Aspirin use is lower among HIV-infected patients compared to HIV-uninfected patients with a pronounced difference among those patients at highest cardiovascular risk. Aspirin use is also lower among HIV-infected patients when used for secondary prevention.
Keywords: aspirin, coronary disease, HIV, prevention, primary care
Abstract
Background
Human immunodeficiency virus (HIV) infection is associated with increased risk of myocardial infarction (MI). The use of aspirin for primary and secondary MI prevention in HIV infection has not been extensively studied.
Methods
We performed a cross-sectional study of 4037 patients infected with HIV and 36 338 demographics-matched control patients in the Partners HealthCare System HIV cohort. We developed an algorithm to ascertain rates of nonepisodic acetylsalicylic acid (ASA) use using medication and electronic health record free text data. We assessed rates of ASA use among HIV-infected and HIV-uninfected (negative) patients with and without coronary heart disease (CHD).
Results
Rates of ASA use were lower among HIV-infected compared with HIV-uninfected patients (12.4% vs 15.3%, P < .001), with a relatively greater difference among patients with ≥2 CHD risk factors (22.1% vs 42.4%, P < .001). This finding was present among men and among patients in the 30–39 and 40–49 age groups. Among patients with prevalent CHD using ASA for secondary prevention, rates of ASA use were also lower among HIV-infected patients compared with HIV-uninfected patients (51.6% vs 65.4%, P < .001).
Conclusions
Rates of ASA use were lower among HIV-infected patients compared with controls, with a greater relative difference among those with elevated CHD risk and those with known CHD. Further studies are needed to investigate the optimal strategies for ASA use among patients infected with HIV.
Because treatment of human immunodeficiency virus (HIV) has become more widespread and effective, there has been an increase in overall life expectancy [1, 2] but also a relative increase in noninfectious complications [3, 4]. Human immunodeficiency virus infection has been associated with increased risk of coronary heart disease (CHD) and myocardial infarction (MI) in multiple studies across different care settings [5–9].
Investigation has been increasingly directed towards understanding the mechanism of CHD in HIV-infected patients, with suggested links including higher rates of traditional MI risk factors [10], use of antiretroviral (ARV) medication [11], chronic inflammation [12], and immune dysfunction [13]. Despite the increased understanding of CHD pathophysiology in HIV, little is known about CHD prevention in this group. In particular, it is unclear whether traditional methods of CHD risk reduction are used in a similar manner or provide a similar benefit in HIV-infected (negative) patients compared with HIV-uninfected patients. Aspirin (acetylsalicylic acid [ASA]) is an inhibitor of platelet aggregation that has been shown to be effective in the primary [14–16] and secondary [17] prevention of MI, although few studies have investigated its use in patients with HIV. Our primary objective was to compare rates of ASA use for both primary and secondary prevention between patients infected with HIV and matched control patients.
METHODS
Study Design
To assess rates of ASA use for primary prevention, we conducted a cross-sectional study of 4037 patients infected with HIV and 36 338 HIV-uninfected patients without known CHD from the Research Patient Data Registry, a clinical care database of patients from Massachusetts General Hospital and Brigham and Women's Hospital, comprising the Partners HealthCare System in Boston, MA. Data from inpatient and outpatient visits are included in the database, as previously described [18]. Human immunodeficiency virus ascertainment was previously validated [19] and was established based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 042 or V08 or corresponding electronic health record (EHR) codes, with initial code occurring before MI if one did occur. The HIV-uninfected group was matched in a 10:1 ratio on age, gender, and race. The time period of analysis started no later than January 1, 2000, the first ICD-9-CM code for HIV when applicable, the first encounter, or the patient's 18th birthday. The analysis period ended no earlier than December 31, 2009, first MI, or last encounter.
We also assessed rates of ASA use among HIV-infected and HIV-uninfected patients with known CHD. We evaluated 410 HIV-infected and 3366 HIV-uninfected patients with prevalent CHD, diagnosed based on ICD-9-CM codes 410–414 before January 1, 2008 and before HIV code, if applicable. For secondary prevention, the analysis period started at the CHD diagnosis date and ended at last encounter or December 31, 2009, whichever was earlier.
