Abstract
Background.
A nationwide outbreak of fungal infections was traced to injection of Exserohilum-contaminated methylprednisolone. We describe our experience with patients who developed spinal or paraspinal infection after injection of contaminated methylprednisolone.
Methods.
Data were assembled from the Michigan Department of Community Health, electronic medical records, and magnetic resonance imaging (MRI) reports.
Results.
Of 544 patients who received an epidural injection from a contaminated lot of methylprednisolone at a pain clinic in southeastern Michigan, 153 (28%) were diagnosed at our institution with probable or confirmed spinal or paraspinal fungal infection at the injection site. Forty-one patients had both meningitis and spinal or paraspinal infection, and 112 had only spinal or paraspinal infection. Magnetic resonance imaging abnormalities included abscess, phlegmon, arachnoiditis, and osteomyelitis. Surgical debridement in 116 patients revealed epidural phlegmon and epidural abscess most often. Among 26 patients with an abnormal MRI but with no increase or change in chronic pain, 19 (73%) had infection identified at surgery. Fungal infection was confirmed in 78 patients (51%) by finding hyphae in tissues, positive polymerase chain reaction, or culture. Initial therapy was voriconazole plus liposomal amphotericin B in 115 patients (75%) and voriconazole alone in 38 patients (25%). As of January 31, 2014, 20 patients remained on an azole agent. Five patients died of infection.
Conclusions.
We report on 153 patients who had spinal or paraspinal fungal infection at the site of epidural injection of contaminated methylprednisolone. One hundred sixteen (76%) underwent operative debridement in addition to treatment with antifungal agents.
Keywords: contaminated steroids, Exserohilum rostratum, fungal epidural infections, fungal meningitis, fungal paraspinal infections
Injection of methylprednisolone manufactured by the New England Compounding Center (NECC) has been linked to a large outbreak of fungal infections in the United States [1–6]. The Centers for Disease Control and Prevention (CDC) has received reports of 751 cases of fungal infection associated with contaminated steroid injections [2]. A total of 264 (35%) occurred in residents of Michigan, 195 of whom (74%) were cared for at Saint Joseph Mercy Hospital (SJMH), a 537-bed community teaching hospital in southeastern Michigan. Of 519 cases for which the CDC received specimens, 153 (29.4%) had evidence of infection with a brown-black mold, Exserohilum rostratum, which was also found in several lots of methylprednisolone distributed by the NECC [5]. Initially, patients presented with symptoms and signs of meningitis [1, 5–8]. As the outbreak evolved, there was a shift to a predominance of patients manifesting symptoms and signs attributable to spinal or paraspinal infection. This phenomenon was especially true of cases seen in Michigan [9]. The purpose of this observational study is to describe the clinical, radiological, surgical, and laboratory findings, as well as the diagnostic and therapeutic challenges presented by patients who developed spinal or paraspinal infection after injection with contaminated methylprednisolone.
METHODS
Patients and Setting
A total of 544 people had received at least 1 epidural or paraspinal injection of methylprednisolone from the highly contaminated NECC lot #06292012@26 between August 9, 2012 and October 2, 2012 at an independent pain clinic in southeastern Michigan. Of the 544, 153 (28%) were admitted to SJMH with probable or confirmed spinal and paraspinal infections and are included in this report. The study was approved by the hospital's Institutional Review Board.
Definitions
Epidemiologic case definitions established by the CDC were used to classify patients. Probable spinal and paraspinal infection cases were defined as patients with evidence of osteomyelitis, abscess, or other infection (eg, soft tissue infection) of unknown etiology in the spinal or paraspinal structures at or near the site of epidural or paraspinal injection with contaminated methylprednisolone on or after May 21, 2012 [1, 9, 10].
Confirmed cases were defined as probable cases in which there was evidence by culture, histopathology, or molecular assay of a fungal pathogen associated with the clinical syndrome. Because patients often had injections on more than 1 occasion, we defined the exposure date as the date of the last spinal injection [4, 5].
