Among HIV-infected, antiretroviral therapy (ART)–naive adults with positive World Health Organization tuberculosis (TB) symptom screening, clinical scoring could categorize patients for the risk of TB. This strategy would reduce the proportion of patients requiring TB testing before starting ART.
Keywords: HIV, tuberculosis, scoring, health center, Ethiopia
Abstract
Background
The World Health Organization (WHO) tuberculosis (TB) symptom screening instrument (WHO-TB) can identify human immunodeficiency virus (HIV)-infected individuals at low risk of tuberculosis (TB); however, many patients report WHO-TB symptoms and require further TB investigations. We hypothesized that further clinical scoring could classify subjects with a positive WHO-TB screening result (WHO-TB+) for the likelihood of TB.
Methods
HIV-infected adults eligible to initiate antiretroviral therapy (ART) were recruited and prospectively followed at 5 Ethiopian health centers. Irrespective of symptoms, all participants underwent sputum bacteriological testing for TB. Symptoms, physical findings, hemoglobin, and CD4 cell count results were compared between subjects with and those without bacteriologically confirmed TB. Variables associated with TB in WHO-TB+ individuals were used to construct a scoring algorithm with multiple logistic regression analysis.
Results
Among 812 participants, 137 (16.9%) had TB. One hundred fifty-nine persons (20%) had a negative WHO-TB screen, 10 of whom had TB (negative predictive value [NPV], 94% [95% confidence interval {CI}, 90%–97.5%]). For WHO-TB+ subjects, the following variables were independently associated with TB, and were assigned 1 point each in the clinical scoring algorithm: cough, Karnofsky score ≤80, mid-upper arm circumference <20 cm, lymphadenopathy, and hemoglobin <10 g/dL. Among subjects with 0–1 points, 20 of 255 had TB (NPV, 92% [95% CI, 89%–95%]), vs 19 of 34 participants with ≥4 points (positive predictive value, 56% [95% CI, 39%–73%]). The use of WHO-TB alone identified 159 of 784 (20%) with a low risk of TB, vs 414 of 784 (53%) using WHO-TB followed by clinical scoring (P< .001). The difference in proportions of confirmed TB in these subsets was nonsignificant (6.3% vs 7.2%; P= .69).
Conclusions
Clinical scoring can further classify HIV-infected adults with positive WHO-TB screen to assess the risk of TB, and would reduce the number of patients in need of further TB investigations before starting ART.
Clinical Trials Registration
Tuberculosis (TB) and human immunodeficiency virus (HIV) have important reciprocal interactions. Hence, HIV testing for subjects with TB, as well as TB screening in people living with HIV (PLHIV), are both strongly recommended [1]. Simple methods with high accuracy are available for HIV screening in TB patients; however, the situation is markedly different for the reverse case—identification of TB in PLHIV [2, 3].
HIV infection affects the clinical presentation of TB; respiratory manifestations may be absent, and nonspecific constitutional symptoms that are also common in advanced HIV disease are often predominant [4, 5]. In addition, the performance of routine diagnostic methods for TB is inferior in HIV-coinfected subjects, with lower sensitivity for sputum smear microscopy [6, 7] and reduced specificity for chest radiography [8]. Consequently, active TB may be missed in PLHIV. Administration of antiretroviral therapy (ART) to patients with unrecognized active TB is associated with increased risk of death and immune reconstitution inflammatory syndrome (IRIS) during ART [9, 10]. Importantly, exclusion of active TB is required before initiating isoniazid preventive therapy (IPT). IPT has been shown to reduce mortality and TB incidence among PLHIV [11]. Despite this, rates of IPT prescription to PLHIV remain low [12], mostly due to difficulties in excluding active TB.
Currently, the World Health Organization TB symptom screening algorithm (WHO-TB) is recommended for TB screening in PLHIV [13]. This screening tool is based on the self-reported presence of any of 4 symptoms found to be associated with active TB in PLHIV (weight loss, fever, night sweats, and cough of any duration). Because WHO-TB has a high negative predictive value (NPV), it is mainly used as a “rule-out” test for TB [4]. Thereby, inappropriate IPT prescription to subjects with active TB can be avoided, and persons who may start ART without additional TB testing can be identified. However, the positive predictive value (PPV) of WHO-TB for detection of TB is low. Because the proportion of PLHIV reporting WHO-TB symptoms can be as high as 80% [4, 14], many PLHIV will require further TB investigations prior to initiation of ART and IPT.
The need for TB investigations for large proportions of PLHIV is a major challenge for health care systems in high-prevalence regions with constrained resources. In such settings, a clinical algorithm for further categorization of individuals who have positive WHO-TB screening results (WHO-TB+) with regard to their likelihood of TB could be of great benefit.