Acetylsalicylic Acid Rate Measurement
Acetylsalicylic acid use rates were ascertained based on an algorithm developed for this study using a combination of medication data and EHR free text notes. Medication data included inpatient and outpatient prescriptions. All EHR clinical notes were loaded into an SQL-Server database, which searched for evidence of ASA use using a strategy that located the terms “aspirin,” “ASA,” “acetylsalicylic acid,” and “Aggrenox” and that eliminated cases in which the surrounding text indicated nonuse (eg, mentions of allergies or instructions to avoid ASA). Other brand names of ASA and possible misspellings of ASA were initially included but yielded no search results. We established a criterion for nonepisodic ASA use combining the medication and note data. Patients were classified as ASA users if they had at least: (1) 2 prescriptions, (2) notes with ASA text on 2 or more days, or (3) a prescription and a note. Patients were classified as using ASA for primary prevention if the notes or prescriptions were dated at least 30 days before the MI event. We classified patients as ASA nonusers if they had no notes and no prescriptions. Those with only one note or one prescription were excluded (n = 3329 of 40 375). We validated this algorithm using physician chart review of 207 charts, selected at random equally from HIV-infected and HIV-uninfected patients. The algorithm yielded a sensitivity of 73%, specificity of 83%, and area under the receiver-operating characteristics curve (AUC) of 0.78. When stratified by HIV status, AUC was 0.85 for HIV-infected and 0.76 for HIV-uninfected patients. For the secondary prevention analysis, patients were required to meet criteria for ASA use after the initial CHD diagnosis but before the end of the analysis period.
Patient characteristics including hypertension, diabetes mellitus, dyslipidemia, thromboembolic disease (deep venous thrombosis or pulmonary embolism), atrial fibrillation, and hepatitis C virus were defined using ICD-9-CM coding (see Table 1 for relevant codes). Additional exposure ascertainment included ICD-9-CM code 571 for chronic liver disease, 578 for gastrointestinal bleed, and 531–534 for ulcer disease. We obtained smoking status using a validated algorithm developed based on a natural language processing tool [20, 21]. For HIV-infected patients, patient characteristics measured included CD4 count, HIV RNA, and history of ARV use, subclassified as protease inhibitor (PI) use, nonnucleoside reverse-transcriptase inhibitor use, or nucleoside reverse-transcriptase inhibitor use. Recent CD4 and HIV RNA were defined as the latest laboratory measurement before the end of the analysis period. All patient characteristics were restricted to occurring before MI, if one occurred.
Table 1.
Baseline Characteristics of Study Participants Without Known CHDa
| HIV-Infected Patients |
HIV Uninfected Patients |
|||||
|---|---|---|---|---|---|---|
| ASA Use | No ASA Use | P Value | ASA Use | No ASA Use | P Value | |
| n = 458 | n = 3240 | n = 5089 | n = 28259 | |||
| Demographics | ||||||
| Age, mean (SD) | 48.0 (11.6) | 40.2 (10.1) | <.001 | 47.9 (11.6) | 40.1 (11.4) | <.001 |
| Women | 123 (26.9) | 1016 (31.4) | .051 | 1327 (26.1) | 10321 (36.5) | <.001 |
| Race | ||||||
| African-American | 104 (22.7) | 680 (21.0) | 1071 (21.1) | 6312 (22.3) | ||
| Caucasian | 249 (54.4) | 1632 (50.4) | 2983 (58.6) | 13936 (49.3) | ||
| Hispanic | 82 (17.9) | 565 (17.4) | .001 | 751 (14.8) | 5312 (18.8) | <.001 |
| Other | 10 (2.2) | 99 (3.1) | 95 (1.9) | 845 (3.0) | ||
| Unknown | 13 (2.8) | 264 (8.2) | 189 (3.7) | 1854 (6.6) | ||
