(1→3)-Beta-D-glucan was detected in high levels in cerebrospinal fluid, and to lesser extent in serum, among HIV-infected persons with cryptococcal meningitis.
Keywords: AIDS, β-glucan, cryptococcal meningitis, HIV, predictive value of tests
Abstract
Background
(1→3)-β-d-Glucan (BDG) is a helpful diagnostic marker for many invasive fungal infections. However, BDG is not thought to be useful in diagnosing cryptococcosis. We evaluated the utility of BDG as an adjunct diagnostic tool for patients infected with human immunodeficiency virus (HIV) and presenting with suspected cryptococcal meningitis.
Methods
The Fungitell assay was used to measure BDG concentrations in cerebrospinal fluid (CSF) (n = 177) and serum (n = 109) of HIV-infected Ugandans and South Africans with suspected meningitis. Correlations between BDG concentrations and quantitative CSF cryptococcal cultures, CSF cryptococcal antigen (CRAG) titers, and 18 different CSF cytokine concentrations were assessed using non-parametric tests. Mixed models evaluated longitudinal changes in CSF BDG concentrations. Survival analyses were used to evaluate BDG's relationship with mortality.
Results
The Fungitell BDG assay provided 89% sensitivity and 85% specificity in CSF for cryptococcal meningitis. Serum sensitivity was suboptimal (79%). Cerebrospinal fluid BDG concentrations at diagnosis were median (interquartile range) 343 (200–597) pg/mL in cryptococcal patients and 37 (23–46) pg/mL in patients without cryptococcosis. Sensitivity in CSF improved to 98% (53 of 54) when initial fungal burdens were ≥10 000 colony-forming units/mL. (1→3)-β-d-Glucan normalized rapidly after initiating antifungal therapy. Baseline BDG concentrations correlated with CSF fungal burden (rho = 0.820; P < .001), CSF CRAG lateral flow assay titers (rho = 0.780, P < .001), and monocyte chemotactic protein-1 levels in CSF (P = .047). In patients with cryptococcal meningitis, BDG ≥500 pg/mL at diagnosis was associated with increased 10-week mortality.
Conclusions
(1→3)-β-d-Glucan is detectable in the CSF of HIV-infected patients with Cryptococcus, and it may provide useful prognostic information. Sensitivity is less than CRAG; however, BDG normalizes rapidly, unlike CRAG, making BDG potentially useful in diagnosing recurrent episodes.
(1→3)-β-d-Glucan (BDG) is a polysaccharide glucose polymer that is a major constituent of the cell wall of many medically important fungi [1, 2]. The Fungitell assay (Associates of Cape Cod, Inc., Falmouth, Massachusetts) has been approved by the US Food and Drug Administration (FDA) for the detection of BDG in serum, and it is helpful in the diagnosis of invasive fungal infections [3, 4]. New data also demonstrate value in measuring BDG concentrations in the cerebrospinal fluid (CSF) of patients with suspected fungal infections of the central nervous system (CNS) [5–7].
Cryptococcus reportedly releases very low levels of BDG [1], thus the FDA label of the Fungitell assay specifies that it does not detect Cryptococcus [8]. However, this assumption is based on limited data from small studies measuring BDG levels in the serum of human immunodeficiency virus (HIV)-negative patients with cryptococcal disease outside of the CNS. In a frequently cited study consisting of 7 patients with pulmonary cryptococcosis, plasma levels of BDG were not elevated [8]. All previous studies evaluating serum BDG have involved small numbers of cryptococcosis cases as part of larger investigations into invasive fungal disease [9–12].
We used the Fungitell assay (Associates of Cape Cod, Inc., East Falmouth, Massachusetts) to assess the diagnostic performance of BDG for cryptococcal meningitis in the CSF and serum of HIV-infected Ugandans and South Africans. We also investigated associations between CSF BDG concentrations and markers of CSF immune response and clinical outcomes.
METHODS
(1→3)-β-d-Glucan testing was performed retrospectively on cryopreserved (−80°C) CSF (n = 177) or serum (n = 109) specimens from persons infected with HIV who were enrolled into 2 prospective cohorts of hospitalized patients with suspected meningitis in Uganda (Kampala and Mbarara) and Cape Town, South Africa, from 2010 to 2013 (ClinicalTrials.gov: NCT01075152; NCT01802385) [13]. All subjects provided written informed consent. Institutional review board approval was obtained from all sites. All subjects received amphotericin B (0.7–1 mg/kg per day) and fluconazole (800–1200 mg/day) during the first 14 days of induction therapy, according to locally established guidelines.
