Abstract
Background
Identifying factors associated with tuberculosis (TB) deaths will inform efforts to prevent deaths.
Methods
We examined deaths among patients with culture-confirmed TB reported to the California TB Registry during 1994–2008. We calculated the age-adjusted percentage of deaths before and during TB treatment and estimated trends. We constructed multivariable logistic regression models to identify factors associated with death during treatment.
Results
Of 40 125 patients with culture-confirmed TB, 4565 (11%) died: 1146 (25%) died before treatment started, and 3419 (75%) died during treatment. The age-adjusted percentage of patients who died before and during treatment declined from 1994 to 2008 (3.5% to 2%, and 10.4% to 7.2%, respectively, both P < .0001). We identified several risk factors for death that may be addressed with public health efforts: acquired multidrug resistance (adjusted odds ratio [aOR] = 4.67; 95% confidence interval [CI], 2.09–10.45); care in the private sector (aOR = 3.08; 95% CI, 2.75–3.44); and an initial treatment regimen of <3 drugs (aOR = 2.07; 95% CI, 1.63–2.64). We identified other risk factors for death that could be used as markers for intensified diagnostic and treatment processes in hospital: human immunodeficiency virus coinfection; meningeal, peritoneal, and disseminated TB; substance use; and abnormal chest radiograph without cavities.
Conclusions
In California, 1 in 9 TB patients died with a potentially curable disease. Public health departments might prevent deaths in patients with TB by strengthening partnerships with private providers, intensifying diagnostic and treatment processes for patients at risk of death in hospital, optimizing treatment regimens for patients with comorbidities, and preventing the acquisition of drug resistance.
Keywords: tuberculosis, death, public health, risk factors, trends
In the United States, many patients with tuberculosis (TB) die despite the availability of advanced diagnostic tests and curative treatment [1]. Clinical treatment trials have demonstrated contributors to death, including older age; extensive lung damage; human immunodeficiency virus (HIV) coinfection; Mycobacterium tuberculosis drug resistance; other comorbidities such as malignancy [2] and iron deficiency anemia [3]; and daily alcohol consumption and unemployment [2]. However, few large population-based studies have examined factors that contribute to death in a modern-era setting with relatively high resources for TB care and low TB incidence.
We used population-based surveillance data to determine the magnitude; trends; and sociodemographic, clinical, treatment, and provider characteristics of TB patients who died in California during 1994–2008, a period of decreasing TB incidence in the United States [4]. We sought to identify risk factors associated with TB deaths in California, particularly those that can be modified or used to focus interventions on patients at highest risk of death.
METHODS
Study Design and Population
We assessed characteristics and trends of patients who died with TB. We retrospectively reviewed surveillance data from TB cases that were reported to the California TB Registry from January 1, 1994 through December 31, 2008. The California TB Registry is part of the National TB Surveillance System, which captures information on all reportable TB cases [5]. The California TB and AIDS registries were matched to identify TB/HIV-coinfected patients.
Definitions
We defined a TB patient as a person who had a culture that was positive for M. tuberculosis and was reported to the California TB Registry. A TB death was a TB patient who died at any time before or during treatment. For TB deaths during treatment, we included data for each TB patient who started treatment until final treatment disposition was assessed (eg, completion of treatment, death, default from treatment). The comparison group for patients who died during treatment was patients who completed treatment; those who defaulted were excluded because final outcomes could not be ascertained. Among TB patients who died during therapy, we further defined early deaths (during the first 60 days of treatment) and late deaths (at least 61 days of treatment). The comparison group for early deaths was patients who completed at least 60 days of anti-TB therapy. The comparison group for late deaths was patients who completed treatment.
Initial drug susceptibility test results were from the first positive culture (sputum or other tissue/body fluid). Follow-up drug susceptibility test results were from a specimen collected at least 30 days later. Initial multidrug-resistant (MDR) TB was defined as resistance to at least isoniazid (INH) and rifampin (RIF) on initial specimens. Acquired MDR-TB was defined as susceptibility to INH and/or RIF on initial susceptibility tests but resistance to at least INH and RIF on follow-up tests. INH or RIF monoresistance was defined as resistance to INH or RIF, respectively, on either initial or follow-up tests, without resistance to pyrazinamide or ethambutol. Other drug resistance included any other pattern of drug resistance not meeting the above definitions.
We assessed the adequacy of the initial treatment regimen, that is, the regimen used during the first 2 weeks after TB diagnosis, by whether the patient was prescribed at least 3 of the 4 first-line drugs (INH, RIF, ethambutol, and pyrazinamide).
Patients with private provider care had the majority of TB care provided outside of the health department. Private providers included county and private hospital providers as well as non–health department providers in outpatient settings. A patient who had TB care provided by both a private provider and a health department provider was defined as having a private provider because the health department did not have sole responsibility for patient care and treatment.