| Duration of observation, mean years (SD) | 5.9 (3.1) | 5.1 (3.4) | <.001 | 6.8 (3.3) | 4.1 (3.6) | <.001 |
| Vascular Risk Factorsb | ||||||
| Hypertension | 290 (63.3) | 813 (25.1) | <.001 | 3219 (63.3) | 4650 (16.5) | <.001 |
| Diabetes mellitus | 185 (40.4) | 486 (15.0) | <.001 | 1547 (30.4) | 1606 (5.7) | <.001 |
| Dyslipidemia | 294 (64.2) | 1128 (34.8) | <.001 | 3168 (62.3) | 4681 (16.6) | <.001 |
| Smoking | 306 (66.8) | 1695 (52.3) | <.001 | 2584 (50.8) | 7657 (27.1) | <.001 |
| Atrial fibrillation | 43 (9.4) | 44 (1.4) | <.001 | 462 (9.1) | 351 (1.2) | <.001 |
| Thromboembolic disease | 34 (7.4) | 61 (1.9) | <.001 | 172 (3.4) | 195 (0.7) | <.001 |
| HCV infection | 146 (31.9) | 656 (20.3) | <.001 | 186 (3.7) | 430 (1.5) | <.001 |
| HIV Parameters | ||||||
| Recent CD4 count (cells/mm3), median (IQR) | 390 (218–621) | 431 (253–648) | .096 | – | – | – |
| Nadir CD4 count (cells/mm3), median (IQR) | 186 (53–340) | 221 (89–389) | .006 | – | – | – |
| Nadir CD4 count <200 | 152 (53.7) | 893 (45.8) | .013 | – | – | – |
| HIV RNA recent, median log copies/mL (IQR)c | 4.0 (2.9–4.8) | 4.0 (2.7–4.8) | .699 | – | – | – |
| HIV RNA <400 (undetectable) | 186 (67.9) | 1141 (61.9) | .055 | – | – | – |
| ARV medication used | 278 (60.7) | 1501 (46.3) | <.001 | – | – | – |
| PI use | 194 (42.4) | 981 (30.3) | <.001 | – | – | |
| NNRTI use | 172 (37.6) | 799 (24.7) | <.001 | – | – | |
| NRTI use | 265 (57.9) | 1432 (44.2) | <.001 | – | – | |
Abbreviations: ARV, antiretroviral; ASA, acetylsalicylic acid; CHD, coronary heart disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; SD, standard deviation.
a Unless indicated otherwise, data are expressed in number (percentage) of patients.
b ICD-9-CM codes used to identify diagnoses: hypertension, 401; diabetes mellitus, 250; dyslipidemia, 272; atrial fibrillation, 427.3; thromboembolic disease, 415.1 (pulmonary embolism) or 453.4 (deep vein thrombosis); HCV infection, 070.5, 070.7, 070.41, or 070.44; CHD, 410–414.
c Among those with detectable viral load.
d ARV use is categorized as ever/never.
Statistical Analysis
For patients without known CHD at start of observation, the frequencies of MI risk factors and HIV parameters (when applicable) were calculated in the HIV-infected and HIV-uninfected groups, stratified by ASA use. The χ2 test was used to compare categorical baseline characteristics between ASA users and nonusers in the both the HIV-infected and HIV-uninfected groups. We measured rates of nonepisodic ASA use using the described algorithm in HIV-infected and HIV-uninfected patients stratified by age group, CHD risk (high risk defined as ≥2 risk factors vs low risk defined as 0–1 risk factors, with risk factors including hypertension, dyslipidemia, diabetes, and smoking), and CD4 nadir in HIV-infected patients. We repeated the analysis for HIV-infected and HIV-uninfected patients with CHD before the start of observation, with the exception of stratification by CD4 nadir.
RESULTS
Demographic Characteristics
Demographic and clinical characteristics of patients without known CHD are outlined in Table 1. Among both HIV-infected and HIV-uninfected patients, ASA users were older and had significantly higher rates of traditional MI risk factors, including hypertension, diabetes, dyslipidemia, and smoking, although the relative differences in risk factors between ASA users and nonusers were greater in HIV-uninfected patients. Human immunodeficiency virus-infected ASA users had a significantly lower nadir CD4 count and a higher proportion of patients on ARV medications. Patients in the HIV-infected group also had higher rates of chronic liver disease (13.1% vs 3.2%), gastrointestinal bleed (11.0% vs 5.5%), and ulcer disease (3.7% vs 1.5%).