We hypothesized that clinically based scoring might identify WHO-TB+ patients with a high or low likelihood of TB. This would reduce the proportion of patients who need further TB investigations, and allow for timely initiation of therapy for both HIV and TB. In this study, we have constructed a scoring algorithm based on data from a cohort of PLHIV recruited in Ethiopian health centers, with bacteriologically confirmed TB for reference. We have compared the performance for TB categorization among PLHIV using this clinical scoring algorithm as a second-step screening for WHO-TB+ individuals to that of screening based solely on WHO-TB.
METHODS
Study Design and Participants
Participants for this cohort study were consecutively recruited at 5 health centers in the Oromia region of Ethiopia from October 3, 2011 to March 1, 2013 [14], representing all public health centers providing ART in an uptake area of about 600 000 inhabitants. All ART-naive HIV-infected persons receiving care at the study sites were screened for eligibility, irrespective of symptoms or clinical suspicion of TB. The following inclusion criteria were applied: age ≥18 years, residence in the uptake area, submission of at least 1 pair of sputum samples, and written informed consent. Patients with previous ART and those who had received anti-TB treatment (ATT) for >2 weeks prior to screening were excluded.
The study was approved by the National Research Ethics Review Committee, Ministry of Science and Technology, Addis Ababa, Ethiopia, and the Regional Ethical Review Board, Lund University, Lund, Sweden. Written informed consent (in the presence of an impartial witness for illiterate participants) was obtained before inclusion.
Procedures
Demographic information and medical history were collected using a structured questionnaire, followed by recording of disease symptoms and findings from physical examination (including Karnofsky performance score, body mass index [BMI], and mid-upper arm circumference [MUAC]). All study procedures were performed by health center nonphysician clinicians who were certified ART providers; additionally, all persons involved in the study procedures received detailed training on the study protocol. Blood samples were obtained for hemoglobin and CD4 cell count.
At inclusion, participants were requested to submit 2 pairs of spontaneously expectorated morning sputum samples. One randomly selected sample of each pair was delivered to International Clinical Laboratories in Addis Ababa for liquid culture (using the BACTEC mycobacterial growth indicator tubes 960 system, BD Diagnostics). The remaining sample was submitted to Adama Regional Laboratory for smear microscopy (using Ziehl-Neelsen staining) and Xpert MTB/RIF testing (using a 4-module GeneXpert instrument, Cepheid). Fine-needle aspirates for liquid culture and Xpert MTB/RIF testing were obtained from subjects with enlarged peripheral lymph nodes (>1 cm). Samples were delivered under cold-chain system to the respective laboratory within 6 hours of collection.
Participants were identified with study-specific codes. Project data managers continuously entered all data into a study database and conducted regular cross-checking of all entries. The investigators performed weekly monitoring throughout the duration of the study. Laboratory staff were blinded to clinical data. Adama Regional Laboratory is an external quality assurance center for all public laboratories in the Oromia region. International Clinical Laboratories is accredited by the Joint Commission International.
Results were regularly delivered from the laboratories throughout the duration of the study. ATT was initiated for participants with positive bacteriological test results for TB. ATT was also prescribed for patients with negative microbiological results who fulfilled clinical criteria for TB diagnosis according to Ethiopian guidelines [15]. Participants were followed for 6 months, with monthly visits for the first 3 months and 1 visit at 6 months. For patients with clinically suspected TB during this follow-up period, repeated investigations according to the procedures described above were recommended.
Statistical Analysis
Participants were categorized as TB and non-TB cases. TB cases were defined as subjects with bacteriologically confirmed pulmonary and/or extrapulmonary TB (at baseline or within 3 months after enrollment). Participants with negative bacteriological results who did not receive empirical ATT within 3 months of enrollment, were defined as non-TB cases. Participants with clinically diagnosed TB (defined as subjects who were prescribed ATT without microbiological confirmation) at baseline or within 3 months of enrollment were excluded from statistical analysis.
All variables considered to have potential association with TB based on biological plausibility (including disease symptoms, Karnofsky performance score, physical findings, biometrical data, hemoglobin, and CD4 cell count) were entered into univariate logistic regression analysis. Univariate and multivariate analyses were performed for all study participants and separately for the subset with positive WHO-TB results. Continuous variables were initially separated by quartiles depending on their distribution. For the subsequent analysis, these items were dichotomized at clinically meaningful cutoff levels, after controlling that the distribution of these variables in the study population was not discordant with such a dichotomization.
To achieve the simplest possible algorithm with the best possible predictive capacity for TB, stepwise backward multivariate logistic regression analysis was performed, including variables with a P value <.3 in univariate analysis. In each step, the least significant variable was excluded until a parsimonious model, only including significant variables (P < .05), was reached. Each significant variable from the final logistic multivariate analysis of WHO-TB+ subjects was assigned 1 scoring point. The resulting scoring system was applied to the cohort to estimate its predictive capacity for TB. Proportions, PPV, and NPV were determined for 3 levels: low, intermediate, and high risk for TB. All statistical analyses were performed using IBM SPSS software, version 20.0.