Imaging
Contrast-enhanced magnetic resonance imaging (MRI) studies were reviewed by 1 of 3 neuroradiologists who used standard definitions as noted previously to classify their findings [11]. In brief, the paraspinal space included paravertebral muscles, facet joints, and structural ligaments. Abscesses, phlegmon, and arachnoiditis, which was defined as nodular or linear enhancement of nerve roots of the cauda equina or spinal cord, were reported. The degree of enhancement was defined as mild when linear changes were noted and moderate or severe when mass-like changes causing architectural distortion were present.
Data Collection and Management
For the 153 patients comprising this study, data were collected from (1) Michigan Department of Community Health case report forms and (2) electronic medical record reviews of the presenting symptoms, comorbidities, radiological findings, intraoperative findings, laboratory test results, and information about antifungal therapy.
Data Analysis
All analyses were performed using SAS, version 9.3 (SAS Institute). Demographic and baseline clinical variables and radiologic, surgical, and pathologic findings were summarized using means, medians, and percentages, as appropriate.
RESULTS
Patients
One hundred fifty-three (28%) of 544 patients who had received a paraspinal or epidural injection with contaminated methylprednisolone were diagnosed at our institution with probable or confirmed fungal infection at the injection site. The mean age of the patients was 64.4 years, and 60% were women. Comorbid conditions, such as hypertension, hyperlipidemia, and diabetes, were common, but few had serious illnesses or immunosuppressive conditions (Table 1). Over 50% of the patients had a body mass index ≥30.
Table 1.
Baseline Clinical and Demographic Information for 153 Patients With Spinal or Paraspinal Fungal Infection
| Characteristic | Frequency (%) |
|---|---|
| Age (mean ± SD) | 64.4 (13.2) |
| Female Sex | 92 (60.1) |
| White | 150 (98.0) |
| BMI ≥30 | 82 (53.6) |
| BMI ≥35 | 40 (26.1) |
| Hypertension | 63 (41.2) |
| Hyperlipidemia | 49 (32.0) |
| Diabetes mellitus | 31 (20.3) |
| Chronic pulmonary disease | 14 (9.2) |
| Renal insufficiency | 7 (4.6) |
| Connective tissue disease | 2 (1.3) |
| Solid tumor | 9 (5.9) |
| Immunosuppressive therapy | 3 (2.0) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Time to Onset of Infection
The median time from last epidural or paraspinal injection to diagnosis was 52 days (range, 21–232 days). Of the 153 patients, 112 (73%) presented with spinal or paraspinal infection alone, and the median time from last injection to diagnosis was 56 days (range, 23–232 days). The remaining 41 cases had both meningitis and spinal or paraspinal infection, and the median time from injection to diagnosis of the spinal or paraspinal infection was 44 days (range, 21–75 days). Fourteen of the 41 patients were diagnosed with spinal or paraspinal infection at the time of initial hospitalization with meningitis. Twenty-seven patients initially had only meningitis and were then readmitted days to weeks later with symptoms of spinal or paraspinal infection. Of note, 8 patients who had meningitis and who had no evidence of spinal or paraspinal infection on an MRI obtained at the time of diagnosis of meningitis showed spinal or paraspinal involvement when an MRI was repeated a median of 26 days (range, 7–38 days) later.
Clinical Findings
Symptoms and signs that patients manifested on presentation varied according to whether they had meningitis plus spinal or paraspinal infection or solely spinal or paraspinal infection (Table 2). Back pain was the most common symptom and was described as either their typical chronic pain or as more severe and unrelenting pain at the injection site. Not surprisingly, headache, neck pain or stiffness, light sensitivity, and nausea and vomiting occurred more commonly in patients who had meningitis. Fever was noted in only 7 patients (17%) who had meningitis and 4 patients (4%) who had only spinal or paraspinal infection.
Table 2.