Rates of Acetylsalicylic Acid Use
Rates of ASA use are outlined in Table 2. Acetylsalicylic acid use was lower in HIV-infected compared with HIV-uninfected patients in the overall group (12.4% vs 15.3%, P < .001) and among men (13.1% vs 17.3%, P < .001), as shown in Figure 1. In age-stratified analyses, ASA use was significantly lower among HIV-infected patients age 30–39 and age 40–49, which was the largest of the age subgroups, but it was significantly higher among HIV-infected men over the age of 70. Rates of ASA used were significantly lower for HIV-infected compared with HIV-uninfected patients in both the high cardiovascular risk (22.1% vs 42.4%, P < .001) and low cardiovascular risk (5.5% vs 6.7%, P = .037) groups, but the relative difference was greater in patients in the high-risk group, as shown in Figure 1. The relatively larger difference in the high-risk group was seen among both women and men. Among HIV-infected patients, the rates of ASA use were significantly higher among those with a nadir CD4 count of <200 compared with those with a nadir CD4 count of ≥200, among the overall group and women. To address temporal changes in rates of ASA use between HIV-infected and HIV-uninfected patients, we compared the distribution of ASA use start years by HIV status and found the mean start year of ASA use to be similar (2005 for each group). To address whether contraindications contributed to lower ASA rates in HIV, we assessed the effect of HIV status on ASA use (using multivariable analysis), controlling for chronic liver disease, ulcer disease, gastrointestinal bleed, and hepatitis C virus. Human immunodeficiency virus remained significantly associated with decreased ASA (odds ratio for ASA, .56; 95% confidence interval, .50–.63).
Table 2.
Rates of ASA Use Among HIV and Control Patients Without Known CHDa
| Overall |
Women |
MEN |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV-Infected | HIV-Uninfected | P Value | HIV-Infected | HIV-Uninfected | P Value | HIV-Infected | HIV-Uninfected | P Value | |
| n = 3698 | n = 33 348 | n = 1139 | n = 11 648 | n = 2559 | n = 21 700 | ||||
| Overall prevalence | 458 (12.4) | 5089 (15.3) | <.001 | 123 (10.8) | 1327 (11.4) | .547 | 335 (13.1) | 3762 (17.3) | <.001 |
| Age group | |||||||||
| 18–29 | 16 (3.0) | 236 (4.2) | .194 | 7 (3.0) | 119 (3.9) | .491 | 9 (3.0) | 117 (4.5) | .231 |
| 30–39 | 88 (7.2) | 1009 (10.0) | .002 | 28 (7.1) | 265 (7.7) | .657 | 60 (7.3) | 744 (11.2) | .001 |
| 40–49 | 171 (13.6) | 1880 (17.8) | <.001 | 48 (14.2) | 446 (14.2) | .983 | 123 (13.4) | 1434 (19.2) | <.001 |
| 50–59 | 116 (22.0) | 1243 (25.1) | .116 | 20 (17.1) | 271 (20.7) | .351 | 96 (23.4) | 972 (26.6) | .152 |
| 60–69 | 47 (39.5) | 496 (34.5) | .271 | 14 (45.2) | 133 (32.4) | 0.147 | 33 (37.5) | 363 (35.3) | .680 |
| >70 | 20 (45.5) | 225 (36.8) | .250 | 6 (30.0) | 93 (35.8) | .603 | 14 (58.3) | 132 (37.5) | .043 |
| CHD risk factorsb | |||||||||
| 0–1 | 119 (5.5) | 1687 (6.7) | .037 | 36 (5.2) | 505 (5.3) | .864 | 83 (5.7) | 1182 (7.5) | .011 |
| ≥2 | 339 (22.1) | 3402 (42.4) | <.001 | 87 (19.7) | 822 (38.5) | <.001 | 252 (23.0) | 2580 (43.8) | <.001 |
| CD4 count nadir | |||||||||
| <200 cells/mm3 | 152 (14.6) | – | .013c | 44 (14.8) | – | .006c | 108 (14.5) | – | .194c |
| ≥200 cells/mm3 | 131 (11.0) | – | 25 (7.8) | – | 106 (12.3) | – | |||
Abbreviations: ASA,acetylsalicylic acid; CHD,coronary heart disease; HIV, human immunodeficiency virus.
a Unless indicated otherwise, data are expressed in number (percentage) of patients.
b CHD risk factors: hypertension, dyslipidemia, diabetes mellitus, smoking.
c P value comparing rates of ASA use among HIV-infected by CD4 nadir.