Patients with disseminated TB had M. tuberculosis identified in the blood and/or in ≥2 noncontiguous organ systems, and/or had evidence of miliary TB on chest radiography.
Patients were reported as homeless or substance users if those characteristics were present within 1 year of TB diagnosis, and reported as incarcerated or living in a long-term-care facility if TB was diagnosed while housed in those settings.
Main Outcomes and Statistical Analysis
We calculated the age-adjusted percentage of deaths among culture-positive TB cases during each reporting year. We defined the 2001 reporting year case population as the standard population and examined secular trends with the Cochran-Armitage trend test. We also used Joinpoint software (Joinpoint Regression Program, version 4.0.4; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute) to identify changes in secular trends. Joinpoint uses a Monte Carlo permutation method to detect statistically significant changes in trends [6]. Because our focus was on interventions that could prevent death after diagnosis, we investigated factors associated with death on treatment. We estimated the odds ratios (ORs) and 95% confidence intervals (95% CIs) in univariate analyses to investigate factors associated with death on treatment (vs completion of treatment). We analyzed continuous variables (eg, age of TB patient) to assess their distributions and used a nonparametric Wilcoxon rank-sum test to compare medians between groups. We examined the distribution of time (days) from treatment initiation to death for TB patients who died during treatment.
We constructed a stepwise multivariable logistic regression model to identify the factors associated with death. We first included covariates that were known confounders of death (ie, age, sex, and race/ethnicity), and then added covariates that had a P value ≤.2 in the univariate analysis. We tested for collinearity among the covariates. We added covariates to the model based on biological plausibility and decreasing magnitude of the univariate OR; covariates remained in the model if the P values associated with their coefficients were ≤.05. We used the Hosmer-Lemeshow test to assess model fit [7].
To further examine the role of provider type on death, we created 2 additional models: for early and for late deaths (see definitions above). Hospitalization data were not collected; patients who were diagnosed in a hospital and died during their initial hospitalization were reported to the TB Registry as patients with private provider care. We reasoned that early deaths were more likely to occur among patients who were hospitalized and did not have an opportunity to receive care elsewhere, and we expected that early deaths would overestimate an association between death and private providers. We also investigated late deaths, when patients who were hospitalized during diagnosis had a greater opportunity than those who died early to seek postdischarge care in their community facilities, and to be categorized as having private provider care or health department care. The models for early and late deaths included confounding covariates and additional covariates that resulted in a ≥10% change in the aOR of the provider type covariate [8].
Age-adjustment and Cochran-Armitage trend tests were performed using SAS version 9.3 (SAS Institute Inc, Cary, North Carolina). Univariate and multivariate analyses were performed using Stata statistical software, version 12SE (StataCorp, College Station, Texas).
Ethical Review
This analysis was conducted as part of the California Department of Public Health's mandate to routinely collect and analyze surveillance data for public health purposes.
RESULTS
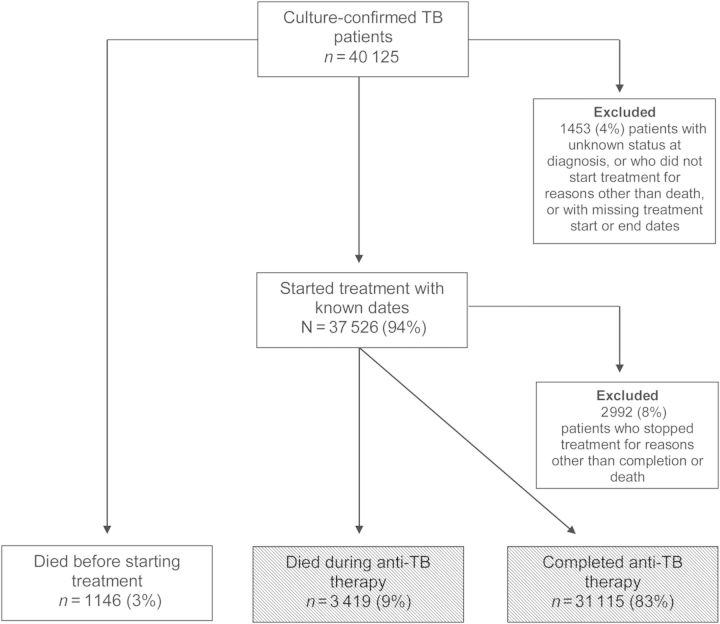
Of 40 125 patients with culture-confirmed TB during 1994–2008, 4565 (11%) died: 1146 (25%) died before treatment started and 3419 (75%) died during treatment. We excluded 1453 patients (4%) from all analyses (Figure 1). Reasons for exclusion included unknown vital status at diagnosis (n = 4); did not start treatment for reasons other than death (n = 342); or started treatment but had missing dates (n = 1107). Of the 37 526 patients who started treatment with known dates, 3419 (9%) died during anti-TB therapy and 31 115 (83%) completed treatment. We excluded 2992 (8%) patients from the multivariate analyses of deaths during treatment because they stopped treatment for other reasons (eg, default), and their final treatment outcome was unknown (Figure 1).
