Abstract
Background
Successful hepatitis C virus (HCV) treatment may reduce cardiovascular disease (CVD) risk and improve levels of CVD biomarkers produced outside the liver (nonhepatic biomarkers).
Methods
Stored serum or plasma from before and 24 weeks after end of HCV treatment (EOT) from human immunodeficiency virus (HIV)/HCV-coinfected subjects who received up to 72 weeks of peginterferon/ribavirin, 27 with and 27 without sustained virologic response (SVR) matched by race, ethnicity and sex, were tested for nonhepatic (soluble intercellular adhesion molecule-1 [sICAM-1], soluble P-selectin [sP-selectin], interleukin [IL]-6, d-dimer, and lipoprotein-associated phospholipase A2 [Lp-PLA2]) and hepatic (cholesterol and high-sensitivity C-reactive protein) CVD and macrophage activation markers (soluble CD163 [sCD163] and soluble CD14). Changes in biomarkers and their association with SVR were examined by t tests or Wilcoxon tests and regression models.
Results
Of the 54 subjects, 30 were white, 24 were black, and 44 were male. Pretreatment levels of nonhepatic biomarkers were high: sICAM-1 overall median, 439.2 ng/mL (interquartile range [IQR], 365.6–592.8]; sP-selectin, 146.7 ng/mL (IQR, 94.1–209.9), and IL-6, 2.32 pg/mL (IQR, 1.61–3.49). Thirty-seven of 52 (71%) subjects had Lp-PLA2 >235 ng/mL. Sustained virologic response was associated with decrease in sICAM-1 (P = .033) and sCD163 (P = .042); this result was attenuated after controlling for changes in the alanine aminotransferase level. At 24 weeks after EOT, 17 (63%) SVRs had Lp-PLA2 >235 ng/mL vs 25 (93%) non-SVRs (P = .021).
Conclusions
Hepatitis C virus clearance may reduce hepatic and, subsequently, systemic inflammation and CVD risk in HIV/HCV coinfection.
Keywords: cholesterol, HIV/HCV coinfection, macrophage activation, sustained virologic response, vascular adhesion molecules
Studies in the current era of effective antiretroviral therapy (ART) demonstrate increased overall mortality in human immunodeficiency virus (HIV)/hepatitis C virus (HCV)-coinfected persons compared with HIV-monoinfected control groups, with cardiovascular disease (CVD) being a leading cause of nonliver, non-acquired immune deficiency syndrome (AIDS) deaths [1, 2]. Large observational studies suggest an increased risk of CVD events in HCV-monoinfected and HIV/HCV-coinfected persons compared with uninfected controls [3–6]. Hepatitis C virus may contribute to CVD risk through several mechanisms, including insulin resistance, hepatic steatosis, and increased chronic inflammation and immune activation [7, 8].
Hepatitis C viral eradication may provide benefits beyond improvement in liver-specific outcomes. A recent study demonstrated the association of sustained virologic response (SVR) to peginterferon alfa and ribavirin (PEG/RBV) with reduced nonliver morbidity and mortality in HIV-infected persons, including CVD [9]. Other studies have demonstrated that cardiovascular risk factors such as hepatic steatosis and insulin resistance, as well as T-cell activation, improve with HCV clearance [8, 10, 11]. Paradoxically, successful HCV treatment may unmask underlying CVD risk, through rise in liver-produced CVD biomarkers such as cholesterol and pro-atherogenic lipoproteins, reaching indication for lipid-lowering therapy in some patients [12, 13].
The optimal method for CVD risk assessment in HIV/HCV-coinfected persons is currently unknown. Total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-sensitivity C-reactive protein (hsCRP), which are routinely used, are reduced in the setting of HCV-associated chronic liver disease [14, 15] and may underestimate CVD risk in coinfected persons. Alternatively, nonhepatically produced markers of endothelial dysfunction and inflammation, such as soluble intercellular adhesion molecule-1 (sICAM-1), soluble P-selectin (sP-selectin), lipoprotein-associated phospholipase A2 (Lp-PLA2), d-dimer, and interleukin-6 (IL-6), may be better CVD risk predictors in this setting. Emerging evidence suggests such markers may improve CVD risk stratification in HIV-infected individuals [16, 17]. Their predictive value in coinfection is unknown.
Additional markers that may be useful in assessing CVD risk in coinfected patients are measures of innate immune activation, which have been linked to all-cause mortality and CVD in the setting of HIV. Soluble CD163 (sCD163), a marker of monocyte/macrophage activation, is associated with the presence or burden of atherosclerotic plaque and arterial wall inflammation in predominantly HIV-monoinfected cohorts [18, 19]. Soluble CD14 (sCD14), another marker of macrophage activation and an endotoxin receptor, predicts mortality [20] and subclinical atherosclerosis progression [21] in patients infected with HIV. Levels of sCD163 and sCD14 are increased in chronic liver disease, including viral hepatitis, in the absence of HIV infection [22, 23]. In the context of chronic HIV infection and associated immune activation, HCV coinfection may further accelerate atherosclerosis through stimulating hepatic inflammation, and specifically macrophage activation, thus promoting systemic inflammation and end organ disease such as CVD.
Our aims were to characterize hepatic and nonhepatic CVD biomarker and macrophage activation levels in a HIV/HCV-coinfected cohort and explore the potential benefit of HCV treatment on cardiovascular outcomes, hypothesizing that HCV clearance would be associated with a favorable reduction in nonhepatic CVD biomarkers and markers of macrophage activation.