RESULTS
Patient Characteristics
Of 873 eligible persons, 812 submitted at least 1 sputum sample for TB testing. Among these, 137 had bacteriologically confirmed TB; 31 (22.6%) of these were smear-positive, 96 (70.1%) Xpert-positive, and 123 (89.8%) culture-positive. All smear-positive cases were also positive by Xpert and/or culture. TB was detected in lymph node aspirates from 10 subjects; 8 of these were also positive by sputum testing. Twenty-one subjects with negative bacteriological results at baseline had clinically diagnosed TB, and were excluded from study analyses (8 at inclusion and 13 within 3 months of enrollment). Between 3 and 6 months after enrollment, 1 additional case of TB was diagnosed (bacteriologically confirmed). Characteristics of the 791 included subjects are shown in Table 1.
Table 1.
Baseline Characteristics of Study Participants With Regard to Bacteriologically Confirmed Tuberculosis
| Characteristics | Total (N = 791) | TB (n = 137) | Non-TB (n = 654) | P Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, y, median (IQR) | 32 (28–40) | 35 (28–40) | 32 (28–39) | .105 |
| Sex | ||||
| Male | 324 (41.0) | 71 (51.8) | 253 (38.7) | .005 |
| Female | 467 (59.0) | 66 (48.2) | 401 (61.3) | |
| Residence | ||||
| Urban | 617 (79.1) | 104 (78.2) | 513 (79.3) | .815 |
| Rural | 163 (20.9) | 29 (21.8) | 134 (20.7) | |
| Permanent home | ||||
| Yes | 601 (76.8) | 97 (71.3) | 504 (77.9) | .117 |
| No | 182 (23.2) | 39 (28.7) | 143 (22.1) | |
| TB-related factors | ||||
| Self-reported history of TB | ||||
| Yes | 49 (6.3) | 4 (2.9) | 45 (7.0) | .082 |
| No | 730 (93.7) | 132 (97.1) | 598 (93.0) | |
| Household members on TB treatment | ||||
| Yes | 13 (1.6) | 2 (1.5) | 11 (1.7) | 1.00 |
| No | 776 (98.4) | 135 (98.5) | 641 (98.3) | |
| Prior TB in household member | ||||
| Yes | 33 (4.2) | 3 (2.2) | 30 (4.6) | .246 |
| No | 753 (75.8) | 134 (97.8) | 619 (95.4) | |
| Behavioral factors | ||||
| Smoking | ||||
| Yes | 35 (4.4) | 10 (7.3) | 25 (3.8) | .105 |
| No | 756 (95.6) | 127 (92.7) | 629 (96.2) | |
| Alcohola | ||||
| Yes | 192 (24.3) | 37 (27.0) | 155 (23.7) | .443 |
| No | 599 (75.7) | 100 (73.0) | 499 (76.3) | |
| Khatb | ||||
| Yes | 60 (7.6) | 16 (11.7) | 44 (6.7) | .052 |
| No | 730 (92.4) | 121 (88.3) | 609 (93.3) | |
| HIV care | ||||
| Enrollment history | ||||
| New | 240 (30.3) | 52 (38.0) | 188 (28.7) | .041 |
| In care | 551 (69.7) | 85 (62.0) | 466 (71.3) | |
| WHO clinical stage | ||||
| 1 or 2 | 383 (48.6) | 40 (29.2) | 343 (52.7) | <.001 |
| 3 or 4 | 405 (51.4) | 97 (70.8) | 308 (47.3) | |
| WHO TB symptom screening | ||||
| Positive | 625 (79.7) | 126 (92.6) | 499 (77.0) | <.001 |
| Negative | 159 (20.3) | 10 (7.4) | 149 (23.0) | |
| On IPT | ||||
| Yes | 19 (2.4) | 1 (0.7) | 18 (2.8) | .224 |
| No | 769 (97.6) | 136 (99.3) | 633 (97.2) | |
| On CPT | ||||
| Yes | 597 (75.9) | 102 (75.0) | 495 (76.0) | .826 |
| No | 190 (24.1) | 34 (25.0) | 156 (24.0) | |
| Hospitalizationc | ||||
| Yes | 11 (1.4) | 3 (2.2) | 8 (1.2) | .416 |
| No | 779 (98.6) | 134 (97.8) | 645 (98.8) | |
| Laboratory characteristics | ||||
| Hemoglobin, g/dL, median (IQR) | 11.6 (10.2–12.7) | 10.4 (9.1–11.9) | 11.9 (10.5–12.9) | <.001 |
| CD4 count, cells/µL, median (IQR) | 212 (119–321) | 172 (91–269) | 221 (126–328) | <.001 |
Abbreviations: CPT, cotrimoxazole preventive therapy; HIV, human immunodeficiency virus; IPT, isoniazid preventive therapy; IQR, interquartile range; TB, tuberculosis; WHO, World Health Organization.
a Defined as consumption of any type and amount of alcohol ≥2 times weekly.
b Defined as the regular chewing of leaves from the endemic plant Catha edulis, which is considered to have mild narcotic properties.
c Self-reported history of hospitalization during the 2 weeks preceding enrollment.