Of the 177 CSF specimens, 117 were obtained at time of diagnosis (67% with cryptococcal meningitis, 33% with undiagnosed aseptic meningitis) and constitute the samples included in the primary analysis of diagnostic performance. All 25 CSF specimens obtained in South Africa (21% of total used in primary analysis) were obtained from patients with cryptococcal meningitis. An additional 60 CSF specimens, obtained from therapeutic lumbar punctures from 3 to 20 days postdiagnosis, were tested to assess the rate of BDG clearance. One hundred seventy-six specimens were included in analyses evaluating the effects of antifungal therapy on fungal burden and BDG concentrations; 1 sample obtained at 20 days postdiagnosis was excluded. Serum specimens from 109 patients, obtained on the day of diagnosis (42% with cryptococcal meningitis), were used in the diagnostic utility analysis. Forty-one subjects contributed both CSF (35% of total) and serum (38% of total) specimens for the primary analysis, whereas the remaining subjects contributed either CSF or serum.
Diagnostic Testing
Diagnosis of cryptococcosis was made on site via CSF cryptococcal antigen (CRAG) lateral flow assay (LFA) (Immy, Inc., Norman, Oklahoma), microscopy, quantitative culture, and/or serum CRAG [14]. Quantitative CSF fungal culture was performed using 100 µL input volume of CSF, cultured undiluted, and with four 1:10 serial dilutions incubated at 30°C for up to 14 days [15, 16]. All culture isolates were independently confirmed as Cryptococcus neoformans var. grubii with multilocus sequence typing, as described previously [17]. Semiquantitative CRAG LFA titers were performed on cryopreserved samples using 2-fold serial dilution, starting at 1:25 dilution. When qualitatively positive but negative at 1:25 titer, 2-fold dilutions were run starting at 1:2. Gram stain, bacterial culture, and stain for acid-fast bacilli were prospectively performed on all CSF specimens. GeneXpert MTB/RIF assay (Cepheid Inc., Sunnyvale, California) was performed on cryptococcal-negative specimens in Uganda. The reference standard for cryptococcal meningitis was defined as either a positive C neoformans CSF culture or CSF CRAG [14].
Laboratory Procedures
The Fungitell assay was performed at Associates of Cape Cod, Inc. laboratories on cryopreserved specimens. Laboratory technicians were blinded to specimen diagnosis. A cutoff BDG concentration of ≥80 pg/mL was considered a positive test result. (1→3)-β-d-Glucan concentrations between 60 pg/mL and 79 pg/mL are indeterminate per the FDA package insert. For assessing diagnostic performance, however, a BDG concentration <80 (including all indeterminate results) was considered negative.
Luminex magnetic bead technology (Bio-Rad Laboratories, Hercules, California) was used to analyze 18 cytokines and chemokines (interleukin [IL]-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17; granulocyte colony-stimulating factor; granulocyte macrophage colony-stimulating factor; interferon-gamma; tumor necrosis factor-alpha [TNF-α]; monocyte chemotactic protein-1 [MCP-1 or CCL2]; macrophage inflammatory protein-1β [MIP-1β or CCL4]; vascular endothelial growth factor [VEGF]) according to the manufacturer's protocols in 89 baseline CSF specimens obtained from patients with (n = 69) and without (n = 20) cryptococcal meningitis.
Statistical Analysis
The primary analysis consisted of the BDG diagnostic performance (sensitivity, specificity, positive and negative predictive values) at time of diagnosis. Secondary analysis examined sensitivity only with stratification by CSF quantitative fungal burden and by country, due to the genetic diversity of Cryptococcus isolates [17, 18]. Post hoc analyses examining important covariates that could explain differences in diagnostic performance across country were conducted, including the Wilcoxon rank-sum test of quantitative fungal burden and a test of equality of sensitivity proportions. Simple and multiple linear regression were used to determine baseline correlations between log-transformed cytokine and BDG concentrations in all patients who completed a quantitative culture. Log-transformed quantitative culture was included as a covariate in multiple linear regression models. Additional analysis included non-parametric correlation of BDG with CRAG titers (n = 112). “Out of range” cytokine measurements were arbitrarily set to the limit of detection divided by 2.