Clinical Signs and Symptoms at the Time of Initial Hospitalization for 153 Patients With Spinal or Paraspinal Fungal Infectiona
| Symptom | Meningitis Plus Spinal or Paraspinal Infection N = 41 | Spinal or Paraspinal Infection Only N = 112 |
|---|---|---|
| Frequency (%) | Frequency (%) | |
| Headache | 30 (73.2) | 39 (34.8) |
| Light sensitivity | 13 (31.7)) | 5 (4.5) |
| Nausea | 18 (43.9) | 14 (12.5) |
| Vomiting | 9 (22.0) | 4 (3.6) |
| Fever | 7 (17.1) | 4 (3.6) |
| Confusion | 4 (9.8) | 4 (3.6) |
| Visual disturbance | 5 (12.2) | 2 (1.8) |
| Back pain | 17 (41.5) | 76 (67.9) |
| Neck pain | 13 (31.7) | 21 (18.8) |
| Stiff neck | 13 (31.7) | 7 (6.3) |
| Leg pain | 0 | 13 (11.6) |
| Numbness | 2 (4.9) | 7 (6.3) |
| Constipation | 0 | 2 (1.8) |
| Urinary retention | 0 | 1 (0.9) |
| Incontinence | 0 | 2 (1.8) |
| Ataxia | 0 | 1 (0.9) |
a Symptoms were abstracted at the time of first admission for probable fungal infection.
Radiological Findings
All 153 patients had at least 1 MRI study. Involvement was noted at the site of injection of contaminated methylprednisolone in the lumbosacral area in 121 patients (79%), the cervicothoracic area in 22 patients (14%), and the thoracic area in 5 patients (3%). One patient each had thoracolumbar and sacral spine involvement, and 3 others had 2 noncontiguous sites of involvement where contaminated steroid had been injected. Probable spinal or paraspinal infection was found on the first MRI that was performed in 132 patients (86%). For the remaining 21 patients, 2–4 additional MRI studies were required to identify spinal or paraspinal abnormalities suggesting infection. The median time to abnormal findings on follow-up imaging in this group was 28 days (range, 7–129 days). In 39 patients (25.5%), an epidural or paraspinal abscess was the prominent finding (Figure 1), and in 53 patients (35%), an epidural or paraspinal phlegmon was the most notable abnormality. Arachnoiditis was noted in 40 patients (26%) (Figure 2). Many patients had several different MRI abnormalities reported (Table 3). In 29 patients, mild enhancement was the only finding. For 10 of these 29 patients, progression to moderate enhancement was noted on subsequent MRI studies. Twelve of 29 patients with only mild enhancement on MRI underwent surgery, and 6 had laboratory-confirmed infection.
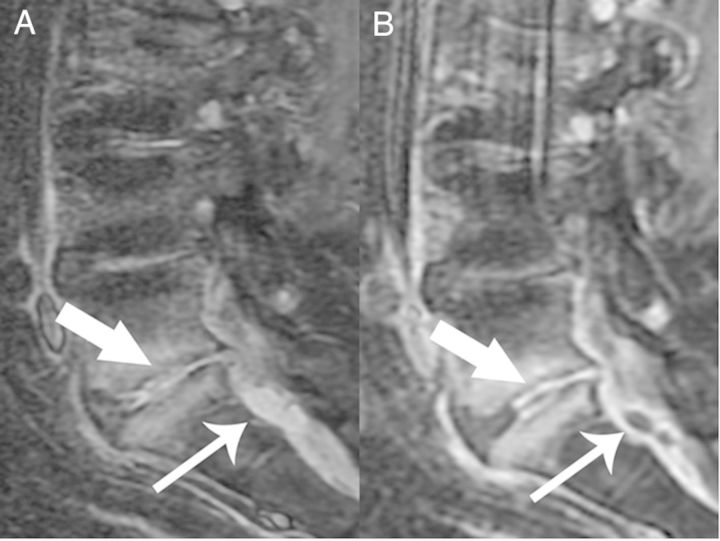
Figure 1.
Sagittal T1 Short T1 Inversion Recovery (A) and postcontrast T1 fat-saturated (B) images demonstrate confluent epidural enhancement with mass effect upon the thecal sac. There are also two small T2 hyperintense, rim-enhancing fluid collections consistent with small epidural abscesses (thin arrows). In addition, there is increased T2 signal and enhancement in the L5-S1disc space consistent with discitis-osteomyelitis (thick arrow).