Factors Associated With TB
Each WHO-TB symptom (cough, fever, weight loss, and night sweats) was independently associated with TB in univariate analysis. Additionally, the following variables showed significant associations with TB: loss of appetite, fatigue, chest pain, dyspnea, Karnofsky score ≤80, conjunctival pallor, peripheral lymphadenopathy, BMI <18.5 kg/m2, MUAC <20 cm, CD4 count <200 cells/µL, and hemoglobin <10 g/dL (Supplementary Material).
In multivariate analysis, the following variables retained statistically significant associations: cough, Karnofsky score ≤80, MUAC <20 cm, hemoglobin <10 g/dL, and peripheral lymphadenopathy.
Factors Associated With TB in WHO-TB+ Subjects
For construction of the clinical scoring algorithm, 159 WHO-TB– participants were excluded, as well as an additional 7 WHO-TB+ persons with incomplete registration of the 4 WHO-TB symptoms. For the remaining 625 subjects included in this analysis, all WHO-TB variables were significantly associated with TB, as well as the additional variables identified for the whole cohort described above.
The following variables retained statistically significant associations with TB in the parsimonious multivariate model: reported cough, Karnofsky score ≤80, MUAC <20 cm, peripheral lymphadenopathy, and hemoglobin <10 g/dL (Table 2). These variables were each assigned 1 point in the clinical scoring algorithm.
Table 2.
Multivariate Analysis of Variables Associated With Tuberculosis (TB) in Subjects With Positive World Health Organization TB Symptom Screening
| Variablesa | Univariate ORb | Multivariate Model ORc |
|---|---|---|
| Cough | 3.4 (2.1–5.5) | 2.1 (1.3–3.3) |
| Karnofsky score ≤80 | 2.8 (1.6–5.0) | 2.8 (1.4–5.7) |
| MUAC <20 cm | 3.5 (2.2–5.7) | 2.0 (1.2–3.5) |
| Peripheral lymphadenopathy | 9.6 (4.0–23) | 7.3 (2.7–19) |
| Hemoglobin <10 g/dL | 3.7 (2.4–5.8) | 2.9 (1.7–4.7) |
Abbreviations: MUAC, mid-upper arm circumference; OR, odds ratio.
a Derived from stepwise backward elimination of least significant variables.
b Adjusted for age group (18–25, 26–35, 36–45, 46–55, >55 years, respectively) and sex.
c Model including all variables and adjustment for age group (18–25, 26–35, 36–45, 46–55, >55 years, respectively) and sex.
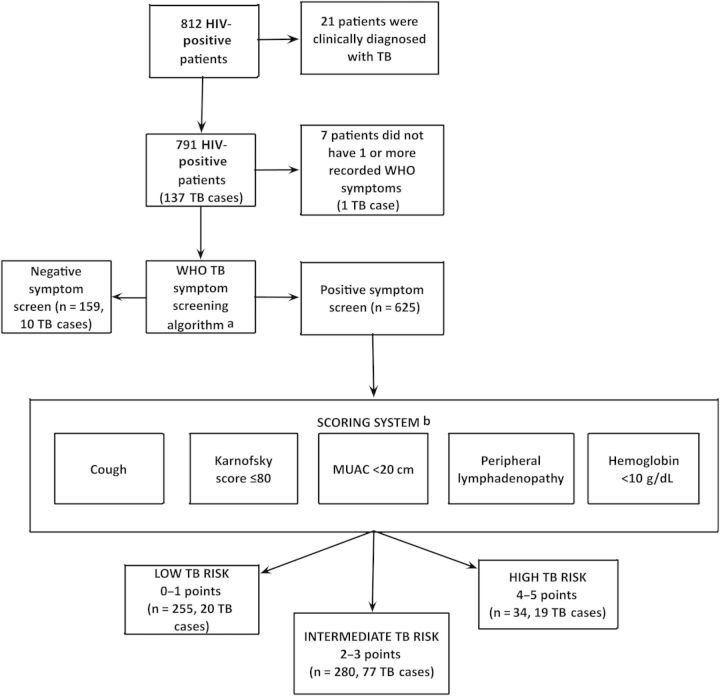
The distribution of participants with regard to the results for WHO-TB and the variables included in the follow-up clinical scoring algorithm is shown in Figure 1.