Figure 1.
Study population of tuberculosis (TB) patients who had a culture that was positive for Mycobacterium tuberculosis, California, 1994–2008. Shaded boxes show comparison groups for multivariate analysis.
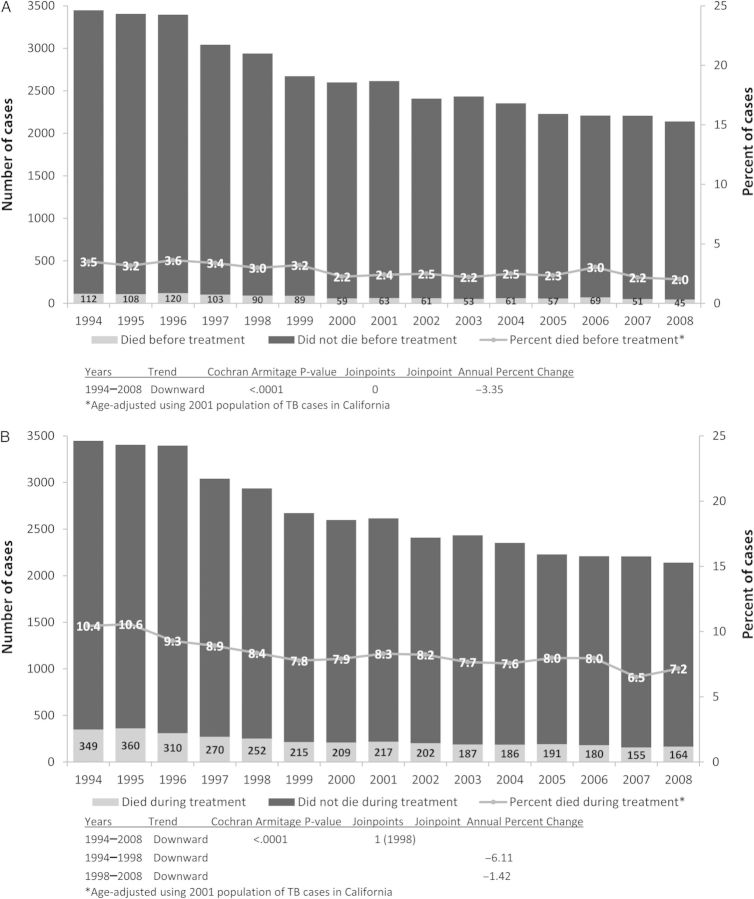
Overall, the median age of patients who died with TB increased from 1994 to 2008 (60 years vs 71 years, P < .0001). Thus, we adjusted for age in our analysis of trends. From 1994 to 2008, the age-adjusted percentage of patients with culture-confirmed TB who died before treatment declined from 3.5% to 2.0% (P < .0001; Figure 2A). The age-adjusted percentage of TB patients who died during treatment declined from 10.4% to 7.2% (P < .0001; Figure 2B). For TB patients who died during treatment, there was an inflection point, or change in the rate of decline (ie, a joinpoint), in 1998. Prior to 1998, the percentage of patients who died declined by 6% per year, whereas after 1998, the percentage of patients who died declined by only 1.4% per year (Figure 2B).
Figure 2.
Tuberculosis (TB) deaths in California, 1994–2008. A, Tuberculosis deaths before treatment. B, Tuberculosis deaths during treatment.
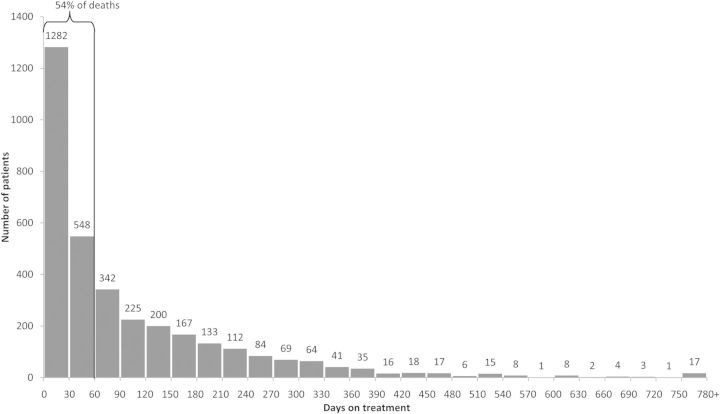
The majority of TB patients who died on treatment died within 60 days of treatment initiation (n = 1830 [54%]; Figure 3). An additional 934 (27%) patients died during the period of 61–180 days of treatment, and the remaining 655 (19%) patients died after 180 days or 6 months on treatment.