Figure 1.
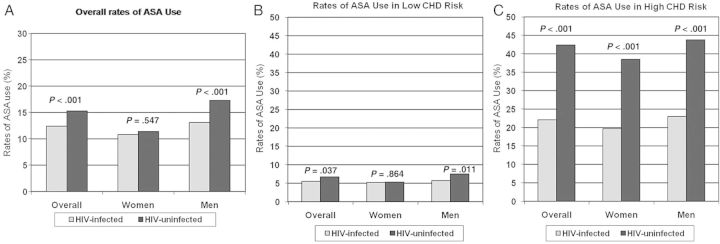
Rates of acetylsalicylic acid (ASA) use in human immunodeficiency virus (HIV)-infected vs HIV-uninfected patients in overall group (A), low coronary heart disease (CHD) risk patients (B), and high CHD risk patients (C). Rates of ASA use are shown in the overall population and within each gender for HIV-infected and HIV-uninfected patients without known CHD. Rates of ASA use are shown in low and high CHD risk (0–1 risk factors and ≥2 risk factors, respectively) HIV-infected and HIV-uninfected patients without known CHD. Coronary heart disease risk factors assessed were hypertension, dyslipidemia, diabetes, and smoking. P values are for comparison of rates between HIV-infected and HIV-uninfected patients within each group.
Rates of ASA use among patients with prevalent CHD at the start of observation are outlined in Table 3. Acetylsalicylic acid use was lower in HIV-infected patients compared with HIV-uninfected patients overall (51.6% vs 65.4%, P < .001) and within each gender (44.6% vs 59.3%, P = .004 among women and 54.3% vs 67.1%, P < .001 among men). In age-stratified analyses, rates of ASA use were significantly lower in the HIV group among patients aged 18–39 (32.3% vs 46.1%, P = .036), 40–49 (50.6% vs 61.9%, P = .006), and 50–59 (57.6% vs 72.7%, P = .001) years but not among patients older than age 59. Similar lower rates of ASA use in age stratified analyses were seen in the subgroup of HIV-infected males. Among all patients with known CHD, lower rates of ASA use were seen in patients with 0–1 CHD risk factors (24.7% vs 36.6%, P = .023) and among patients with ≥2 risk factors (59.7% vs 75.2%, P < .001).
Table 3.
Rates of ASA Use Among HIV And Control Patients With Known CHDa
| Overall |
Women |
Men |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV-Infected | HIV-Uninfected | P Value | HIV-Infected | HIV-Uninfected | P Value | HIV-Infected | HIV-Uninfected | P Value | |
| n = 403 | n = 3331 | n = 110 | n = 700 | n = 293 | n = 2631 | ||||
| Overall prevalence | 208 (51.6) | 2179 (65.4) | <.001 | 49 (44.6) | 415 (59.3) | .004 | 159 (54.3) | 1764 (67.1) | <.001 |
| Age group | |||||||||
| 18–39 | 21 (32.3) | 215 (46.1) | .036 | 9 (33.3) | 46 (38.3) | .628 | 12 (31.6) | 169 (48.8) | .043 |
| 40–49 | 82 (50.6) | 675 (61.9) | .006 | 19 (40.4) | 127 (57.2) | .036 | 63 (54.8) | 548 (63.1) | .083 |
| 50–59 | 68 (57.6) | 754 (72.7) | .001 | 12 (54.6) | 121 (66.9) | .252 | 56 (58.3) | 633 (74.0) | .001 |
| >59 | 37 (63.8) | 535 (72.5) | .156 | 9 (64.3) | 121 (68.4) | .753 | 28 (63.6) | 414 (73.8) | .144 |
| CHD risk factorsb | |||||||||
| 0–1 | 23 (24.7) | 309 (36.6) | .023 | 5 (20.0) | 61 (30.8) | .265 | 18 (26.5) | 248 (38.3) | .054 |
| ≥2 | 185 (59.7) | 1870 (75.2) | <.001 | 44 (51.8) | 354 (70.5) | .001 | 141 (62.7) | 1516 (76.4) | <.001 |
Abbreviations: ASA, acetylsalicylic acid; CHD, coronary heart disease; HIV, human immunodeficiency virus.