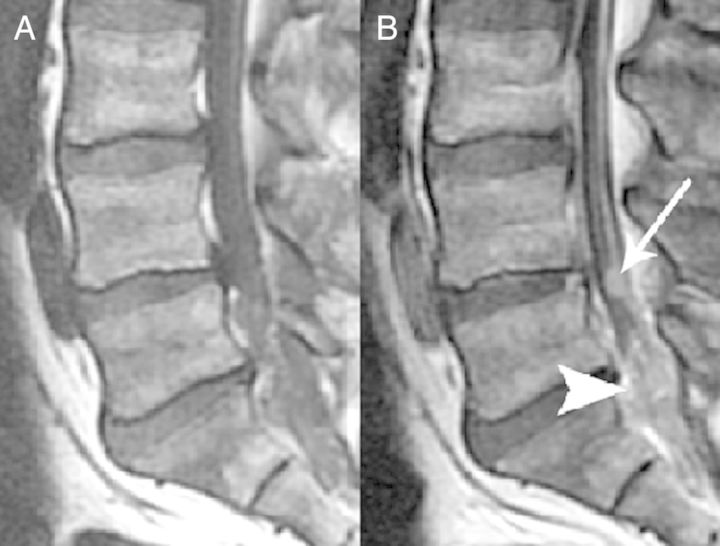
Figure 2.
Sagittal T1 (A) and postcontrast T1-weighted images (B) demonstrate nodular (thin arrow) and confluent (arrowhead) intrathecal enhancement consistent with arachnoiditis.
Table 3.
Results of MRI Studies in 152 Patients With Spinal or Paraspinal Fungal Infectiona
| MRI Findings | First Abnormal MRI Frequency (%)b |
|---|---|
| Epidural abscess | 25 (16.4) |
| Paraspinal abscess | 15 (9.9) |
| Epidural phlegmon | 52 (34.2) |
| Paraspinal phlegmon | 37 (24.3) |
| Arachnoiditis | 40 (26.3) |
| Epidural enhancement | 93 (61.2) |
| Mild | 38 |
| Moderate | 42 |
| Severe | 13 |
| Intraspinal enhancement | 39 (25.7) |
| Mild | 10 |
| Moderate | 20 |
| Severe | 9 |
| Paraspinal enhancement | 93 (61.2) |
| Mild | 43 |
| Moderate | 39 |
| Severe | 11 |
| Osteomyelitis | 24 (15.8) |
Abbreviation: MRI, magnetic resonance imaging.
a One patient with spinal/paraspinal infection alone had an abnormal initial MRI, but it was performed without contrast; results are not included in the table.
b Many patients had more than 1 abnormal finding.
Surgical Intervention
A total of 116 patients (76%) underwent a surgical procedure to decompress neural elements, remove infectious material, and obtain tissue for diagnostic purposes. Most patients underwent either total laminectomy or hemilaminectomy. Operations were performed on the lumbosacral spine in 93 patients (80%), the cervicothoracic spine in 19 patients (16%), and the thoracic spine in 4 patients (3%). Seventy-nine patients (68%) had surgery within 1 week of starting antifungal medication; the other 37 patients were treated with antifungal agents a median of 33 days (range, 9–279 days) before surgery.
Epidural phlegmon with no discrete abscess was the most common finding (70 patients), followed by epidural abscess (30 patients) (Table 4). Collections of purulent material were found in facet joints, paraspinal tissues, and disc spaces. Only 6 patients who had arachnoiditis underwent surgery; in all 6, intradural extension of infection with foci of purulent material and clumping of nerve roots encased in inflammatory tissue were found.
Table 4.
Intraoperative Findings in 116 Patients Who Underwent a Surgical Procedure for Spinal or Paraspinal Fungal Infection
| Surgical Findings | Number (%)a |
|---|---|
| Epidural phlegmon | 70 (60.3) |
| Epidural abscess | 30 (25.9) |
| Epidural fluid | 2 (1.7) |
| Intradural nerve root clumping and purulence | 6 (5.2) |
| Disk infection | 3 (2.6) |
| Paraspinal purulence | 15 (12·9) |
a Several patients had more than 1 abnormal finding.
Before surgery, 80 patients had increased pain at the injection site without neurologic findings (new onset weakness, numbness, urinary incontinence), 9 had increased pain and neurologic signs, 1 had neurologic signs only, and 26 had only MRI abnormalities and no change in their chronic pain. Among these patients, 19 (73%) had evidence of infection found at surgery; epidural phlegmon was identified in 12, epidural abscess in 3, and paraspinal purulence in 4. Intradural infection was not identified in any of these 19 patients.