Among patients diagnosed with cryptococcal meningitis, changes in BDG over time and associations between pretreatment BDG concentrations and 1- and 10-week mortality were examined using a linear mixed model with random intercepts and Cox proportional hazard models, respectively. Four patients were excluded from the survival analysis due to enrollment ineligibility and consequently had no follow-up information. The survival analyses stratified BDG concentrations at the approximately highest quartile (≥500 pg/mL), with adjustment for mental status (Glasgow Coma Scale score <15) and colony-forming units (CFU)/mL. All analyses were conducted in Stata/IC version 12.1 (StataCorp, College Station, Texas). Statistical significance was defined as alpha <0.05.
RESULTS
One hundred seventeen subjects with suspected meningitis were enrolled in Uganda and South Africa: 78 had cryptococcal meningitis (n = 76 culture positive; n = 78 CRAG LFA positive) and 39 were negative by CSF culture, CRAG, and microscopy with Gram stain and/or India ink.
(1→3)-β-d-Glucan in Cerebrospinal fluid
The median (interquartile range [IQR]) CSF BDG concentration in persons with cryptococcal meningitis was 343 (200–597) pg/mL. The Fungitell BDG assay provided 89% (69 of 78) sensitivity and 85% (33 of 39) specificity compared with the composite reference standard of culture or CRAG LFA (Table 1). One negative BDG result occurred in 1 individual with no known history of cryptococcal meningitis that was diagnosed by a positive CSF CRAG but with a sterile CSF culture. Of the 9 false-negative results, 5 had BDG CSF levels of <60 pg/mL and 4 had a BDG level between 60 pg/mL and 79 pg/mL, which is considered indeterminate per manufacturer guidelines for serum. If we considered indeterminate values as positive rather than negative, the overall sensitivity of the assay in CSF increased to 94% (73 of 78) without any loss in specificity.
Table 1.
Diagnostic Performance of (1→3)-β-d-Glucan in CSF and in Serum for Cryptococcal Meningitis, Using a Positive Cutoff of ≥80 pg/mL*
| Specimen Type | N | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | 95% CI | ||
| CSF | 117 | 89% (69 of 78)† | 85% (33 of 39) | 92% (69 of 75) | 79% (33 of 42) |
| 81%–95% | 70%–94% | 83%–97% | 66%–91% | ||
| Serum | 109 | 79% (37 of 47)‡ | 61% (38 of 62) | 61% (37 of 61) | 79% (38 of 48) |
| 64%–89% | 48%–73% | 47%–73% | 65%–90% |
Significant values are represented in bold.
Abbreviations: CI, confidence interval; CSF, cerebrospinal fluid; NPV, negative predictive value; PPV, positive predictive value.
* Cryptococcal meningitis was defined by CSF positivity of cryptococcal antigen (n = 78) and/or culture (n = 76).
† In CSF, 4 specimens from patients diagnosed with cryptococcal meningitis were in the indeterminate range (60–79 pg/mL) according to the package insert. These were considered negative for purposes of diagnostic performance.
‡ In serum, 5 specimens from patients diagnosed with cryptococcal meningitis and 4 specimens from patients without cryptococcal meningitis were in the indeterminate range (60–79 pg/mL) according to the package insert.
The median (IQR) CSF BDG concentration among patients with meningitis not due to Cryptococcus was 37 (23–46) pg/mL (Figure 1). Six patients without cryptococcal meningitis had putative false-positive CSF BDG. One individual that was CRAG-positive in serum but without evidence of meningitis (CSF culture and CRAG negative with a normal CSF opening pressure and no CSF pleocytosis) had a CSF BDG concentration of 130 pg/mL. Three persons with CSF BDG ≥80 pg/mL without cryptococcosis had normal CSF profiles and normal opening pressures. Two persons with false positives had lymphocytic meningitis of unknown etiology, 1 with elevated opening pressure of 42 cm H2O.
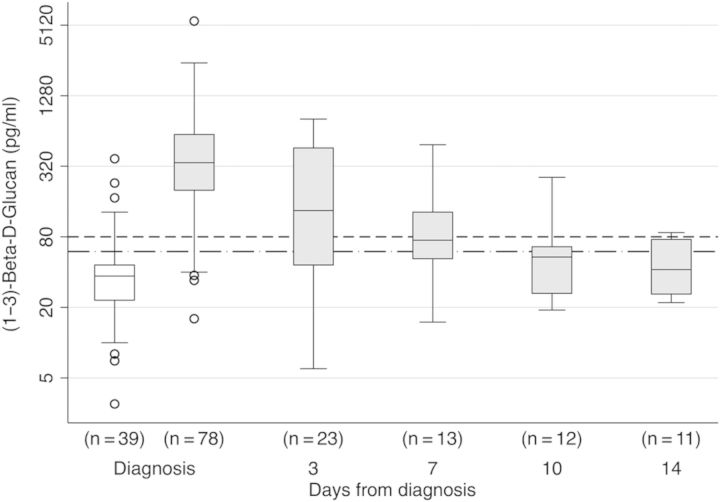
Figure 1.