Figure 3.
Time from tuberculosis treatment start to death, among patients who died, California, 1994–2008 (n = 3419).
Compared with TB patients who completed treatment, patients who died on treatment differed in demographic, sociobehavioral, clinical, and treatment characteristics (Table 1). After adjusting for age, sex, and race/ethnicity, there were many independent risk factors for death during anti-TB therapy (Table 2). Factors previously known to be associated with death included HIV coinfection, substance use, and extensive and/or specific extrapulmonary sites of TB disease. Factors not widely known from previous studies to be associated with death included private provider TB care (adjusted OR [aOR], 3.08; 95% CI, 2.75–3.44), an initial TB treatment regimen of <3 drugs (aOR, 2.07; 95% CI. 1.63–2.64), and abnormal chest radiograph without cavities (normal chest radiograph = reference; aOR, 1.58; 95% CI, 1.32–1.90). The percentage of patients who had an initial TB treatment regimen of <3 drugs declined during the study period from 4% in 1994 to 0.1% in 2008 (P < .0001).
Table 1.
Study Population Characteristics, Culture-Confirmed Tuberculosis Patients Who Died on Treatment vs Patients Who Completed Treatment—California, 1994–2008
| Characteristic | Died on Anti-TB Treatment, No. (%) (n = 3419) |
Completed Anti-TB Treatment No. (%) (n = 31 115) |
OR (95% CI) |
|---|---|---|---|
| Demographic/social | |||
| Age, y, median (IQR) | 68 (51–79) | 45 (31–61) | 1.05 (1.047–1.051) |
| Sex | |||
| Male | 2354 (69) | 18 842 (61) | 1.44 (1.33–1.55) |
| Female | 1065 (31) | 12 272 (39) | Ref |
| Nativity | |||
| US-born | 1239 (37) | 7.703 (25) | 1.75 (1.63–1.89) |
| Foreign-born | 2140 (63) | 23 343 (75) | Ref |
| Race/ethnicity | |||
| White, non-Hispanic | 653 (19) | 3392 (11) | Ref |
| Black, non-Hispanic | 444 (13) | 2995 (10) | 0.77 (0.68–0.88) |
| Hispanic | 1052 (31) | 10 881 (35) | 0.50 (0.45–0.56) |
| Asian/Pacific Islander | 1233 (36) | 13 635 (44) | 0.47 (0.42–0.52) |
| Native American | 20 (0.6) | 113 (0.4) | 0.19 (0.18–0.21) |
| Homeless | |||
| Yes | 279 (8) | 2018 (7) | 1.30 (1.14–1.48) |
| No | 3063 (92) | 28 734 (93) | Ref |
| Incarcerated | |||
| Yes | 43 (1) | 873 (3) | 0.44 (0.33–0.60) |
| No | 3366 (99) | 30 199 (97) | Ref |
| Long-term care | |||
| Yes | 368 (11) | 555 (2) | 6.66 (5.81–7.64) |
| No | 3040 (89) | 30 526 (98) | Ref |
| Injection drug use | |||
| Yes | 146 (5) | 839 (3) | 1.79 (1.50–2.15) |
| No | 2797 (95) | 28 834 (97) | Ref |
| Non–injection drug use | |||
| Yes | 203 (7) | 2061 (7) | 1.00 (0.86–1.16) |
| No | 2704 (93) | 27 450 (93) | Ref |
| Excess alcohol use | |||
| Yes | 428 (15) | 3544 (12) | 1.26 (1.13–1.40) |
| No | 2496 (85) | 26 001 (88) | Ref |
| Clinical | |||
| Previous TB | |||
| Yes | 240 (7) | 1915 (6) | 1.17 (1.02–1.34) |
| No | 3117 (93) | 29 082 (94) | Ref |
| Site of disease | |||
| Pulmonary only | 2387 (70) | 23 235 (75) | Ref |
| Pulmonary and extrapulmonary | 431 (13) | 2205 (7) | 1.90 (1.70–2.13) |
| Extrapulmonary only | 601 (18) | 5674 (18) | 1.03 (0.94–1.13) |
| Chest radiograph findings | |||
| Normal | 246 (7) | 3934 (13) | Ref |
| Abnormal, no cavities | 2571 (79) | 19 826 (66) | 2.07 (1.81–2.37) |
| Abnormal with cavities | 452 (14) | 6372 (21) | 1.13 (0.97–1.33) |
| Sputum smear status | |||
| Positive | 1698 (58) | 13 972 (53) | 1.24 (1.15–1.34) |
| Negative | 1231 (42) | 12 571 (47) | Ref |
| Extent of disease | |||