Figure 1.
Flow chart of participants included for the development of the clinical scoring algorithm. aWorld Health Organization tuberculosis symptom screening instrument (WHO-TB) includes fever, cough, and night sweats of any duration and weight loss. bFifty-six subjects had ≥1 missing scoring system variable and were excluded in the analysis. Abbreviations: HIV, human immunodeficiency virus; MUAC, mid-upper arm circumference.
Performance of the WHO-TB Algorithm and Follow-up Clinical Scoring for TB Categorization
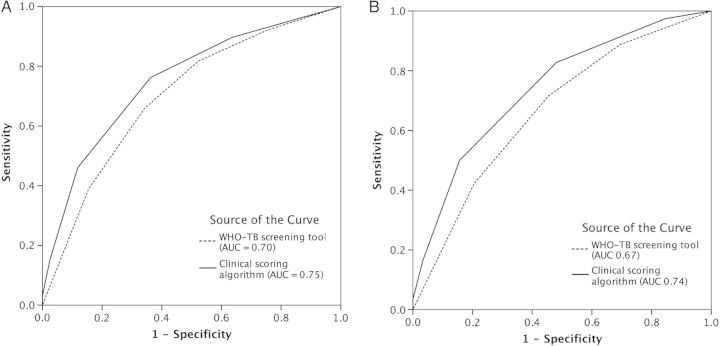
The respective performance of the WHO-TB symptom screening algorithm alone and combined with the clinical scoring algorithm is shown in Figure 2. The area under the curve of the combined strategy (WHO-TB and the clinical scoring algorithm) was slightly better than WHO-TB alone, both for the whole population (0.75 vs 0.70) and for WHO-TB positive participants (0.74 vs 0.67).
Figure 2.
Receiver operating characteristic area under the curve (AUC) showing the performance for tuberculosis identification using 2 different algorithms: World Health Organization tuberculosis (WHO-TB) symptom screening alone and WHO-TB symptom screening followed by clinical scoring for WHO-TB+ subjects. A, Predictive performance for all patients. B, Predictive performance for WHO-TB+ patients.
Using WHO-TB screening alone, 159 (20%) participants were categorized as being at low risk of TB; 10 (6%) of these had TB (NPV, 94% [95% confidence interval {CI}, 90%–97.5%]). These TB cases were characterized by lower age, higher MUAC, higher BMI, and higher CD4 cell count than WHO-TB+ cases.
Among the 625 WHO-TB+ participants, 569 (91%) had recordings for all the variables included in the clinical scoring algorithm (Figure 1). For the 56 (9%) subjects with ≥1 of these variables missing, the main reason was absence of results for MUAC and/or hemoglobin (Supplementary Material). These patients were excluded from this analysis; they did not differ significantly from those included with regard to sex, age, or WHO-TB symptoms.
Based on the distribution of clinical scoring points, subjects were divided into 3 categories with regard to the likelihood of TB (Figure 1). In participants with 0–1 scoring points, 20 of 255 had TB (NPV, 92% [95% CI, 89%–95%]); low-risk group). Among subjects with ≥4 scoring points, 19 of 34 had TB (PPV, 56% [95% CI, 39%–73%]; high-risk group). For participants with 2–3 points, 77 of 280 had TB (intermediate-risk group).
Using WHO-TB alone, 159 of 784 (20%) subjects would be excluded from further TB investigations. By applying the clinical scoring algorithm to WHO-TB+ individuals, an additional 255 subjects with 0–1 scoring points could be exempted from further TB testing (in total, 414/784 [53%]; P < .001). The proportions of subjects who would be falsely classified as TB-negative using these strategies are 10 of 159 (6.3%) and 30 of 414 (7.2%), respectively (P = .69).
The TB detection rate of all diagnostic methods (smear, Xpert MTB/RIF, and culture) increased with increasing scoring points (Table 3).
Table 3.
Performance of the World Health Organization Tuberculosis (WHO-TB) Screening Instrumenta Alone and WHO-TB Screening Followed by the Clinical Scoring Algorithmb for Categorization for the Likelihood of TB in HIV-Infected Adults at Ethiopian Health Centers
| Categorization for Likelihood of TB | No. of Positive Variables | No. of Subjects | TB Cases | Smear-Positivec | Xpert-Positivec | Culture-Positived |
|---|---|---|---|---|---|---|
| WHO TB screening algorithmc (n = 784) | 0 | 159 | 10 (6) | 1 (10) | 4 (40) | 9 (90) |
| 1–2 | 309 | 38 (12) | 8 (21) | 23 (61) | 36 (95) | |
| 3–4 | 316 | 88 (28) | 22 (25) | 69 (78) | 77 (88) | |
| Clinical scoring algorithme (n = 569) | 0–1 | 255 | 20 (8) | 4 (20) | 11 (55) | 17 (85) |
| 2–3 | 280 | 77 (28) | 16 (21) | 58 (75) | 69 (90) | |
| 4–5 | 34 | 19 (56) | 7 (37) | 16 (84) | 17 (89) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis; WHO, World Health Organization.