Laboratory Findings
Seventy-eight of the 153 cases (51%) had laboratory-confirmed infection. Thirty-seven of the 41 patients who had meningitis and spinal or paraspinal infection had cerebrospinal fluid (CSF) tested by polymerase chain reaction (PCR), and 20 (54%) were positive for E rostratum. These patients had CSF white blood cell counts ranging from 6 to 7900 cells/μL (median, 171 cells/μL).
A total of 69 of 116 patients (59.5%) who underwent surgery had confirmation of fungal infection (Table 5). Specimens from 59 of 113 patients (52%) in whom histopathological findings were reported revealed pigmented hyphae, many of which were distorted, lying free in tissues or within macrophages (Figure 3). The tissue reaction was granulomatous or neutrophilic in most specimens, but in some, only minimal inflammation was found. Of 49 patients in whom tissue was sent for PCR testing, 18 (37%) were reported as positive for E rostratum. In 2 other patients, Alternaria species, which were of unclear significance, were detected. Of the 29 patients for whom the PCR assay on tissues was negative, 11 had hyphae seen on histopathological examination. Of the 115 patients in whom cultures were performed, E rostratum was found in only 17 (15%).
Table 5.
Results of Diagnostic Tests for Fungal Pathogens on Specimens Obtained at the Time of Surgical Intervention in Patients With Spinal and Paraspinal Fungal Infection
| Diagnostic Test | Number Tested | Number Positive (%) |
|---|---|---|
| Hyphae on pathology | 113 | 59 (52.2) |
| Fungal culture | 115 | 17 (14.8) |
| Tissue PCR | 49 | 20 (40.81) |
Abbreviation: PCR, polymerase chain reaction.
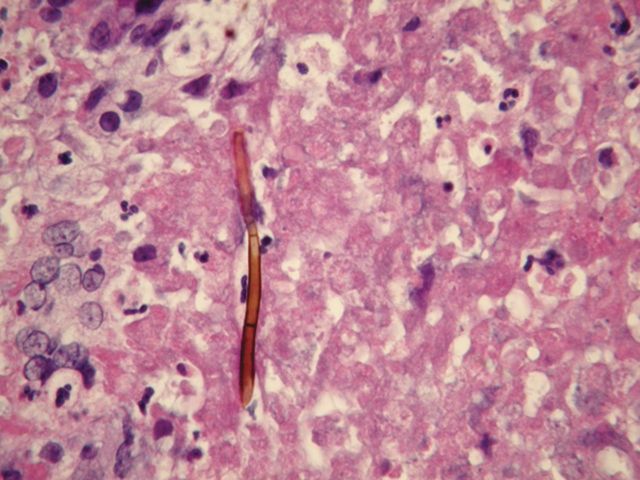
Figure 3.
Pigmented septate hyphal form in epidural abscess found at surgery. Hematoxylin and eosin stain. Original magnification ×400.
Laboratory tests confirmed infection in 63 of the 101 patients in whom abnormalities suggesting infection were found at surgery. For those 15 patients whose intraoperative findings did not suggest infection, fungal infection was confirmed in 6 (40%). Tissue from all 6 showed hyphae on histopathological examination, culture yielded Exserohilum species in 3, and PCR was positive for Exserohilum species in 1.
Antifungal Therapy
Initial antifungal therapy in the 153 patients was voriconazole and liposomal amphotericin B in 115 (75%) and voriconazole alone in 38 patients (25%). The liposomal amphotericin B dose ranged from 5 to 7.5 mg/kg daily, and the voriconazole dose was 6 mg/kg twice daily for the first 5–7 days. After the first week, voriconazole serum concentrations were monitored every 1–2 weeks and the daily dose was modified to achieve a serum concentration between 2 and 5 μg/mL.