METHODS
Study Design
We conducted a retrospective case control study analyzing stored serum and plasma samples and clinical data from HIV/HCV-coinfected subjects who participated in the AIDS Clinical Trials Group study A5178, a study of maintenance PEG for reducing fibrosis progression [24]. All subjects included in this analysis achieved an early virologic response (defined as ≥2 log decrease in or undetectable HCV RNA at 12 weeks of treatment) and were assigned to an extended treatment arm that was offered a 72-week course of PEG/RBV. Serum and plasma samples from before treatment at study entry (baseline) and from 24 weeks after end of treatment (EOT+24 weeks) were tested for nonhepatic (sICAM-1, sP-selectin, IL-6, d-dimer, Lp-PLA2) and hepatic (hsCRP and lipids) CVD risk biomarkers and markers of macrophage activation (sCD163 and sCD14). Included were 54 subjects with available stored serum and plasma, 27 with SVR (defined as HCV RNA <60 IU/mL at 24 weeks after EOT) and 27 nonresponders/relapsers (non-SVR), matched simultaneously on race, ethnicity and sex, to exclude their potential confounding [25].
Clinical Data
Baseline and on-treatment data abstracted from the original study dataset included: age; gender; race and ethnicity; presence or absence of hypertension and diabetes; concomitant medications; body mass index; intravenous drug use history; fasting glucose and insulin; aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels; comorbid disease; HCV treatment history; HCV RNA levels at baseline, EOT, and 24 weeks after EOT; HCV genotype; hepatic fibrosis scores by liver biopsy (scoring systems included Knodell, Ludwig, METAVIR, Modified HAI, and Scheuer); CD4 cell count; and HIV RNA level. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as fasting glucose (mg/dL) × fasting insulin (µU/mL)/405.
Biomarkers
Biomarker testing of stored serum and EDTA plasma specimens was performed at a central laboratory. Serum sICAM-1 and plasma sP-selectin were measured by enzyme-linked immunosorbent assay (ELISA) (SearchLight Custom Human ICAM-1 and P-Selectin Assays, Pierce Biotechnology, Inc.); plasma Lp-PLA2 mass was measured by ELISA (PLAC Test, diaDexus, Inc.); plasma d-dimer was measured by immuno-turbidimetric assay (STA-Liatest D-DI, Diagnostica Stago); serum IL-6 was measured by ELISA (Quantikine HS ELISA, R&D Systems, Inc.); plasma sCD163 and sCD14 were measured by ELISA (Macro163, Trillium Diagnostics, LLC and Quantikine Human sCD14 ELISA, R&D Systems, Inc.); serum hsCRP was measured by immunonephelometry (CardioPhase hsCRP, Siemens Healthcare Diagnostics, Inc.); and serum lipid panel was measured by automated enzymatic automated spectrophotometry (Beckman Coulter analyzer). Forty-one of the 54 available subjects had fasting lipids, insulin, and glucose levels available from the parent ACTG study for analysis. A lipid panel was repeated on all subjects, including nonfasting lipids on the 13 subjects without fasting samples.
Data Analysis
The primary outcome was the change in sICAM-1 levels from baseline to 24 weeks after EOT. Secondary outcomes were changes in sP-selectin, IL-6, d-dimer, Lp-PLA2, sCD163, sCD14, total cholesterol, high-density lipoprotein cholesterol, LDL, and triglyceride levels between the same timepoints. Biomarkers below the limit of detection were assigned the lowest level of detection. Analyses of lipid levels were done separately for fasting lipids collected during A5178 and combining retesting of fasting samples and nonfasting samples. Lp-PLA2 levels were analyzed as a continuous variable and categorized into high (>235 ng/mL), intermediate (200–235 ng/mL), or low predicted CVD risk (<200 ng/mL). Liver fibrosis scores were dichotomized as <3 or ≥3, regardless of scoring method. Baseline characteristics and biomarker levels between the SVR and non-SVR groups were compared by Fisher's exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Log10 transformations were utilized for biomarkers with skewed distribution. Associations between biomarkers and baseline variables were examined by simple linear regressions. Within each group, change in each biomarker from baseline to 24 weeks after EOT was compared to zero by paired sample t test or Wilcoxon signed-rank test and between groups by two-sample t test or Wilcoxon test. Linear regressions for the association between SVR and change in each biomarker were performed, adjusting for (1) sex and race or ethnicity to account for the matching in study design, (2) baseline variables that were significantly different by SVR status at a 0.10 significance level with a backward variable selection procedure, (3) change in ALT from baseline to 24 weeks after EOT, (4) duration of PEG/RBV treatment, (5) occurrence of serious infections on study, and (6) concomitant medication use (grouped a priori as immunomodulators, antiplatelet/aspirin, antihypertensives, nonsteroidal anti-inflammatories, and lipid-lowering agents), to examine the potential confounding effect of these covariates. Sensitivity analyses excluding subjects with use of these medications were also conducted to evaluate their effect on the results of analyses. Given concern that HIV viremia might confound biomarker levels, sensitivity analyses were also conducted limiting analyses to those with documented HIV virologic suppression (<50 copies/mL) for the entire duration of the study. Given the expected reduction in power with sensitivity analysis, parameter estimates in these subsets were examined for large shifts.
Sample Size Determination
The study was powered to detect a difference in change in sICAM-1 between the SVR and non-SVR groups. We anticipated a decrease of 45 ng/mL in the change of sICAM-1 from baseline to 24 weeks after EOT in SVRs compared with no change for non-SVRs, the difference associated with improved cardiovascular outcome was reported by Hwang et al [25]. Assuming a standard deviation (SD) of 40.4 for both SVR and non-SVR groups, 20% correlation between baseline and 24 weeks after EOT, and 20% reduction in the SD by matching on race or ethnicity and sex, 54 subjects provided 95% power to detect the projected difference in change in sICAM-1.
Institutional Review Board Approval
Informed consent was obtained from participants for the parent A5178 study, including use of stored samples for research testing. The current analysis was reviewed and approved by the University of California, Los Angeles Institutional Review Board.