| Disseminated | 334 (10) | 1106 (4) | 2.94 (2.58–3.34) |
| Not disseminated | 3085 (90) | 30 009 (96) | Ref |
| Extrapulmonary sites | |||
| Meningeal | 143 (4) | 343 (1) | 3.92 (3.21–4.78) |
| Peritoneal | 99 (3) | 358 (1) | 2.56 (2.04–3.21) |
| Pleural | 816 (24) | 3965 (13) | 2.15 (1.97–2.34) |
| Lymphatic | 160 (5) | 3179 (10) | 0.43 (0.37–0.51) |
| Genitourinary | 104 (3) | 819 (3) | 1.16 (0.94–1.43) |
| Drug resistance | |||
| Pandrug susceptible | 2885 (87) | 26 512 (86) | Ref |
| INH monoresistant | 162 (5) | 2274 (7) | 0.65 (0.56–0.77) |
| RIF monoresistant | 25 (0.8) | 70 (0.2) | 3.28 (2.08–5.19) |
| Initial MDR | 59 (2) | 326 (1) | 1.66 (1.26–2.20) |
| Acquired MDR | 14 (0.4) | 30 (0.1) | 4.29 (2.27–8.10) |
| Other DR | 173 (5) | 1477 (5) | 1.08 (0.92–1.27) |
| HIV/AIDS match | |||
| Yes | 515 (15) | 1890 (6) | 2.74 (2.47–3.05) |
| No | 2904 (85) | 29 225 (94) | Ref |
| Treatment regimen | |||
| Initial regimen <3 drugs | 165 (5) | 675 (2) | 2.29 (1.92–2.73) |
| Initial regimen ≥3 drugs | 3249 (95) | 30 435 (98) | Ref |
| Any DOT | |||
| Yes | 2313 (71) | 21 332 (70) | 1.07 (0.98–1.15) |
| No | 952 (29) | 9355 (30) | Ref |
| Provider type | |||
| Health department | 698 (20) | 15 644 (50) | Ref |
| Any private (private only and both) | 2720 (80) | 15 451 (50) | 3.95 (3.62–4.30) |
| Period | |||
| 1994–1999 | 1761 (52) | 14 737 (47) | 1.18 (1.10–1.27) |
| 2000–2008 | 1658 (48) | 16 378 (53) | Ref |
Abbreviations: CI, confidence interval; DOT, directly observed therapy; DR, drug resistance; HIV, human immunodeficiency virus; INH, isoniazid; IQR, interquartile range; MDR, multidrug resistance; Ref, reference group; RIF, rifampin; TB, tuberculosis; US, United States.
Table 2.
Multivariate Analysis of Death at Any Time During Treatment Among Culture-Confirmed Patients Who Died on Treatment vs Completed Treatment—California, 1994–2008
| Factor | aOR (95% CI) | Forest Plot of aOR and 95% CI |
|---|---|---|
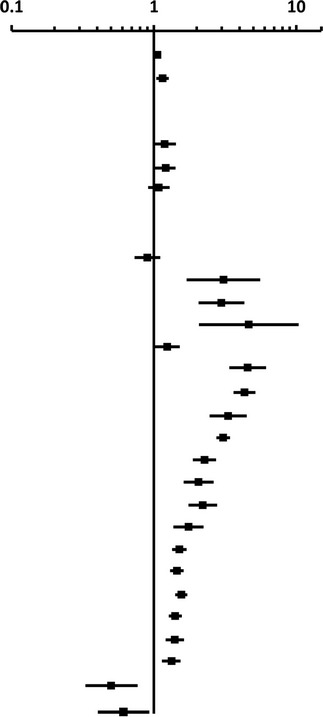 |
||
| Age | 1.06 (1.055–1.062) | |
| Male sex | 1.14 (1.03–1.26) | |
| Race/ethnicity | ||
| White non-Hispanic | Ref | |
| Black non-Hispanic | 1.19 (0.99–1.43) | |
| Hispanic | 1.20 (1.02–1.42) | |
| Asian/PI non-Hispanic | 1.08 (0.91–1.29) | |
| Drug resistance | ||
| Pandrug sensitive | Ref | |
| INH monoresistant | 0.90 (0.73–1.11) | |
| RIF monoresistant | 3.07 (1.69–5–57) | |
| Initial MDR | 2.96 (2.04–4.30) | |
| Acquired MDR | 4.67 (2.09–10.45) | |
| Other DR | 1.25 (1.02–1.54) | |
| Meningeal TB | 4.59 (3.40–6.20) | |
| HIV | 4.39 (3.68–5.24) | |
| Peritoneal TB | 3.36 (2.48–4.54) | |
| Private provider | 3.08 (2.75–3.44) | |
| Disseminated TB | 2.30 (1.82–2.91) | |
| Long-term care | 2.27 (1.89–2.73) | |
| Initial regimen <3 drugs | 2.07 (1.63–2.64) | |
| Injection drug use | 1.75 (1.37–2.23) | |
| Abnormal CXR, no cavities | 1.58 (1.32–1.90) | |
| Sputum smear positive | 1.52 (1.38–1.69) | |
| Pleural TB | 1.49 (1.33–1.68) | |
| DOT | 1.40 (1.26–1.56) | |
| US-born | 1.40 (1.21–1.62) | |
| Excess alcohol use | 1.33 (1.14–1.54) | |
| Incarceration | 0.50 (0.33–0.77) | |
| HIV and disseminated TB | 0.62 (0.43–0.91) | |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; CXR, chest radiograph; DOT, directly observed therapy; DR, drug resistance; HIV, human immunodeficiency virus; INH, isoniazid; IQR, interquartile range; MDR, multidrug resistance; PI, Pacific Islander; RIF, rifampin; TB, tuberculosis; US, United States.