Cerebrospinal fluid levels of (1→3)-β-d-glucan (BDG) by diagnosis and change with antifungal therapy. Boxplot for log2 BDG concentration by meningitis diagnosis, and the effect of induction therapy on BDG concentrations in patients with cryptococcal meningitis. The median BDG level was below the assay negative cutoff value of 60 pg/mL (dotted dashed line) in meningitis patients without cryptococcosis (white box). The median BDG levels in cryptococcal meningitis patients (gray boxes) was above the assay positive cutoff value of 80 pg/mL (dashed line) at diagnosis, but it rapidly normalized with induction therapy.
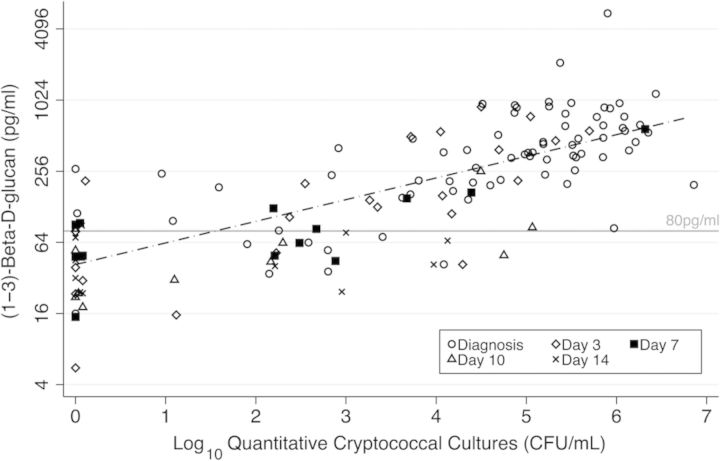
(1→3)-β-d-Glucan levels correlated with CSF fungal burden by quantitative culture (rho = 0.820; P < .001) (Figure 2) and CRAG LFA titers (rho = 0.780, P < .001) at baseline. Sensitivity improved with higher initial fungal burden by quantitative culture (Table 2). We observed a sensitivity of 98% (53 of 54) when initial fungal burdens were ≥10 000 CFU/mL, and we noted only 61% (11 of 18) sensitivity when initial fungal burdens were below 10 000 CFU/mL. We also observed better sensitivity of BDG in CSF in Uganda (94%; 95% confidence interval [CI], 87%–98%; n = 53) compared with South Africa (80%; 95% CI, 64%–96%; n = 25) (P = .10). Ugandan patients tended to have higher CSF burdens, with 80% (40/50) in Uganda vs 58% (14/24) in South Africa (P = .05) presenting with an initial quantitative culture ≥ 10 000 CFU/mL.
Figure 2.
Correlation between cerebrospinal fluid (CSF) quantitative culture and CSF (1→3)-β-d-glucan levels. (1→3)-β-d-Glucan concentrations relative to quantitative cryptococcal cultures among all 78 patients diagnosed with cryptococcal meningitis at diagnosis (hollow circle), and day 3 (hollow diamond), day 7 (square), day 10 (hollow triangle), and day 14 (small x) (n = 137).
Table 2.
Sensitivity of CSF (1→3)-β-d-Glucan by CSF CRAG LFA or Quantitative CSF Fungal Culture at Time of Diagnosis, Using a Positive Cutoff Value of ≥80 pg/mL (n = 78)
| Cryptococcal Status | n | Median (IQR) pg/mL | Sensitivity | 95%CI |
|---|---|---|---|---|
| Culture negative, CSF CRAG negative | 39 | 37 (23–46) | N/A | |
| Culture negative, CSF CRAG+ only | 2 | 144 (16–271) | 50% (1 of 2) | 0–100* |
| CSF culture <10 000 CFU/mL | 18 | 121 (63–202) | 61% (11 of 18) | 36–83 |
| CSF culture 10 000–100 000 CFU/mL | 18 | 349 (215–531) | 100% (18 of 18) | 81–100* |
| CSF culture >100 000 CFU/mL | 36 | 524 (336–769) | 97% (35 of 36) | 85–100 |
| CSF culture positive, any† | 72† | 360 (201–645) | 89% (64 of 72) | 79–95 |
Abbreviations: CFU, colony forming units; CRAG, cryptococcal antigen; CSF, cerebrospinal fluid; IQR, interquartile range; LFA, lateral flow assay; N/A, not applicable; 95% CI, binomial exact confidence interval.