The median duration of liposomal amphotericin B therapy for the 115 patients who have completed treatment with this agent was 13 days. This agent was stopped in most patients following recommendations from the CDC suggesting that voriconazole was likely adequate therapy for Exserohilum infection [10]. However, 22 patients (20%) were treated with amphotericin B for at least 4 weeks. The expected toxicities of amphotericin B---rising creatinine, hypokalemia, hypomagnesemia, and infusion reactions---were seen to varying degrees in all patients who received this agent.
The median duration of voriconazole therapy was 177 days. Adverse effects were frequent and included photopsia, visual hallucinations, nausea, and a feeling of “fogginess” and trouble thinking that were especially noticeable for 1–4 h after taking voriconazole. Chapped lips, nail changes, or alopecia developed after several months on voriconazole therapy in many of the patients. Some patients developed pain in their ribs or other long bones; on further workup with bone scan and serum fluoride levels in 21 patients, this pain was found to be due to periostitis. In 59 patients, these adverse effects led to a change in treatment to itraconazole solution or capsules. Three other patients were treated with posaconazole suspension.
Because of the location of infection primarily in the epidural space and our concern that extension or persistence of infection could have very severe consequences, we elected to treat most patients who had epidural abscess or phlegmon infection for a minimum of 6 months with oral azole therapy. Most patients who had arachnoiditis or bony involvement were treated with azoles for a minimum of 12 months.
As of January 31, 2014, 20 patients, all of whom had arachnoiditis or osteomyelitis, remained on azole therapy. One patient, who had severe arachnoiditis and osteomyelitis and who had required 3 surgical procedures, continued to receive outpatient infusion of liposomal amphotericin B until January 30, 2014 in addition to itraconazole.
Outcomes
Eight patients had a protracted course with a poor response to antifungal therapy and repeated imaging studies showing progressive infection. For 3 patients, surgery was undertaken 3–4 months after antifungal treatment had begun. On histopathological examination of surgical specimens from all 3 patients, hyphae were seen in tissues. Another 3 patients underwent surgery after 4–6 months of antifungal treatment: 1 patient had a worsening MRI only and 2 had increasing pain and progressive changes on MRI. Purulence was noted at surgery in all 3 patients, and hyphae were seen on histopathological examination of 2 of the 3 intraoperative specimens. Two patients underwent surgery after 8 and 9 months of antifungal therapy because of persistent pain and worsening MRI findings. Purulent material that showed hyphae was found in both patients.
More than half of the patients had multiple hospital admissions for complications of the infection, the antifungal therapy, or to undergo surgical procedures. Seventy-three were admitted ≥3 times and 12 had ≥6 admissions.
Five patients died of their fungal infection, including 4 who had both meningitis and spinal or paraspinal infection. Three patients, 2 of whom had intradural extension of infection, died within 3 months of diagnosis. Two patients, who each had multiple underlying conditions, did not improve with antifungal therapy and chose comfort care measures 4 months and 5 months after diagnosis, respectively. Most of the patients who had epidural abscess or phlegmon have returned to their baseline functional status. Many continue to have the chronic back pain for which they originally were treated with epidural steroid injections. However, many of the patients who developed arachnoiditis continue to experience perineal pain and radiculopathy symptoms. To date, there have been no relapses of infection.
DISCUSSION
Our study population is unique in that a high proportion (at least 28 per 100 cases exposed) had spinal or paraspinal infections compared with the national estimate of development of meningitis and spinal or paraspinal infections (5.9 per 100 cases exposed) [3, 5]. We can only estimate a lower bound on the incidence because some patients may have been diagnosed at other hospitals in the area. The relatively high incidence could relate, in part, to the fact that all of our patients received lot #06292012@26, which has been shown to have the highest lot-specific attack rate [4–6]. It is also possible that the type of injection given could be related to a higher incidence of spinal or paraspinal infections [9]. It was noted in the series from Tennessee that patients who received methylprednisolone through a translaminar approach had a higher risk of developing infection than those who were received injections by the transforaminal route [4]; most of their patients had meningitis and not spinal or paraspinal infection. Further study has verified a significantly increased risk of development of central nervous system (CNS) disease (meningitis, stroke, arachnoiditis) with the translaminar approach, but studies have also shown increased risk for spinal or paraspinal infection with the transforaminal approach [6, 9]. We did not have patient-specific data, but the pain clinic involved has been noted to prefer transforaminal injections.
