e1001469. doi: 10.1371/journal.pmed.1001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vassall A, van Kampen S, Sohn H, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews JR, Lawn SD, Dowdy DW, Walensky RP. Challenges in evaluating the cost-effectiveness of new diagnostic tests for HIV-associated tuberculosis. Clin Infect Dis. 2013;57:1021–6. doi: 10.1093/cid/cit412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimbabwe Health System Assessment. USAID. 2010. Available at: http://www.healthsystems2020.org/content/resource/detail/2812/ . Accessed 25 November 2013.
- 39.Lim E, Brown A, Helmy A, et al. Composite outcomes in cardiovascular research: a survey of randomized trials. Ann Intern Med. 2008;149:612–7. doi: 10.7326/0003-4819-149-9-200811040-00004. [DOI] [PubMed] [Google Scholar]
- 40.Greenland S. Nonsignificance plus high power does not imply support for the null over the alternative. Ann Epidemiol. 2012;22:364–8. doi: 10.1016/j.annepidem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Bossuyt PM, Reitsma JB, Linnet K, Moons KG. Beyond diagnostic accuracy: the clinical utility of diagnostic tests. Clin Chem. 2012;58:1636–43. doi: 10.1373/clinchem.2012.182576. [DOI] [PubMed] [Google Scholar]
- 42.Weyer K, Mirzayev F, Migliori GB, et al. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J. 2013;42:252–71. doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 43.Denkinger CM, Nicolau I, Ramsay A, et al. Are peripheral microscopy centres ready for next generation molecular tuberculosis diagnostics? Eur Respir J. 2013;42:544–7. doi: 10.1183/09031936.00081113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.