a Unless indicated otherwise, data are expressed in number (percentage) of patients.
b CHD risk factors: hypertension, dyslipidemia, diabetes mellitus, smoking.
DISCUSSION
In this large clinical care cohort comprised of more than 40 000 patients, we found that among patients infected with HIV, ASA was underused in primary and secondary prevention of MI compared with HIV-uninfected patients. Using a validated algorithm to ascertain nonepisodic ASA use, we showed that rates of ASA use were significantly lower in patients infected with HIV compared with matched controls, and that this difference was relatively greater among those at higher cardiovascular risk and those with known CHD.
Prior studies have shown ASA to be under utilized for the primary prevention of CHD in patients infected with HIV, with only 17% of patients who met criteria based on US Preventive Services Task Force recommendations receiving ASA [22]. Another study documented less than 2% of HIV-infected patients using ASA, although it was indicated by guidelines for approximately 31% [23]. However, neither of these studies included an HIV-uninfected population. In addition, the study was not able to compare ASA use in stratified models with patients comprehensively phenotyped for CVD risk and separated by gender. Moreover, these studies have not compared ASA use in primary and secondary prevention. We determined rates of ASA use in HIV-infected and HIV-uninfected patients, with stratification by age, gender, and CHD risk group. We ensured that there was at least a 30-day gap between ASA use documentation and initial MI to eliminate cases in which ASA use was initiated as a consequence of MI. Aspirin use for primary prevention was lower among HIV-infected patients compared with HIV-uninfected patients overall and among males, but not among females. There were also striking differences in rates of ASA use by CHD risk group, with ASA rates increased 20% in HIV-uninfected vs HIV-infected patients at low risk but nearly double for patients at high risk. Aspirin use for secondary prevention of MI was also significantly lower among HIV-infected patients compared with HIV-uninfected patients overall and among both women and men.
The reason for lower rates of ASA use among HIV-infected patients is unclear. One possible reason for the discrepancy is the lack of CHD prevention guidelines specific to the HIV population. Although recent HIV primary care guidelines do provide information on statin use, particularly as they pertain to drug interactions as well as recommendations for diabetes screening, they do not provide guidance on starting pharmacologic prevention measures such as ASA or statins [24]. This omission likely reflects a lack of studies of the role of ASA or statins, specifically in HIV. Moreover, CHD prevention strategies for the general population continue to evolve, as evidenced by recently released cholesterol treatment guidelines [25]. New CHD risk calculators that underlie the guideline changes merit validation in the HIV population, given novel risk factors in the pathogenesis of CHD in HIV infection before formalized adoption of the new cholesterol guidelines in the HIV-infected population.
This discrepancy in rates of ASA use could also reflect a generalized under emphasis by HIV providers on noninfectious chronic disease complications of HIV in the earlier years included in the time period of the study. As the paradigm of HIV care in developed countries shifts from preventing acquired immune deficiency syndrome-related complications and treating opportunistic infections to the chronic disease management of relatively healthier patients, clinical practice may take time to incorporate the vast number of changes needed. As noted, some of the chronic disease complications are reflected in recent HIV primary care guidelines that provide guidance on topics including diabetes, colon cancer, and bone density screening [24]. One recent study showed that breast cancer screening was lower among HIV-infected patients compared with HIV-uninfected patients and that HIV-infected patients were less likely to receive a colonoscopy purely for colorectal cancer screening purposes [26]. Another study showed low rates of successful treatment of hypertension among men and women infected with HIV [27]. Human immunodeficiency virus providers must continue to be informed of updates in primary care in addition to their specialty knowledge, including advances in HIV care and an expanded array of ARV medications. An additional reason for lower rates of ASA use in patients infected with HIV could be relatively higher rates of conditions that might increase bleeding risk. In our cohort, patients infected with HIV had higher rates of chronic liver disease, gastrointestinal bleed, and ulcer disease, any of which could represent a relative contraindication to ASA use. The finding that HIV status remained associated with decreased rates of ASA use when controlling for conditions that predispose to bleeding suggests that these factors alone are not likely to explain the lower rates of ASA use observed in HIV.