* 1-sided 97.5% CI.
† n = 4 missing quantification of initial CSF culture.
(1→3)-β-d-Glucan normalized rapidly after initiating antifungal therapy, with a −0.23 (95% CI: −0.27, −0.19) average change in log2 BDG concentrations for each day of follow-up (Figure 1). Alternatively stated, there was on average a ∼50% reduction (ie, −0.92 log2) in CSF BDG concentrations after 4 days of therapy. In this cohort, the average rate of early fungicidal activity was −0.31 log10 CFU/mL CSF/day [13]. Among CSF specimens collected on day 7 onwards, culture-positive CSF specimens trended towards having higher BDG levels than the culture-negative specimens (101 vs 50 pg/mL, respectively; P = .055).
Among patients diagnosed with cryptococcal meningitis, we noted that pretreatment BDG concentrations ≥500 pg/mL were associated with increased acute mortality through 10 weeks (hazard ratio, 2.9; 95% CI, 1.5–5.9; P = .003) (Figure 3). When we further included baseline quantitative fungal culture (n = 68) into a multivariable model, pretreatment CSF BDG levels ≥500 pg/mL remained independently associated withincreased mortality (hazard ratio, 2.54; 95% CI, 1.01–6.35; P = .047). When we adjusted for altered mental status, we found that there was no impact on hazard estimates.
Figure 3.
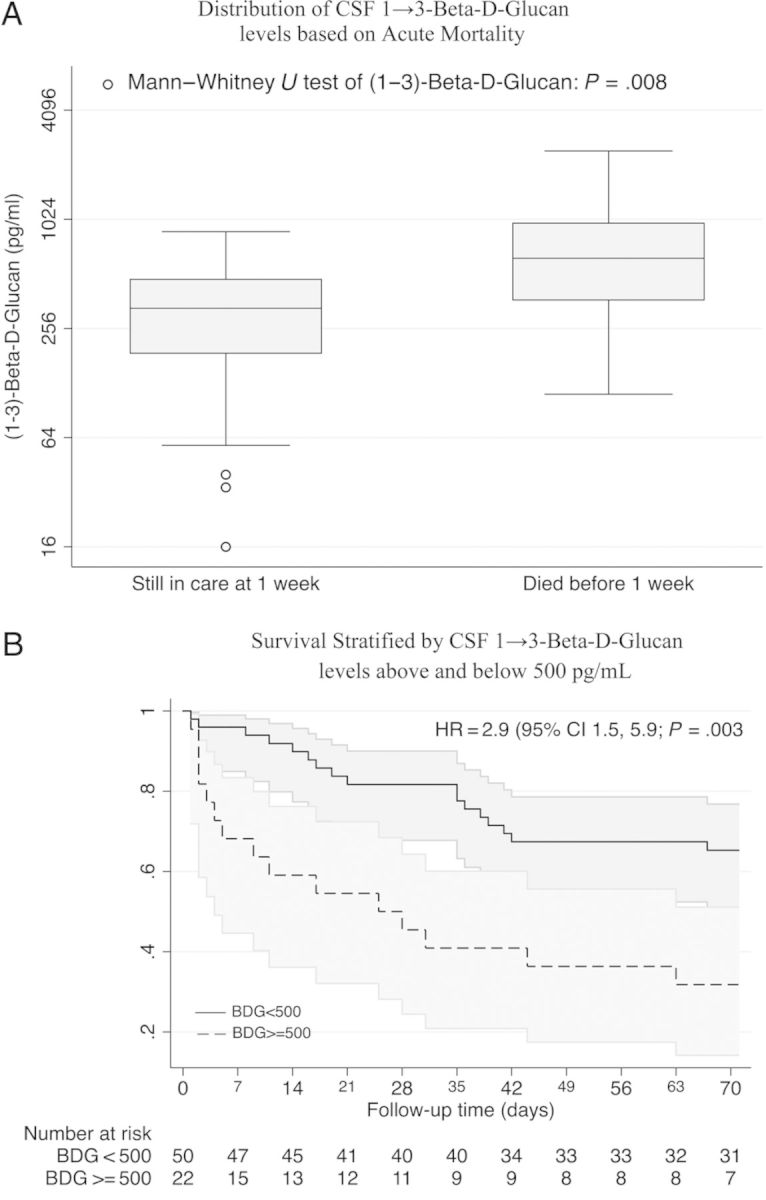
Cerebrospinal fluid (1→3)-β-d-glucan (BDG) associations with mortality. (A) The BDG concentrations were significantly higher in patients who died within 1 week of starting therapy for cryptococcal meningitis (P = .008). (B) A Kaplan–Meier survival curve showing 10-week survival from time of diagnosis in the 72 Ugandan patients with cryptococcal meningitis included in the analysis.