a The WHO-TB symptom screening algorithm includes 4 symptoms (fever, weight loss, night sweats, and/or cough of any duration); persons reporting any of these symptoms are categorized as WHO-TB+.
b The clinical scoring algorithm is intended for use in WHO-TB+ subjects, and includes 5 variables (cough, Karnofsky score ≤80, mid-upper arm circumference <20 cm, hemoglobin <10 g/dL, and peripheral lymphadenopathy). Each of these variables confers 1 point in the score (possible range, 0–5 points).
c Seven hundred eighty-four persons with all 4 recorded symptoms were included in the analysis; 136 had TB.
d Percentage was calculated from bacteriologically confirmed TB cases with the same range of variables or clinical scores.
e Including WHO-TB+ subjects (n = 625). Five hundred sixty-nine persons with recordings for all variables were included in the analysis; 116 had TB.
DISCUSSION
We developed a clinical scoring algorithm for further categorization of PLHIV with positive WHO-TB symptom screening with regard to their likelihood of TB, and compared the performance of this combined diagnostic strategy to that of using WHO-TB alone in a cohort of PLHIV eligible to start ART. The clinical scoring algorithm could identify subsets of WHO-TB+ subjects with a low likelihood of TB; such a strategy would reduce the need of further TB investigations before ART initiation.
We could confirm the projected performance of the WHO-TB symptom screening algorithm for ruling out active TB among PLHIV [4]. Our investigation was performed at Ethiopian public health centers, a setting that is typical for where most TB/HIV-coinfected patients globally receive care. This external validation of WHO-TB provides support for its use in primary health care in sub-Saharan Africa. As demonstrated in the original study [4] and in other reports [16], the WHO-TB algorithm has its principal use in identifying subjects with a low likelihood of TB; the NPV was 94% in our population, with an overall TB prevalence of 17%. However, the PPV was low; overall, around 80% of our study participants were WHO-TB+, and would require further TB investigations according to existing recommendations.
For the development of the clinical scoring algorithm, we used multiple logistic regression [17], with bacteriologically confirmed TB as criterion for concurrent validation [18]. Although regression models are widely used for risk stratification in different fields of medicine [19], alternative methods might be considered, such as algorithmic approaches or criteria based on expert opinion [8]. A parsimonious logistic regression model has advantages of simplicity and robustness for measuring the degree of correlations, while accounting for the influence of confounding variables. For these reasons, such a model was considered to be best suited for our objectives, in particular for the construction of an algorithm intended for use in decentralized care in low-income countries.
Currently, there is a lack of practical diagnostic methods for TB case finding among PLHIV in resource-limited settings [20]. This constitutes a substantial problem for health systems, with both direct financial costs and potential negative effects with delayed ART initiation and administration of IPT for persons who first have to undergo testing for TB [2, 21]. The need for TB investigations that require laboratory resources and technical facilities is an important obstacle for efficient provision of HIV services and IPT at the health center level.
The disease manifestations in HIV-associated TB are often vague and overlap with those of HIV/AIDS [4, 5]. A prominent feature is pronounced wasting (“slim disease”); subjects with TB coinfection have lower BMI, MUAC, and hemoglobin level [22]. We hypothesized that markers of wasting could be useful to assess the risk of TB among PLHIV eligible to start ART. The presence of TB was associated with cough, low Karnofsky performance score, low MUAC, anemia, and peripheral lymph node enlargement in multivariate analysis; these variables were included in the scoring system.
Among the 255 WHO-TB+ patients with 0–1 scoring points (45% of the WHO-TB+ group), the risk of TB was low (20/255 [8%]; NPV = 92%). For such individuals, both ART and IPT might be initiated without further TB investigations. Conversely, for the 34 subjects with ≥4 scoring points, the likelihood of TB was 56%, whereas subjects with 2–3 points had an intermediate risk of TB (27%). For the latter group, further diagnostic TB testing is clearly indicated. This should also be considered for those with higher scores, although in settings with restricted access to TB diagnostic resources, it could be reasonable to initiate empirical treatment for active TB before starting ART in such patients [23].
The collection of the scoring variables requires neither great expertise from care providers nor advanced technical resources, apart from measurement of hemoglobin. An important advantage of a scoring system is that it can be used to indicate both a low and high likelihood of having a certain condition.