The gap in ASA use for secondary prevention in HIV-infected patients compared with HIV-uninfected patients may also be accounted for by a lack of specific ASA-related guidelines or an under emphasis by HIV providers. However, the overall low rates in both HIV-infected and HIV-uninfected groups are striking considering the strong evidence favoring ASA use for secondary prevention. The low rates observed among both HIV-infected and HIV-uninfected patients may reflect underuse of this treatment modality, which has been demonstrated for the general population [28]. However, the underlying reasons for low rates of ASA use for secondary prevention in the general population remain unknown. There is a clear need for targeted interventions to increase ASA use in this high-risk population of both HIV-infected and HIV-uninfected patients.
Indications for and optimal rates of ASA use in patients infected with HIV remain unknown. In the general population, multiple meta-analyses of randomized trials of primary prevention have shown that ASA was associated with reduction in vascular events, including MI, but not necessarily a reduction in mortality [15, 16, 29]. The evidence supporting ASA use for secondary prevention of MI in the general population is stronger, with a meta-analysis showing antiplatelet therapy to be associated with a 22% reduction in the risk of serious vascular event and reduction in vascular mortality [17]. Whether this data on the efficacy of ASA use for event reduction applies to patients infected with HIV remains incompletely understood and is a focal point for further investigation.
Mechanistic evidence on platelet activation and endovascular damage in patients infected with HIV-infected suggests that ASA might play at least a partial role in CHD prevention for this group. Platelet activation and aggregation are necessary steps for thrombus formation and arterial occlusion in acute coronary syndrome [30], and ASA reduces platelet production of thromboxane A2, thereby decreasing platelet aggregation. Early evidence suggested that compared with HIV-uninfected controls, patients infected with HIV had increased platelet activation, as measured by flow cytometric analysis of markers P-selectin and CD63 [31]. Further study has shown differential effects between HIV-infected and HIV-uninfected patients in response to various agonists of platelet aggregation, with unclear effects on the overall association between HIV infection and platelet aggregation [32]. There are only limited data on the effects of ASA on platelet aggregation in patients infected with HIV; however, in vitro data suggest that ASA reduces platelet aggregation in patients infected with HIV, although in slightly different ways than for HIV-uninfected patients, and also reduces a generalized marker of immune activation in HIV-infected patients [33]. Overall, it remains unclear whether ASA will have the same effects in HIV-infected patients as in HIV-uninfected patients given the novel CHD-related risk factors in HIV, which may not be mitigated by ASA. This underlying issue of whether traditional CHD prevention measures such as ASA or statin therapy have a different role in HIV infection remains an open question. Furthermore, the role of novel therapies targeting chronic inflammation and immune activation to decrease CHD risk in HIV infection requires investigation in addition to study of more conventional CHD prevention strategies in HIV populations.
Strengths of our study include the use of a well validated, large data registry, previously shown to perform well in the characterization of MI, CHD risk rates, and stroke in HIV-infected vs HIV-uninfected populations [6, 13, 34]. Using this database, we were able to generate a tailored control group matched to the HIV cohort, from the same background population. Therefore, for the first time, we were able to assess rates of ASA use in patients infected with HIV in comparison with matched control patients. We also developed and validated an algorithm utilizing EHR free text search to accurately identify ASA use, an exposure that can be difficult to ascertain using prescription medication data alone. Limitations of our study include the incomplete ability to assess dosing of and adherence to ASA. We were also unable to evaluate over-the-counter ASA use if it was not explicitly mentioned in provider notes. Nonetheless, we expect similar limitations for both HIV-infected patients and matched control patients.