Interestingly, having a TB diagnosis while incarcerated or having both HIV and disseminated TB was associated with decreased risk of death (aOR, 0.50; 95% CI, 0.33–0.77 and aOR, 0.62; 95% CI, 0.43–0.91, respectively; Table 2). We further investigated the interaction between HIV infection and disseminated TB (compared to either condition alone or neither condition). Patients with both HIV infection and disseminated TB were more common in the earlier, pre–highly active antiretroviral therapy (HAART) years of the study period (1.6% during 1994–2001 vs 1.0% during 2002–2008, P < .001). Additionally, among patients with HIV and disseminated disease, more patients met the definition of disseminated disease by positive blood culture in the pre-HAART period (67.9% during 1994–2001 vs 38.3% during 2002–2008, P < .001), at a time when more blood cultures were done (1.0% of patients during 1994–2001 vs 0.4% during 2002–2008, P < .001).
Private provider care was an independent risk factor in both early deaths (aOR, 3.70; 95% CI, 3.19–4.29) and late deaths (aOR, 2.80; 95% CI, 2.46–3.19).
Patients who died before starting treatment were similar to those who died during treatment, except that they were older (median age, 70.5 years with interquartile range [IQR], 53.5–81 vs 68 years [IQR], 51–79; Wilcoxon rank-sum test P = .0015), more likely of white non-Hispanic race (22% vs 19%, P = .017), less likely of Asian/Pacific Islander race (32% vs 36%, P = .025), and less likely to have had INH-monoresistant M. tuberculosis isolates (3.2% vs 4.8%, P = .023).
DISCUSSION
Our large population-based study showed that, despite a slow decline since 1994, death with TB is a common outcome. Eleven percent of patients diagnosed with TB in California died, which is higher than the percentage of TB patients who died in the United States in 2010 [893 of 11 163 (8%)] [1]. Although the trend in deaths among patients with TB in California is consistent with studies showing that TB mortality has continuously declined in the United States since 1990 [9, 10], the rate of decrease is slowing [11]. We speculate that one reason that the decline in deaths is slower since 1998 is the increasing prevalence of comorbid conditions among an aging population of TB patients. However, we did not test this hypothesis. Treatment for HIV infection was not captured in the TB surveillance system, and we could not directly assess the impact of HAART on TB deaths. Nonetheless, the introduction of HAART for persons living with HIV was a contributing factor to the decline in TB deaths. TB rates have been declining among persons with HIV in California since 1993, and death rates decreased after HAART became available. However, death rates with TB for HIV-coinfected patients remained twice that for TB patients without HIV infection [12]. Rapid initiation of HAART in HIV-coinfected patients who present with TB may prevent deaths in this group [13].
We found that one-quarter of all patients who died with TB died before treatment started, and presumably, before there was sufficient opportunity for the healthcare system to intervene. More than half of the deaths on treatment occurred in the first 60 days of treatment. These findings, and the increased risk for death associated with significant comorbidity (eg, HIV infection) or extensive, severe disease (eg, disseminated disease, meningeal TB) likely indicate that many patients who die present late, when their disease has become difficult to treat successfully. Although these characteristics of extensive and disseminated disease and comorbidities themselves may not be modifiable, their presence could indicate the need to intensify clinical care and consultation in an effort to prevent death. These findings, however, also may indicate that treatment of TB infection prior to progression to active TB disease is needed to prevent TB deaths. Prevention efforts that focus on finding and treating TB infection among persons who also have a comorbidity such as HIV or diabetes mellitus, or who are being treated with immunosuppressive medications [14, 15], may be effective at preventing deaths because these comorbidities are associated with TB death and extensive, severe disease [13, 16–18] .
There were 3 potentially modifiable factors that conferred an increased risk of death during treatment: private provider care, inadequate initial treatment regimen, and acquisition of MDR-TB during treatment. Among these modifiable factors, private provider care had a strong association with TB deaths, with an adjusted odds for death >3 times higher than patients who received care exclusively from the health department. Although ours is not the first study to identify an association of patient death with private sector care [19], it confirms and extends the finding to a large, diverse population of TB patients. Public health departments are charged with ensuring prompt and appropriate treatment [20, 21] and have historically cared for most TB patients. Therefore, health departments may be more likely than private providers to maintain clinical expertise in TB care at a time when TB cases are becoming less common, and to have an infrastructure for providing case management, treatment support, and monitoring.