RESULTS
Baseline characteristics of the cohort are summarized in Table 1. Median age overall was 48 years (interquartile range [IQR], 44–52). Forty-four of the 54 subjects were male, 30 were white, 24 were black, and all were non-Hispanic. Median CD4 cell count was similar between the groups, and the majority (78%) had HIV-1 RNA <50 copies/mL at baseline. Antiretroviral therapy did not differ between the groups (P = .498) (Table 1). The HOMA-IR, AST, and ALT levels were significantly higher in non-SVR subjects at baseline (see Table 1). Few subjects (3 in the SVR group, 4 in the non-SVR group) had known CVD at baseline. Only 2 of the 54 subjects, both in the non-SVR group, had cirrhosis; 5 (19%) of the SVR group and 13 (48%) of the non-SVR group had significant fibrosis, with a fibrosis score of at least 3. At baseline, 19 (35%) subjects were taking antihypertensive agents, 5 (9%) were taking antiplatelet medications including aspirin, 24 (44%) were taking nonsteroidal anti-inflammatory drugs (NSAIDs), 4 (7%) were taking 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), and 4 (7%) were taking other lipid-lowering agents. Twenty-four subjects (44%) received immunomodulators on study, primarily granulocyte colony-stimulating factor. Median duration of PEG/RBV treatment for the SVR and non-SVR groups was 71.9 (IQR, 71.0–72.1) and 71.4 (IQR, 47.3–72.3) weeks. Of 48 subjects with baseline ART information available, 38 (79%; 21 of 25 with SVR and 17 of 25 non-SVRs) did not change their ART while on study. No subjects initiated ART while on study. No subjects reported a CVD event during the course of the study. Two subjects, both in the non-SVR group, developed diabetes during the study. Eight subjects (15%) experienced a serious infection (3 SVR, 5 non-SVR), including bacterial pneumonia, sepsis, pulmonary histoplasmosis, and cutaneous varicella zoster.
Table 1.
Baseline Characteristics of the Study Cohort
| Characteristic | Overall (n = 54) Median (Q1, Q3) or n (%) | SVR (n = 27) Median (Q1, Q3) or n (%) | Non-SVR (n = 27) Median (Q1, Q3) or n (%) | P Value (SVR vs Non-SVR)* |
|---|---|---|---|---|
| Age (years) | 48 (44, 52) | 47 (42, 52) | 48 (45, 52) | .069 |
| Sex: Male | 44 (81) | 22 (81) | 22 (81) | N/A (matched) |
| Race | ||||
| White | 30 (56) | 15 (56) | 15 (56) | N/A (matched) |
| Black | 24 (44) | 12 (44) | 12 (44) | |
| Body mass index | 25.9 (23.8, 29.5) | 26.1 (23.2, 30.0) | 25.5 (24.1, 28.5) | .592 |
| HIV-1 RNA Undetectable (<50 copies/mL) | 42 (78) | 20 (74) | 22 (81) | .745 |
| CD4 cell count | 544 (370, 734) | 571 (378, 747) | 536 (357, 734) | .616 |
| HCV genotype | ||||
| 1 | 41 (76) | 18 (87) | 23 (85) | .145 |
| 2 | 9 (17) | 7 (26) | 2 (7) | |
| 3 | 3 (6) | 2 (7) | 1 (4) | |
| 4 | 1 (2) | 0 (0) | 1 (4) | |
| Cirrhosis | 2 (4) | 0 (0) | 2 (7) | .491 |
| Fibrosis stage ≥3 | 18 (33) | 5 (19) | 13 (48) | .042 |
| HCV RNA (log10 copies/mL) | 6.60 (6.10, 6.98) | 6.41 (5.73, 6.92) | 6.70 (6.31, 7.04) | .084 |
| History of prior HCV treatment | 15 (28) | 4 (15) | 11 (41) | .066 |
| History of CVD | 7 (13) | 3 (11) | 4 (15) | 1.000 |
| Hypertension | 14 (26) | 5 (19) | 9 (33) | .352 |
| Diabetes | 7 (13) | 2 (7) | 5 (19) | .420 |
| History of injection drug use | ||||
| Current | 1 (2) | 1 (4) | 0 (0) | .785 |
| Former | 30 (56) | 14 (52) | 16 (59) | |
| Never | 23 (43) | 12 (44) | 11 (41) | |
| HOMA-IR (SVR, n = 22; non-SVR, n = 16) | 4.00 (1.65, 6.27) | 2.97 (1.43, 4.49) | 6.11 (3.73, 8.77) | .007 |
| AST (U/L) | 50 (33, 72) | 41 (30, 60) | 63 (42, 85) | .007 |
| ALT (U/L) | 64 (43, 79) | 58 (37, 73) | 68 (51, 91) | .045 |
| Antiretroviral therapy (ART) | ||||
| Unboosted protease inhibitor | 15 (28) | 9 (33) | 6 (22) | .498 |
| Ritonavir-boosted protease inhibitor | 13 (24) | 5 (19) | 8 (30) | |
| No protease inhibitor | 19 (35) | 11 (41) | 8 (30) | |
| No ART | 1 (2) | 0 (0) | 1 (3) | |
| Unknown | 6 (11) | 2 (7) | 4 (15) | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CVD, cardiovascular disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HOMA-IR, homeostatic model assessment of insulin resistance; N/A, not applicable; SVR, sustained virologic response.
* Categorical variables compared by Fisher exact test, continuous variables by Wilcoxon test.
Baseline (pretreatment) levels of the biomarkers by SVR status are shown in Table 2. Overall, levels of the nonhepatic biomarkers (sICAM-1, sP-selectin, IL-6, and Lp-PLA2) and macrophage activation markers were high. Thirty-seven of 52 (71%) subjects with available Lp-PLA2 measures had Lp-PLA2 level >235 ng/mL. Of note, 24 (44%) subjects had undetectable d-dimer (<0.22 mcg/mL) at baseline (13 SVR, 11 non-SVR). Biomarker levels were similar between the SVR and non-SVR groups for all biomarkers except sCD163, total cholesterol, and LDL. Baseline sCD163 levels were significantly lower in the SVR group (median, 2056 ng/mL [IQR, 1172–3006] vs 2933 ng/mL [IQR, 2094–4129]; P = .013) and were positively associated with fibrosis stage (P < .001) and baseline ALT (P < .001) in univariate analysis. Adjusting for fibrosis, there was no significant difference in baseline sCD163 by SVR status (P = .094). Subjects with a fibrosis score of 3 or greater also had a significantly lower total cholesterol level (median, 157 mg/dL [IQR, 135–157]) than those with less fibrosis (177 mg/dL [IQR, 146–207]; P = .034). Likewise, LDL level was lower among subjects with higher fibrosis score (86 mg/dL [IQR, 51–109] vs 103 mg/dL [IQR, 78–124]), but the difference did not reach statistical significance (P = .130).