It is likely that the proactive approach that we took to perform MRI imaging in exposed patients resulted in the earlier identification of cases. Because more patients were admitted with spinal or paraspinal infections, some of whom were being treated for meningitis, a program was implemented to reach out to all patients who had received injections with lot #06292012@26 to offer them a screening MRI of the injection site, regardless of the presence of symptoms [11]. This program led to the identification of probable spinal or paraspinal infection in 35 patients, 24 of whom ultimately underwent surgical intervention and 17 of whom had laboratory evidence of fungal infection.
We observed that a single MRI study was not always sufficient to rule out infection. Twenty-one patients, for whom the initial MRI showed no evidence of infection, were later found to have phlegmon or abscess when the MRI study was repeated. Screening led to the diagnosis in 35 patients as long as 192 days after the injection [11]. Increasing pain at the site of injection correlated with MRI abnormalities; however, MRI identified several patients who had radiological evidence of spinal or paraspinal disease without an increase in baseline pain symptoms.
Early in the course of defining spinal and paraspinal infections, surgery was limited to patients who had both an abnormal MRI and worsening symptoms. Over time, surgical intervention was offered to patients who had an abnormal MRI without worsening of their chronic back pain symptoms in the belief that surgical debulking could help reduce the organism burden, especially given the impressive purulence noted intraoperatively in several patients who had minimal symptoms before surgery. Of the 116 patients who underwent surgery, 101 had intraoperative findings that were highly suggestive of infection. Laboratory studies on intraoperative specimens from 6 of the 15 patients in whom direct observation showed only minimal changes confirmed fungal infection.
We found that no single diagnostic test was sensitive enough to establish the etiology of infection in all patients. Cultures were infrequently positive for fungi, even in those operative specimens in which hyphae were seen by histopathological examination and in which PCR tests identified E rostratum. The combination of culture, PCR, and histopathology failed to confirm the etiology in 38 patients (33%) in whom a surgical procedure showed an abscess or phlegmon. The insensitivity of culture methods for mold infections of the CNS has been documented previously for Aspergillus and other molds [12–14]. The PCR test for E rostratum, which was developed expeditiously by the CDC after this mold was discovered to be the cause of the outbreak, proved more useful than culture but still had a sensitivity of only 29% [14, 15].
There are several limitations to our report. This a single-center experience and thus may not be generalizable to other centers. We were unable to procure patient-specific records from the pain clinic regarding the number of injections given, the doses of methylprednisolone administered, or the injection approach (transforaminal or translaminar). This information may have allowed further definition of the risks for spinal or paraspinal infection. Quality-of-life measures and pain scores were not systematically collected. Thus, we were unable to quantify the level of improvement with therapy. An ongoing multicenter study sponsored by the CDC and the Mycoses Study Group may help to establish long-term outcomes.
Management of spinal and paraspinal infections that developed at the site of contaminated methylprednisolone injection required a multifaceted diagnostic approach and complex and prolonged treatment regimens. Nearly half of the patients were readmitted to hospital, and several patients required repeated surgical intervention. Prolonged antifungal therapy with voriconazole was associated with many adverse events that, in some patients, proved to be almost as debilitating as the infection for which they were being treated. Most patients have completed therapy with no relapse of infection, but the long-term prognosis of these patients remains unclear.
Notes
Acknowledgments. We thank Jack Sobel, MD, for scientific input and assistance with study design; Mark Cowen, MD, SHMH, his assistance with the Quality Institute database and review of the study design. We also thank our spine-surgical colleagues Geoffrey M. Thomas, MD, Martin J. Buckingham, MD, Mark H. Falahee, MD, Douglas F. Geiger, MD, and Anthony Cucchi, MD, for invaluable expertise and for providing ongoing clinical care for patients; Jeffrey Sanfield, MD, and Thomas M. Shehab, MD, MMM, CPE, for continuing support in organizing a response to this healthcare crisis; the Michigan Department of Community Health for their efforts with the clinical response and for assistance with chart review and data acquisition; and Suzanne Bradley, MD, and Laraine Washer, MD, for volunteering time and clinical expertise during this outbreak
Potential conflicts of interest. V. M. has served on the advisory board for Gilead and Merck and on the Speaker's Bureau for Cubist. A. N. M. has served on the Speaker's Bureau for Cubist and is a shareholder in Pfizer. L. K. is a shareholder in Johnson and Johnson and Merck. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Meningitis and stroke associated with potentially contaminated product. Available at: http://emergency.cdc.gov/HAN/han00327.asp . Distributed via the CDC Health Alert Network October 4, 2012. Accessed 16 July 2013.