Although we were not able to directly adjust for deaths occurring among hospitalized patients who did not survive long enough to receive health department care, private provider TB care was associated with both early deaths (when hospitalized patients were more likely to be categorized as having private provider care because there was little opportunity for the patient to receive care from the health department after diagnosis) and late deaths (when patients who were hospitalized during diagnosis had a greater opportunity than those who died early to seek postdischarge care in their community facilities, and to be categorized as having private provider care or health department care). There are opportunities for health departments to influence TB outcomes among patients who are cared for in the private sector: by intensifying linkages for hospitalized and privately managed patients, by serving as a resource during the diagnostic process, and perhaps by earlier and increased involvement in treatment management and support. Improved case management might be especially important for patients with specific risks for death such as HIV coinfection, primary drug resistance, meningeal TB, and substance use. California law currently requires that providers report patients suspected to have TB within 24 hours and that hospitals submit a treatment and follow-up plan to the local health officer for all TB patients discharged from the hospital. Health departments could use the initial report and the discharge plan to identify patients at risk for death and prioritize these patients for health department–hospital consultation at the time of TB workup in hospital through discharge. Collaboration between health departments and the private sector is likely to become more important as the number of TB patients receiving private health insurance under the Affordable Care Act increases. However, declining health department resources [22, 23] for TB control will need to be rectified to ensure the capacity of health departments to provide advice or service to privately managed patients.
Two other potentially modifiable risk factors identified in our analysis included an inadequate initial treatment regimen and acquired M. tuberculosis drug resistance. Patients who initiated therapy with <3 of the recommended 4 first-line anti-TB drugs were at increased risk of death. The percentage of patients using <3 of the 4 first-line anti-TB drugs declined during the study period, suggesting improved adherence to TB treatment guidelines over time. However, use of fewer anti-TB drugs may have been a marker of unmeasured comorbidities, such as liver disease, that increased the risk of death and prevented the use of the recommended first-line regimen. Further investigation of appropriate treatment regimens for patients with comorbid conditions is warranted.
MDR-TB acquired during treatment was a risk factor for death, and, although infrequent in California, can be prevented with careful and appropriate clinical management and directly observed therapy (DOT) for patients with baseline drug resistance, cavities on chest radiograph, or HIV infection [24].
The 2 factors associated with decreased risk of death deserve additional discussion. First, we speculate that the reason that patients with both HIV coinfection and disseminated TB disease were less likely to die was rapid diagnosis, possibly because of more frequent use of mycobacterial blood cultures during diagnostic workup of HIV-infected hospitalized patients in the pre-HAART era. Second, further investigation of the characteristics of TB patient health status and TB diagnosis and care in correctional facilities may provide insight into the reasons for lower risk of death for TB patients diagnosed while incarcerated. Incarcerated patients may have been more likely to have been adherent to treatment and monitoring than nonincarcerated patients.
We, like researchers in Washington [11], found that DOT was associated with death. In California, not all patients receive TB treatment by DOT, as guidelines recommend prioritizing DOT for TB patients at risk for nonadherence to treatment (eg, psychiatric disorder or memory impairment), and/or for poor treatment outcomes (eg, immunosuppression) [25]. We did not measure these specific risks (eg, immunosuppression unassociated with HIV coinfection). Thus, in our study population, DOT was likely a marker of these unmeasured risk factors and its association with death could be explained by residual confounding.
Our surveillance-based study had some limitations. The proportion of patients for whom TB disease was a cause of death was unknown. However, California participated in a national study that demonstrated that 72% of deaths among TB patients reported to the national surveillance system in 2005–2006 were TB related [26]. Thus, TB likely contributed to the vast majority of deaths in our study. We also did not have information on the level of oversight provided by the health department for privately managed patients. If the level of oversight and partnership between health departments and private providers was strong during the study period, then additional efforts to strengthen partnerships may not reduce deaths in future. Another potential limitation is that the surveillance system did not collect all relevant comorbidities (eg, malignancy) that could have also contributed to TB patient death. Future investigations should focus on the contribution of comorbidities to death, and the prevention of death with optimized treatment regimens and strategies. Additionally, we had limited information for patients who died prior to treatment and were unable to fully characterize those patients. Patients with a delayed diagnosis of TB also deserve further investigation.
In conclusion, public health departments might prevent deaths in patients with TB by strengthening partnerships with private providers, increasing involvement in diagnostic and treatment processes for patients at risk of death while hospitalized, optimizing treatment regimens for patients with comorbidities, and preventing the acquisition of drug resistance.