Table 2.
Baseline Biomarker Levels by SVR Status
| Biomarker | Overall (n = 54) Median (Q1, Q3) | SVR (n = 27) Median (Q1, Q3) | Non-SVR (n = 27) Median (Q1, Q3) | P Value (SVR vs Non-SVR)* |
|---|---|---|---|---|
| sICAM-1 (ng/mL) | 439.2 (365.6, 592.8) | 428.6 (355.1, 478.8) | 476.6 (366.9, 744.5) | .156 |
| sP-selectin (ng/mL) | 146.7 (94.1, 209.9) | 153.6 (110.8, 225.9) | 146.4 (89.4, 170.6) | .276 |
| IL-6 (pg/mL)† | 2.32 (1.61, 3.49) | 2.43 (1.55, 3.60) | 2.32 (1.61, 3.49) | .943 |
| Lp-PLA2 (ng/mL)† | 312.5 (234.5, 382.5) | 315.5 (231.0, 394.0) | 297.5 (235.0, 371.0) | .934 |
| d-dimer (mcg/mL) | 0.25 (0.22, 0.48) | 0.24 (0.22, 0.36) | 0.31 (0.22, 0.52) | .239 |
| sCD163 (ng/mL)† | 2464 (1522, 3480) | 2056 (1172, 3006) | 2933 (2094, 4129) | .013 |
| sCD14 (ng/mL)† | 1894 (1719, 2484) | 2032 (1622, 2484) | 1866 (1724, 2591) | .950 |
| Total cholesterol (mg/dL) | 168 (139, 192) | 188 (169, 222) | 153 (135, 167) | .002 |
| LDL (mg/dL)† | 95 (76, 124) | 112 (85, 125) | 81 (68, 108) | .015 |
| Triglycerides (mg/dL) | 143 (102, 225) | 144 (107, 243) | 141 (97, 215) | .665 |
| HDL (mg/dL) | 36 (31, 48) | 38 (31, 50) | 36 (31, 46) | .545 |
| hsCRP (mg/L) | 1.10 (0.70, 2.60) | 1.15 (0.70, 3.20) | 1.00 (0.60, 2.60) | .476 |
Abbreviations: HDL, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein cholesterol; Lp-PLA2, lipoprotein-associated phospholipase A2; sCD14, soluble CD14; sCD163, soluble CD163; sICAM-1, soluble ICAM-1; sP-selectin, soluble P-selectin; SVR, sustained virologic response.
* Wilcoxon rank-sum test.
† One subject in each group missing Lp-PLA2, one subject in SVR group missing IL-6, one subject in SVR group missing sCD163 and sCD14.
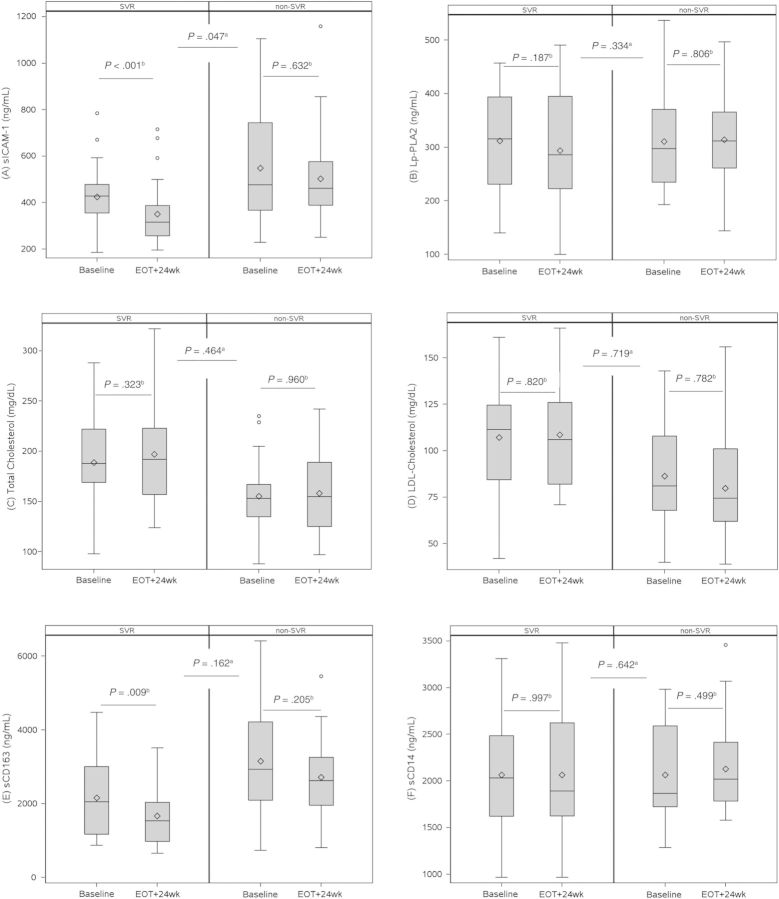
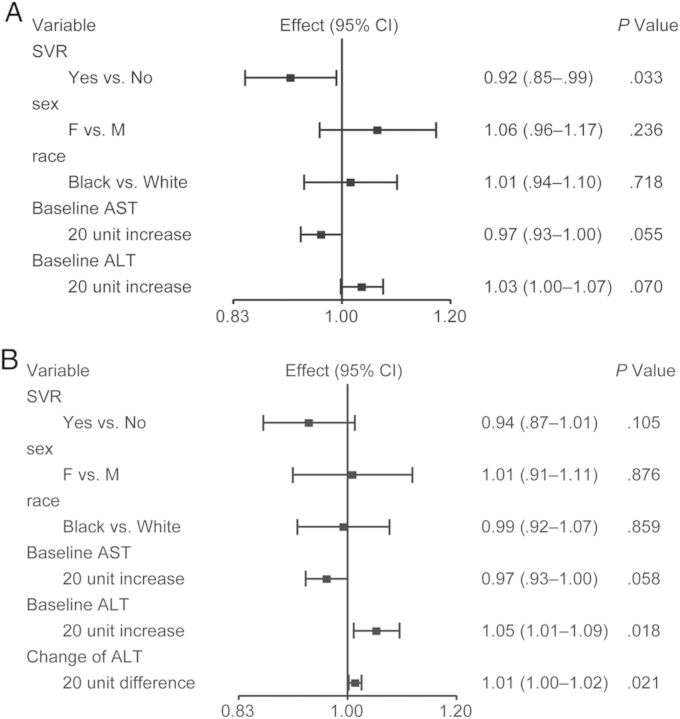
Box plots depicting the change in biomarker levels by SVR status are provided in Figure 1 and Supplementary Figure 1. Supplementary Figure 2 provides spaghetti plots of the within-subject change in biomarker levels. Levels of sICAM-1 decreased significantly from week 0 to 24 weeks after EOT in the SVR group (mean change in log10 sICAM-1 = −0.09 [SD = 0.13]; P < .001), but they remained unchanged in the non-SVR group (mean change in log10 sICAM-1 = −0.01 [SD = 0.14]; P = .632). The change in sICAM-1 comparing SVR and non-SVR groups was statistically significant (P = .047). In regression analysis controlling for sex and race, SVR status was significantly associated with decrease in log10 sICAM-1 (P = .042). After a backward variable selection procedure including liver fibrosis stage, HCV RNA level, HCV treatment history, and baseline AST and ALT level, which differed between the SVR and non-SVR groups at baseline, only baseline AST and ALT were retained, and SVR status remained statistically significantly associated with decrease in log10 sICAM-1 (P = .033) (Figure 2A). After further adjusting for change in ALT from baseline to 24 weeks after EOT, SVR status was no longer associated with the change in log10 sICAM-1 (P = .105) (Figure 2B). Change in ALT was significantly associated with change in log10 sICAM-1 (P = .021). There was no association between duration of PEG/RBV treatment and change in sICAM-1.