- 2.Centers for Disease Control and Prevention. CDC fungal meningitis outbreak case count. Available at: http://www.cdc.gov/hai/outbreaks/meningitis-map-large.html . Accessed 10 December 2013.
- 3.Kauffman CA, Pappas PG, Patterson TF. Fungal infections associated with contaminated methylprednisolone injections. N Engl J Med. 2013;368:2495–500. doi: 10.1056/NEJMra1212617. [DOI] [PubMed] [Google Scholar]
- 4.Kainer MA, Reagan DR, Nguyen DB, et al. Fungal infections associated with contaminated methylprednisolone in Tennessee. N Engl J Med. 2012;367:2194–203. doi: 10.1056/NEJMoa1212972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RM, Schaefer MK, Kainer MA, et al. Fungal infections associated with contaminated methylprednisolone injections. N Engl J Med. 2013;369:1598–609. doi: 10.1056/NEJMoa1213978. [DOI] [PubMed] [Google Scholar]
- 6.Chiller TM, Roy M, Nguyen D, et al. Clinical findings for fungal infections caused by methylprednisolone injections. N Engl J Med. 3012;369:1610–9. doi: 10.1056/NEJMoa1304879. [DOI] [PubMed] [Google Scholar]
- 7.Lyons JL, Gireesh ED, Trivedi JB, et al. Fatal Exserohilum meningitis and central nervous system vasculitis after cervical epidural methylprednisolone injection. Ann Intern Med. 2012;157:835–6. doi: 10.7326/0003-4819-158-1-201212040-00557. [DOI] [PubMed] [Google Scholar]
- 8.Kerkering TM, Grifasi ML, Baffoe-Bonnie AW, et al. Early clinical observations in prospectively followed patients with fungal meningitis related to contaminated epidural steroid injections. Ann Intern Med. 2013;158:154–61. doi: 10.7326/0003-4819-158-3-201302050-00568. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Spinal and paraspinal infections associated with contaminated methylprednisolone acetate injections – Michigan, 2012–2013. MMWR Morb Mortal Wkly Rep. 2013;62:377–81. [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Interim treatment guidance for central nervous system and parameningeal infections associated with injection of contaminated steroid products. Available at: http://www.cdc.gov/hai/outbreaks/clinicians/guidance_cns.html . Accessed 10 December 2013.
- 11.Malani AN, Vandenberg DM, Singal BM, et al. Magnetic resonance imaging screening to identify spinal and paraspinal infections associated with injections of contaminated methylprednisolone acetate. JAMA. 2013;309:2465–72. doi: 10.1001/jama.2013.6293. [DOI] [PubMed] [Google Scholar]
- 12.Hummel M, Spiess B, Kentouche K, et al. Detection of Aspergillus DNA in cerebrospinal fluid from patients with cerebral aspergillosis by nested PCR assay. J Clin Microbiol. 2006;44:3989–93. doi: 10.1128/JCM.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottfredsson M, Perfect JR. Fungal meningitis. Semin Neurol. 2000;20:307–20. doi: 10.1055/s-2000-9394. [DOI] [PubMed] [Google Scholar]
- 14.Gade L, Scheel CM, Pham CD, et al. Detection of fungal DNA in human body fluids and tissues during a multistate outbreak of fungal meningitis and other infections. Eukaryot Cell. 2013;12:677–83. doi: 10.1128/EC.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockhart SR, Pham CD, Gade L, et al. Fungal infections associated with contaminated methylprednisolone injections-preliminary laboratory report. J Clin Microbiol. 2013;51:2654–61. doi: 10.1128/JCM.01000-13. [DOI] [PMC free article] [PubMed] [Google Scholar]