Acknowledgments
We thank Varsha Nimbal, Saul Kanowitz, Phil Lowenthal, and Peter Oh for analytic and figure development assistance, and Alex Golden, Allison Kelley, and Christy Pak for formatting assistance.
This work was partially supported by the National Institutes of Health under Award Number DP2OD006452 (K. D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Centers for Disease Control and Prevention (CDC) Atlanta, GA: Department of Health and Human Services; 2013. Reported tuberculosis in the United States, 2012. [Google Scholar]
- 2.Sterling TR, Zhao Z, Khan A, et al. Mortality in a large tuberculosis treatment trial: modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis. 2006;10:542–9. [PubMed] [Google Scholar]
- 3.Isanaka S, Mugusi F, Urassa W, et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. 2012;142:350–7. doi: 10.3945/jn.111.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Trends in tuberculosis—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:181–5. [PubMed] [Google Scholar]
- 5.Curtis AB, McCray E, McKenna M, et al. Completeness and timeliness of tuberculosis case reporting. A multistate study. Am J Prev Med. 2001;20:108–12. doi: 10.1016/s0749-3797(00)00284-1. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: John Wiley and Sons, Inc; 2000. [Google Scholar]
- 8.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–9. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung RS, Bennion JR, Sorvillo F, et al. Trends in tuberculosis mortality in the United States, 1990–2006: a population-based case-control study. Public Health Rep. 2010;125:389–97. doi: 10.1177/003335491012500307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Quickstats: age-adjusted death rates from tuberculosis, by race and sex—National Vital Statistics System, United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2013;62:865. [Google Scholar]
- 11.Barnes RF, Moore ML, Garfein RS, et al. Trends in mortality of tuberculosis patients in the United States: the long-term perspective. Ann Epidemiol. 2011;21:791–5. doi: 10.1016/j.annepidem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalfe JZ, Porco TC, Westenhouse J, et al. Tuberculosis and HIV co-infection, California, USA, 1993–2008. Emerg Infect Dis. 2013;19:400–6. doi: 10.3201/eid1903.121521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcy O, Laureillard D, Madec Y, et al. Causes and determinants of mortality in HIV-infected adults with tuberculosis: an analysis from the CAMELIA ANRS 1295-CIPRA KH001 randomized trial. Clin Infect Dis. 2014;59:435–45. doi: 10.1093/cid/ciu283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49:1–51. [PubMed] [Google Scholar]
- 15.Kaplan JE, Benson C, Holmes KK, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 16.Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC. 2014;9:1–15. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wobeser W, Yuan L, Naus M. Outcome of pulmonary tuberculosis treatment in the tertiary care setting—Toronto 1992/93. Tuberculosis Treatment Completion Study Group [see comments] CMAJ. 1999;160:789–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Skogberg K, Ruutu P, Tukiainen P, et al. Effect of immunosuppressive therapy on the clinical presentation and outcome of tuberculosis. Clin Infect Dis. 1993;17:1012–7. doi: 10.1093/clinids/17.6.1012. [DOI] [PubMed] [Google Scholar]
- 19.Horne DJ, Hubbard R, Narita M, et al. Factors associated with mortality in patients with tuberculosis. BMC Infect Dis. 2010;10:258. doi: 10.1186/1471-2334-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.California Department of Public Health, California Tuberculosis Control Branch, California Tuberculosis Controllers Association. Responsibilities of public health departments to control tuberculosis. http://www.ctca.org/fileLibrary/file_494.pdf . Accessed February 27, 2014.
- 21.American Thoracic Society, Centers for Disease and Prevention, Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1–77. [PubMed] [Google Scholar]
- 22.Association of State and Territorial Health Officials. Budget cuts continue to affect the health of Americans. http://www.astho.org/budget-cuts-dec-2012/ . Accessed February 27, 2014.
- 23.National Association of County and City Health Officials. Local health department job losses and program cuts: findings from the 2013 Profile Study. http://naccho.org/topics/infrastructure/lhdbudget/upload/survey-findings-brief-8-13-13-4.pdf . Accessed February 27, 2014.
- 24.Porco TC, Oh P, Flood JM. Antituberculosis drug resistance acquired during treatment: an analysis of cases reported in California, 1994–2006. Clin Infect Dis. 2013;56:761–9. doi: 10.1093/cid/cis989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.California Department of Public Health. Information for physicians regarding directly observed therapy (DOT) for active tuberculosis (TB) http://www.cdph.ca.gov/programs/tb/Documents/TBCB-PMD-DOT.pdf . Accessed February 27, 2014. [Google Scholar]
- 26.Beavers S, Flood J, Weinfurter P, et al. Tuberculosis mortality in the United States: how can it be prevented?. American Thoracic Society International Conference; San Francisco, California. 2012. [Google Scholar]