Figure 1.
Effect of SVR on biomarker levels. The length of the box represents the interquartile range (distance between the 25th and 75th percentiles), the horizontal lines within the boxes represent the group median, the diamonds represent the group mean, the whiskers represent the minimum and maximum values within a distance of 1.5 times the IQR below the 25th percentile and above the 75th percentile, and the open circles represent outliers. P values provided are based on abetween-group and bwithin-group t tests; for sICAM-1 and sCD163, t tests were conducted on log10-transformed values. Abbreviations: EOT, end of HCV treatment; LDL, low-density lipoprotein cholesterol; Lp-PLA2, lipoprotein-associated phospholipase A2; sCD163, soluble CD163; sCD14, soluble CD14; sICAM-1, soluble intercellular adhesion molecule-1; SVR, sustained virologic response.
Figure 2.
(A) Regression of change in log10 soluble intercellular adhesion molecule-1 (sICAM-1) after backward variable selection. Variables included in backward variable selection: baseline hepatic fibrosis stage, baseline hepatitis C virus (HCV) RNA level, HCV treatment history (naive or experienced), baseline aspartate aminotransferase (AST), and baseline alanine aminotransferase (ALT) level. (B) Regression of change in log10 sICAM-1 with backward variable selection and adjusting for change in ALT level from baseline to 24 weeks after end of HCV treatment.
On-study occurrence of serious infections was not significantly associated with SVR status (P = .365). Adjusting for occurrence of serious infection, change in log10 sICAM-1 remained significantly associated with SVR (P = .025). Because more than 20% of subjects received medications that may have affected biomarker levels (24 with immunomodulators, 26 with antihypertensives, and 32 with NSAIDS), sensitivity analyses were conducted excluding these subjects. Statistically significant between-group differences in log10 sICAM-1 persisted with exclusion of subjects with antihypertensive use. No statistically significant between-group differences were seen after excluding subjects with immunomodulator use (n = 25) or with NSAID use (n = 20). Fourteen SVR subjects and 8 non-SVR subjects had documented HIV virologic suppression for the entire PEG/RBV treatment period. Restricting analyses to these subjects, the mean change in log10 sICAM-1 among SVRs was −0.09 (SD = 0.12) compared with 0.02 (SD = 0.17) among non-SVRs (within group P = .015 and P = .750; between-group P = .129).