CONCLUSIONS
In summary, our study demonstrates lower rates of ASA use among patients infected with HIV compared with controls, particularly in patients at high underlying cardiovascular risk and those with known CHD. Our findings underscore the need for the development of evidence-based, tailored CHD prevention strategies in the HIV population.
Acknowledgments
We are grateful the Center for AIDS Research Biostatistical Core for statistical consultation and the Partners HealthCare Research Patient Data Registry group for facilitating use of their database.
Disclaimer. The funding sources had no role in the design and conduct of the study.
Financial support. This work was supported by the National Institutes of Health (NIAID AI 007433 [to S. S.], NIH K01 AI07310 [to V. A. T.], K24 DK080140 [to J. B. M.], and K24 DK064545 [to S. K. G.]).
Potential conflicts of interest. S. K. G. received research support from Amgen, Bristol-Myers Squibb, Gilead, and Theratechnologies. He also served as a consultant to Aileron.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.van Sighem AI, Gras LA, Reiss P, et al. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24:1527–35. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- 2.Lewden C, Bouteloup V, De Wit S, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41:433–45. doi: 10.1093/ije/dyr164. [DOI] [PubMed] [Google Scholar]
- 3.Sackoff JE, Hanna DB, Pfeiffer MR, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 4.Neuhaus J, Angus B, Kowalska JD, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–12. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 6.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–30. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 8.Klein D, Hurley LB, Quesenberry CP, et al. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30:471–7. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 9.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 11.Holmberg SD, Moorman AC, Williamson JM, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–8. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 12.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 13.Triant VA, Regan S, Lee H, et al. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55:615–9. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 15.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seshasai SR, Wijesuriya S, Sivakumaran R, et al. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172:209–16. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 17.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triant VA, Brown TT, Lee H, et al. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–73. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng QT, Goryachev S, Weiss S, et al. Extracting principal diagnosis, co-morbidity and smoking status for asthma research: evaluation of a natural language processing system. BMC Med Inform Decis Mak. 2006;6:30. doi: 10.1186/1472-6947-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regan S, Meigs JB, Grinspoon SK, et al. Determinants of smoking and quitting in an HIV cohort using a validated natural language processing tool. Abstract P168 of the Epidemiology and Prevention and Nutrition, Physical Activity and Metabolism meeting; San Diego. 2012. 13–16 March. [Google Scholar]
- 22.Burkholder GA, Tamhane AR, Salinas JL, et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis. 2012;55:1550–7. doi: 10.1093/cid/cis752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tornero C, Ventura A, Mafe M. Aspirin is indicated for primary prevention of cardiovascular events in HIV-infected patients. J Acquir Immune Defic Syndr. 2010;54:560. doi: 10.1097/QAI.0b013e3181d913fd. [DOI] [PubMed] [Google Scholar]
- 24.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:1–10. doi: 10.1093/cid/cit757. [DOI] [PubMed] [Google Scholar]
- 25.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 26.Momplaisir F, Mounzer K, Long JA. Preventive cancer screening practices in HIV-positive patients. AIDS Care. 2014;26:87–94. doi: 10.1080/09540121.2013.802276. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein KA, Armon C, Buchacz K, et al. Provider compliance with guidelines for management of cardiovascular risk in HIV-infected patients. Prev Chronic Dis. 2013;10:E10. doi: 10.5888/pcd10.120083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005;2:e353. doi: 10.1371/journal.pmed.0020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol. 2011;107:1796–801. doi: 10.1016/j.amjcard.2011.02.325. [DOI] [PubMed] [Google Scholar]
- 30.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–94. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 31.Holme PA, Muller F, Solum NO, et al. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 1998;12:79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 32.Satchell CS, Cotter AG, O'Connor EF, et al. Platelet function and HIV: a case-control study. AIDS. 2010;24:649–57. doi: 10.1097/QAD.0b013e328336098c. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien M, Montenont E, Hu L, et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr. 2008;63:280–8. doi: 10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow FC, Regan S, Feske S, et al. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]