A proportion of TB cases were missed by the WHO-TB screening algorithm and the clinical scoring algorithm (10 WHO-TB– patients and 20 WHO-TB+ persons with 0–1 points). This may be explained by the occurrence of subclinical TB, which has been reported in settings with high TB prevalence [24]. Such cases might not be possible to identify by clinically based screening. It is likely that patients with TB not detected through our scoring strategy have less advanced disease with better prognosis [14, 25]. However, this approach could lead to inadvertent administration of IPT to subjects with active TB, and might also increase the risk of unmasking TB-IRIS for those who initiate ART. Although TB cases with initial negative scoring results might be identified through repeated scoring, most such persons would probably have started ART during this interval. Because exclusion of active TB using WHO-TB has been shown to be inferior in ART recipients, we do not propose use of the clinical scoring algorithm for such subjects [16].
Because several scoring variables reflect the severity of wasting, it is likewise plausible that subjects identified by clinical scoring are those with most advanced disease. For example, low MUAC has been shown to predict mortality in HIV/TB-coinfected subjects [26]. Interestingly, several components in the clinical scoring algorithm are similar to those included in the TBscore I and II [27, 28]. This scoring system was constructed to estimate TB disease severity and prognosis, but could also be useful for TB case finding [29].
Other techniques, independent of clinical manifestations, exist for TB case finding among PLHIV. In particular, rapid polymerase chain reaction technology (Xpert MTB/RIF) shows promise in the field of TB diagnostics, and has been validated for intensified case finding in PLHIV eligible to start ART [14, 33]. Yet, widespread access to Xpert MTB/RIF testing may be difficult to achieve [30]. Furthermore, this test fails to detect TB in approximately one-third of culture-confirmed cases [14, 25]. The role of chest radiography for TB screening among PLHIV has been assessed in different settings; its value for the confirmation of TB in PLHIV is low, especially in the absence of qualified interpretation [8, 31]. Another option for TB screening could be point-of-care biomarker testing; for instance, a level of C-reactive protein <10 mg/L has been shown to have a high NPV for exclusion of TB among individuals with suspected TB [32].
This study shows that the potential for clinically based categorization for the risk of TB among PLHIV has not been exhausted. Through prospective follow-up, we could estimate the proportion of TB cases presenting in patients not diagnosed at inclusion. Most of these cases occurred in subjects starting ART, and probably represent occult TB prevalent at baseline, a phenomenon that may lead to misclassification of subjects in cross-sectional studies. In our population, incident TB during 6-month follow-up was rare, illustrating the benefit of intensified TB case finding at ART initiation. All health centers providing integrated TB and HIV care in a defined uptake area of central Ethiopia participated in the study, and we consider the risk of inclusion bias into the cohort to be low.
This study has certain limitations. Before being considered for routine use, external validation of the scoring system is required. The performance of the clinical scoring algorithm is likely to be reduced in regions with lower TB endemicity, and the distribution and correlations between clinical variables and prevalent TB could be different in other settings. We did not assess interobserver variability for recording of variables, another factor that could affect performance. Our diagnostic protocol focused on pulmonary TB, and required submission of morning sputum samples. A proportion of subjects (61/873 [7%]) did not submit such samples, and were not included in the study; we consider this proportion to be small and unlikely to affect the results. Our protocol did not include investigations for extrapulmonary TB apart from peripheral lymphadenitis; hence, it is possible that some cases of extrapulmonary TB may have been missed.
In conclusion, by performing clinical scoring for HIV-infected adults with positive WHO-TB screening, the proportion of persons in need of further TB investigations could be significantly reduced. This classification strategy would be particularly useful in TB-endemic settings with restricted resources. By the combined use of these 2 clinically based instruments, a decision on the likelihood of active TB could be reached for a majority of PLHIV with access to health center–based ART, allowing for early initiation of treatment for both HIV and TB.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Notes
Acknowledgments. We extend our sincere gratitude to the patients participating in this study. We also thank all study investigators, laboratory staff, and our data management team, led by Gadisa Merga, for their committed work efforts. Furthermore, we appreciate the key support from the study health centers, Adama Regional Laboratory, and Oromia Health Bureau.
Financial support. This work was supported by the Swedish Civil Contingency Agency, the Swedish International Development Cooperation Agency, Region Skåne, and the Swedish Medical Association.
Potential conflicts of interest. All authors: No potential conflicts.