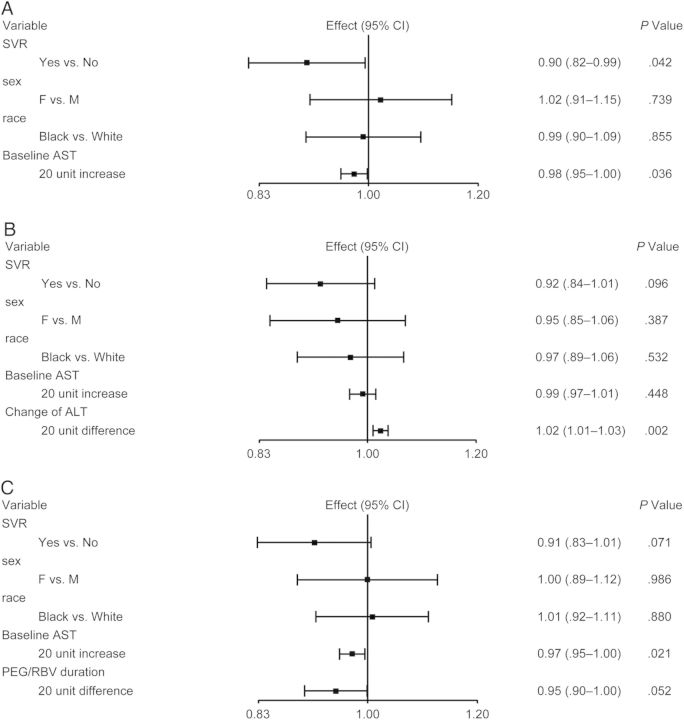
Soluble CD163 levels decreased significantly from week 0 to 24 weeks after EOT in SVRs (mean change in log10 sCD163 = −0.11 [SD = 0.19]; P = .009) but not in non-SVRs (mean change in log10 sCD163 = −0.04 [SD = 0.15]; P = .205). After backward variable selection, only baseline AST, in addition to race and sex, were retained. Sustained virologic response remained significantly associated with change in log10 sCD163 (P = .042) (Figure 3A). Adjusting further for change in ALT resulted in loss of the association between SVR status and change in log10 sCD163 (P = .096), as was seen with sICAM-1 models; in this model, change in ALT was significantly associated with change in sCD163 (P = .002) (Figure 3B). With further adjustment for duration of PEG/RBV, SVR and duration of PEG/RBV were associated with change in sCD163, but statistical significance was not reached (P = .071 and P = .052, respectively) (Figure 3C).
Figure 3.
(A) Regression of change in log10 soluble CD163 (sCD163) after backward variable selection. Variables included in backward variable selection: baseline hepatic fibrosis stage, baseline hepatitis C virus (HCV) RNA level, HCV treatment history (naive or experienced), baseline aspartate aminotransferase (AST), and baseline alanine aminotransferase (ALT) level. (B) Regression of change in log10 sCD163 with backward variable selection and adjusting for change in alanine aminotransferase (ALT) level from baseline to 24 weeks after end of HCV treatment. (C) Regression of change in log10 sCD163 with backward variable selection and adjusting for duration of pegylated interferon plus ribavirin (PEG/RBV) therapy.
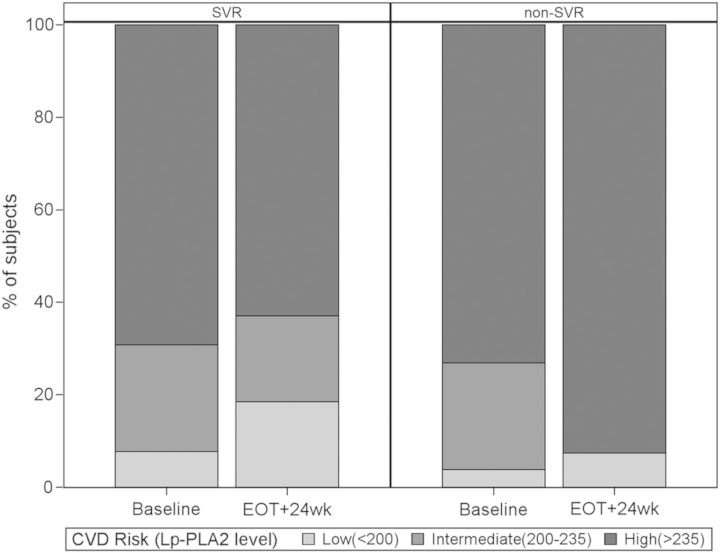
At baseline, there was no difference in CVD risk class by Lp-PLA2 level, whereas after PEG/RBV treatment, Lp-PLA2 levels decreased, although not statistically significantly, among SVRs (median change = −17.5 ng/mL [IQR, −67.0 to 47.0]) but not non-SVRs (median change = 9.50 ng/mL [IQR, −49.0 to 67.0]). There was a statistically significant difference in CVD risk class distribution by Lp-PLA2 level at 24 weeks after EOT (Figure 4), with 25 of 27 (93%) of the non-SVR group in the high-risk category compared with 17 of 27 (63%) of the SVR group (P = .021). There were no statistically significant differences in the other CVD biomarkers comparing SVR and non-SVR groups.
Figure 4.
Effect of SVR on cardiovascular risk class by Lp-PLA2 level. The Lp-PLA2 levels are provided in ng/mL. Abbreviations: EOT, end of hepatitis C virus treatment; Lp-PLA2, lipoprotein-associated phospholipase A2; SVR, sustained virologic response.
DISCUSSION
Prior to HCV treatment, subjects had high levels of the nonhepatic CVD biomarkers sICAM-1, sP-selectin, IL-6, and Lp-PLA2, exceeding levels in HIV-uninfected persons at high risk for CVD or with known baseline CVD [25–29], suggesting higher CVD risk in HIV/HCV coinfection. Levels of sICAM-1, sP-selectin, and IL-6 in our analysis were consistent with those seen in other studies of coinfected persons [30, 31]. Soluble ICAM-1 levels were similar to and sP-selectin levels higher than those seen in an HIV-infected cohort (of which 7% were coinfected) on long-term ART [32]. Interleukin-6 and d-dimer levels were similar to those seen in recent studies of HIV-infected persons who went on to have CVD and other serious non-AIDS-defining events or death [33, 34]. The majority of subjects had Lp-PLA2 levels that would classify them as high risk. The high levels of inflammatory biomarkers in our study may be driven by a combination of HCV replication, fibrotic liver disease, comorbid conditions including insulin resistance, dyslipidemia, and smoking, and ART. Residual HIV replication may also have contributed, although the majority of subjects had suppressed HIV RNA. In contrast to the high levels of nonhepatic biomarkers, total cholesterol and LDL levels were low or optimal in the study participants, with higher levels in the SVR compared with the non-SVR group, driven in part by degree of hepatic fibrosis. The lack of association of the nonhepatic CVD biomarkers with degree of liver disease suggests they may be less confounded by liver disease effects and more readily interpretable in HCV-coinfected patients, compared with hepatic markers. Their incorporation into risk prediction paradigms for the general population has been limited, because they do not, in uninfected persons, provide incremental risk discrimination. In the context of perturbed traditional risk markers such as cholesterol in chronic HCV infection, there may be a distinct role for these biomarkers in HIV/HCV-coinfected or HCV-monoinfected persons.
Levels of sCD163 in our study were higher than those seen in cohorts of predominantly HIV-infected persons on ART with significant subclinical atherosclerotic disease [18, 19] and levels of sCD14 similar to those seen in studies of HIV-monoinfected persons, where sCD14 levels predicted subclinical atherosclerotic disease progression [21]. We found sCD163 levels, but not sCD14 levels, were associated with degree of hepatic fibrosis and baseline ALT (greater fibrosis and higher ALT with higher sCD163 levels). Different results observed between sCD163 and sCD14 suggest differential sensitivity of these 2 macrophage activation markers to hepatic inflammation and extent of liver disease. Interpretation of sCD163 levels in HIV-infected persons must take into account degree of liver disease, which may be confounding. Our analyses also support a complex relationship among chronic viral coinfection, liver disease, and immune dysregulation with systemic immune activation.
The magnitude of the change in sICAM-1 observed with HCV clearance was greater than the difference in sICAM-1 observed in studies comparing subjects who went on to have a coronary disease event and those who did not [25, 35]. The association of SVR with sICAM-1 and sCD163 no longer existed after adjusting for change in ALT, suggesting that the effect of SVR on sICAM-1 and sCD163 levels may be mediated by reduction in hepatic inflammation. This result is a distinct and new finding from other studies that have explored the effect of HCV treatment and eradication on biomarkers such as sICAM-1, and it is a new observation for sCD163 [36, 37]. Also suggestive is that SVR was associated with stable Lp-PLA2 levels over 96 weeks, whereas non-SVRs had a shift to higher CVD risk class by Lp-PLA2 level. It is possible that HCV virologic clearance attenuates atherosclerotic disease progression in persons infected with HIV. We observed that hepatically produced markers did not change significantly with HCV treatment and remained low, possibly due to persistent effects from fibrotic liver disease or limited power. The reliability of cholesterol levels as CVD risk predictors in the context of HCV-associated liver disease remains unknown.
Liver-related deaths alone do not explain excess mortality in HIV/HCV-coinfected patients on effective ART. Chronic and high levels of viral replication and possibly increased gut permeability and microbial translocation/endotoxemia are associated with systemic immune activation in HIV, and they are thought to be major drivers of HIV-1 disease progression. Hepatitis C virus coinfection and ongoing HCV viremia may augment such immune activation, complicating the interaction between HIV and host innate immune response. Impaired lipopolysaccharide tolerance in the setting of chronic HCV infection may lead to chronic intrahepatic monocyte and macrophage activation, hepatic inflammation, and further systemic immune activation and inflammation [38]. As such, it may be that HCV acts additively, or even synergistically, to drive immune activation and extrahepatic complications. Treatment and clearance of HCV may reduce hepatic and, subsequently, systemic inflammation and its associated complications. This finding is supported by several studies to date describing decreased all-cause and nonliver mortality with SVR in HCV-monoinfected and coinfected patients [9, 39, 40].
Limitations of the study include its retrospective nature with use of frozen samples for which the stability of all the assays has not been confirmed; lack of smoking history, which is relevant to the outcomes of interest, although we would not expect there to be between-group differences in smoking status that would affect biomarker levels or changes; inclusion of a select group of interferon-responsive individuals, such that generalizability may be limited; and possible confounding by HIV viremia or concomitant medication use, which we explored in sensitivity analyses (finding similar point estimates for change in biomarker levels, with substantially reduced sample size likely limiting our power to find statistically significant differences between the groups). The study was powered to detect a significant change in sICAM-1 levels, and it may have been underpowered for evaluation of the other biomarkers. Interpretation of the favorable changes in sICAM-1 and Lp-PLA2 is limited given the small size of our cohort, lack of validation of these biomarkers for CVD risk prognostication in HIV/HCV coinfection, and inability to correlate biomarker levels with hard outcomes such as CVD events or validated surrogate measures of cardiovascular risk.
Despite these limitations, our data remain suggestive and further investigation into characterizing the predictive utility of both nonhepatic and hepatic CVD biomarkers and quantifying the potential benefit of HCV treatment on nonliver outcomes such as CVD is warranted. Today, aging HIV-infected patients on effective ART are at increasing risk for non-AIDS complications including CVD, which may be further accelerated by HCV coinfection. Successful HCV treatment may be a viable method for CVD risk reduction and an additional indication for earlier HCV treatment in HIV/HCV-coinfected persons.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Dr William A. Meyer III and Mr Larry Hirsch of Quest Diagnostics, Inc. for assistance with performance of the laboratory assays included in this analysis.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support. This work was supported by seed grants from the UCLA AIDS Institute/Center for AIDS Research, National Institute of Allergy and Infectious Diseases at the National Institutes of Health P30AI028697 and the Campbell Foundation (Award Number 6-11). Support was also provided by the AIDS Clinical Trials Group, Award Number UM1AI068636 from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH) and National Institute of Dental and Craniofacial Research (NIDCR), as well as the National Institutes of Health Awards K24DK078772 (to R. T. C.) and T32MH080634 (support for K. W. C.).