References
- 1.World Health Organization. Geneva, Switzerland: WHO: 2013. Global tuberculosis report. [Google Scholar]
- 2.Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375:1906–19. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, Harrington M, O'Brien R, et al. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–9. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 4.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis. 2011;204:1159–67. doi: 10.1093/infdis/jir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theron G, Peter J, van Zyl-Smit R, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184:132–40. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 7.Parsons LM, Somoskövi Á, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries : challenges and opportunities. Clin Microbiol Rev. 2011;24:314–50. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cain KP, Mccarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362:707–16. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 9.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 10.Lawn SD, Harries AD, Meintjes G, et al. Reducing deaths from tuberculosis in antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2012;26:2121–33. doi: 10.1097/QAD.0b013e3283565dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons (review) 2010:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alarcon E, Bissell K, Boillot F, et al. Isoniazid preventive therapy for people living with HIV: public health challenges and implementation issues. Int J Tuberc Lung Dis. 2015;13:927–35. [PubMed] [Google Scholar]
- 13.World Health Organization. Geneva, Switzerland: WHO: 2011. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource constrained settings. [Google Scholar]
- 14.Balcha TT, Sturegård E, Winqvist N, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLoS One. 2014;9:e85478. doi: 10.1371/journal.pone.0085478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health of Ethiopia. 2012. Guidelines for clinical and programmatic management of TB, leprosy and TB/HIV in Ethiopia. 5th ed. http://www.etharc.org/resources/download/finish/33/709 . Accessed 23 April 2014. [Google Scholar]
- 16.Khan F, Verkuijl S, Parrish A, et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS. 2014;28:1–10. doi: 10.1097/QAD.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNamee R. Regression modelling and other methods to control confounding. Occup Environ Med. 2005;62:500–6. doi: 10.1136/oem.2002.001115. 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streiner DL, Norman GR. Health measurement scales. New York, USA: Oxford University Press; 2008. [Google Scholar]
- 19.Wilson PWF, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 20.Dorman SE. New diagnostic tests for tuberculosis: bench, bedside, and beyond. Clin Infect Dis. 2010;50(suppl 3):S173–7. doi: 10.1086/651488. [DOI] [PubMed] [Google Scholar]
- 21.Corbett EL, Macpherson P. Tuberculosis screening in high human immunodeficiency virus prevalence settings: turning promise into reality. Int J Tuberc Lung Dis. 2013;17:1125–38. doi: 10.5588/ijtld.13.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skogmar S, Schön T, Balcha TT, et al. CD4 cell levels during treatment for tuberculosis (TB) in Ethiopian adults and clinical markers associated with CD4 lymphocytopenia. PLoS One. 2013;8:e83270. doi: 10.1371/journal.pone.0083270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis. 2011;15:287–95. [PubMed] [Google Scholar]
- 24.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–7. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Kerkhoff AD, Vogt M, et al. Characteristics and early outcomes of patients with Xpert MTB/RIF-negative pulmonary tuberculosis diagnosed during screening before antiretroviral therapy. Clin Infect Dis. 2012;54:1–9. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafson P, Gomes VF, Vieira CS, et al. Clinical predictors for death in HIV-positive and HIV-negative tuberculosis patients in Guinea-Bissau. Infection. 2007;35:69–80. doi: 10.1007/s15010-007-6090-3. [DOI] [PubMed] [Google Scholar]
- 27.Wejse C, Gustafson P, Nielsen J, et al. TBscore: signs and symptoms from tuberculosis patients in a low-resource setting have predictive value and may be used to assess clinical course. Scand J Infect Dis. 2008;40:111–20. doi: 10.1080/00365540701558698. [DOI] [PubMed] [Google Scholar]
- 28.Rudolf F, Lemvik G, Abate E, et al. TBscore II: refining and validating a simple clinical score for treatment monitoring of patients with pulmonary tuberculosis. Scand J Infect Dis. 2013;45:1–12. doi: 10.3109/00365548.2013.826876. [DOI] [PubMed] [Google Scholar]
- 29.Rudolf F, Haraldsdottir TL, Mendes MS, et al. Can tuberculosis case finding among health-care seeking adults be improved? Observations from Bissau. Int J Tuberc Lung Dis. 2014;18:277–85. doi: 10.5588/ijtld.13.0517. [DOI] [PubMed] [Google Scholar]
- 30.Walusimbi S, Bwanga F, De Costa A, et al. Meta-analysis to compare the accuracy of GeneXpert , MODS and the WHO 2007 algorithm for diagnosis of smear-negative pulmonary tuberculosis. BMC Infect Dis. 2013;13:1. doi: 10.1186/1471-2334-13-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holtz TH, Kabera G, Mthiyane T, et al. Use of a WHO-recommended algorithm to reduce mortality in seriously ill patients with HIV infection and smear-negative pulmonary tuberculosis in South Africa: an observational cohort study. Lancet Infect Dis. 2007;11:533–40. doi: 10.1016/S1473-3099(11)70057-3. [DOI] [PubMed] [Google Scholar]
- 32.Yoon C, Davis JL, Huang L, et al. Point-of-care C-reactive protein testing to facilitate implementation of isoniazid preventive therapy for people living with HIV. J Acquir Immune Defic Syndr. 2014;65:551–6. doi: 10.1097/QAI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.