Potential conflicts of interest. K. W. C., L. B., and J. W. A. report no conflicts of interest. L. H. is now employed by Vertex Pharmaceuticals, Inc. and may own stock or stock options in Vertex Pharmaceuticals, Inc. D. B. has received research support from Vertex Pharmaceuticals, Inc. A. A. B. is the recipient of investigator-initiated grants from Merck & Co. and Pfizer. R. T. C. has received grant support from Gilead, Mass Biologics, Vertex, and has served as a consultant for Abbvie. J. S. C. has received grant support from Merck & Co. and served as a consultant for Viiv and Serono. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hernando V, Perez-Cachafeiro S, Lewden C, et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J Hepatol. 2012;57:743–51. doi: 10.1016/j.jhep.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Branch AD, Van Natta ML, Vachon ML, et al. Mortality in hepatitis C virus-infected patients with a diagnosis of AIDS in the era of combination antiretroviral therapy. Clin Infect Dis. 2012;55:137–44. doi: 10.1093/cid/cis404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt AA, Xiaoqiang W, Budoff M, et al. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–32. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CC, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4:425–32. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsui JI, Whooley MA, Monto A, et al. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fail. 2009;15:451–6. doi: 10.1016/j.cardfail.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao CC, Su TC, Sung FC, et al. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One. 2012;7:e31527. doi: 10.1371/journal.pone.0031527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs A, Al-Harthi L, Christensen S, et al. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197:1402–7. doi: 10.1086/587696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez VD, Falconer K, Blom KG, et al. High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol. 2009;83:11407–11. doi: 10.1128/JVI.01211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berenguer J, Rodriguez E, Miralles P, et al. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with human immunodeficiency virus and hepatitis c virus. Clin Infect Dis. 2012;55:728–36. doi: 10.1093/cid/cis500. [DOI] [PubMed] [Google Scholar]
- 10.Castera L, Hezode C, Roudot-Thoraval F, et al. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53:420–4. doi: 10.1136/gut.2002.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conjeevaram HS, Wahed AS, Afdhal N, et al. Changes in Insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology. 2011;140:469–77. doi: 10.1053/j.gastro.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Corey KE, Kane E, Munroe C, et al. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030–7. doi: 10.1002/hep.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaguarnera M, Giugno I, Trovato BA, et al. Lipoprotein(a) concentration in patients with chronic active hepatitis C before and after interferon treatment. Clin Ther. 1995;17:721–8. doi: 10.1016/0149-2918(95)80048-4. [DOI] [PubMed] [Google Scholar]
- 14.Polgreen PM, Fultz SL, Justice AC, et al. Association of hypocholesterolaemia with hepatitis C virus infection in HIV-infected people. HIV Med. 2004;5:144–50. doi: 10.1111/j.1468-1293.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 15.Reingold J, Wanke C, Kotler D, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–8. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–17. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelesidis T, Kendall MA, Yang OO, et al. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rode A, Nicoll A, Moller HJ, et al. Hepatic macrophage activation predicts clinical decompensation in chronic liver disease. Gut. 2013;62:1231–2. doi: 10.1136/gutjnl-2012-304135. [DOI] [PubMed] [Google Scholar]
- 23.Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–30. doi: 10.1053/j.gastro.2011.06.063. 1230.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung RT, Umbleja T, Chen JY, et al. Extended therapy with pegylated interferon and weight-based ribavirin for HCV-HIV coinfected patients. HIV Clin Trials. 2012;13:70–82. doi: 10.1310/hct1302-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1997;96:4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–5. doi: 10.1161/01.cir.103.4.491. [DOI] [PubMed] [Google Scholar]
- 28.Caslake MJ, Packard CJ, Robertson M, et al. Lipoprotein-associated phospholipase A(2), inflammatory biomarkers, and risk of cardiovascular disease in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Atherosclerosis. 2010;210:28–34. doi: 10.1016/j.atherosclerosis.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Blankenberg S, Rupprecht HJ, Bickel C, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336–42. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 30.Masia M, Padilla S, Robledano C, et al. Evaluation of endothelial function and subclinical atherosclerosis in association with hepatitis C virus in HIV-infected patients: a cross-sectional study. BMC Infect Dis. 2011;11:265. doi: 10.1186/1471-2334-11-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masia M, Robledano C, Lopez N, et al. Treatment for hepatitis C virus with pegylated interferon-{alpha} plus ribavirin induces anti-atherogenic effects on cardiovascular risk biomarkers in HIV-infected and -uninfected patients. J Antimicrob Chemother. 2011;66:1861–8. doi: 10.1093/jac/dkr202. [DOI] [PubMed] [Google Scholar]
- 32.Ronsholt FF, Ullum H, Katzenstein TL, et al. Persistent inflammation and endothelial activation in HIV-1 infected patients after 12 years of antiretroviral therapy. PLoS One. 2013;8:e65182. doi: 10.1371/journal.pone.0065182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210:1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordell AD, McKenna M, Borges AH, et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3:e000844. doi: 10.1161/JAHA.114.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt C, Hulthe J, Fagerberg B. Baseline ICAM-1 and VCAM-1 are increased in initially healthy middle-aged men who develop cardiovascular disease during 6.6 years of follow-up. Angiology. 2009;60:108–14. doi: 10.1177/0003319708316899. [DOI] [PubMed] [Google Scholar]
- 36.Guzman-Fulgencio M, Berenguer J, de Castro IF, et al. Sustained virological response to interferon-alpha plus ribavirin decreases inflammation and endothelial dysfunction markers in HIV/HCV co-infected patients. J Antimicrob Chemother. 2011;66:645–9. doi: 10.1093/jac/dkq518. [DOI] [PubMed] [Google Scholar]
- 37.de Castro IF, Micheloud D, Berenguer J, et al. Hepatitis C virus infection is associated with endothelial dysfunction in HIV/hepatitis C virus coinfected patients. AIDS. 2010;24:2059–67. doi: 10.1097/QAD.0b013e32833ce54d. [DOI] [PubMed] [Google Scholar]
- 38.Rotman Y, Liang TJ. Coinfection with hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. J Virol. 2009;83:7366–74. doi: 10.1128/JVI.00191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 40.Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–16.e1. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.