Cerebrospinal Fluid Biomarkers
Of the 89 baseline CSF specimens further analyzed for immune biomarkers (Supplemental Table S1), CSF BDG concentrations correlated with IL-8 (β = 0.27, P < .01), MIP-1β (or CCL4; β = 0.38, P = .02), MCP-1 (or CCL2; β = 0.48, P = < .001), and TNF-α (β = 0.32, P = .02). However, these chemokines also correlated with quantitative fungal burden, and after adjusting for quantitative CSF fungal burden, only MCP-1 (CCL2) maintained an independent relationship with BDG in a multivariable model (β = 0.15, P = .047).
(1→3)-β-d-Glucan in Serum
In serum, BDG demonstrated 79% (37 of 47) sensitivity and 61% (38 of 62) specificity for cryptococcal meningitis, using the manufacturer's cutoff of 80 pg/mL (Table 1). Nine samples (n = 5 with Cryptococcus) would be considered indeterminate according to the manufacturer (BDG 60–79 pg/mL). If we considered indeterminate values as positive rather than negative, the overall sensitivity of the assay in serum increased to 89% (42 of 47), but this result was at the expense of specificity, which decreased to 55% (34 of 62). Although diagnostic performance in serum was suboptimal, it demonstrated that a positive serum BDG result can occur with cryptococcosis.
Among the 41 individuals who had both serum and CSF available at the time of diagnosis, Fungitell BDG results agreed in 83% of patients (Cohen's κ 0.39, showing fair agreement). (1→3)-β-d-Glucan concentrations in CSF and serum among these samples were correlated (Spearman's rho = 0.34; P = .03).
DISCUSSION
This is the first study to specifically evaluate BDG concentrations in patients infected with HIV with cryptococcal meningitis. We observed that the Fungitell BDG assay is moderately sensitive and specific in CSF for the diagnosis of cryptococcal meningitis. Although BDG diagnostic performance characteristics are inferior to those of the CRAG LFA for first episodes of cryptococcal diagnosis, we observed that BDG correlated with immune responses, rapidly decreased with effective antifungal therapy, and that higher concentrations were independently associated with worse short-term survival. Our findings are contrary to established dogma, which asserts that Cryptococcus produces insufficient amounts of BDG to be detected. Although it is not an optimal diagnostic test, a positive BDG result is probable in any given patient with cryptococcal meningitis, particularly among those with higher fungal burdens.
Previous studies on the utility of BDG in cryptococcal disease have focused on the detection of BDG in serum, with variable performance reported [8–11, 19, 20]. Our results, which constitute the largest sampling to date, demonstrate that BDG is detectable in patients with cryptococcal meningitis, although BDG in serum is neither as sensitive nor as specific as BDG in CSF. As has been observed in prior studies, BDG may be detected in the CSF but not serum of patients with fungal infections of the CNS [6]. (1→3)-β-d-Glucan traffic between compartments, relative clearance rates, and organism-related impacts remain to be clarified. Although BDG measurement in serum does not have adequate sensitivity or specificity to guide therapy at screening, a positive result should guide further diagnostic testing. Our results suggest that cryptococcosis should remain in the differential diagnosis of any patient with a positive BDG assay being screened for possible invasive fungal infection.
More importantly, ours is the first study to measure BDG levels in the CSF of a large group of patients with cryptococcal meningitis. Greater sensitivity of BDG in CSF compared with serum may partially explain the poor sensitivity observed in previous studies evaluating BDG in the diagnosis of cryptococcosis, which have involved patients primarily with pulmonary cryptococcosis.
The ability to detect BDG seems to be dependent on fungal burden. In cases of meningitis, Cryptococcus is concentrated in the CSF, whereas serum may contain lower levels of fungal products reflecting disseminated infection. Therefore, it may be expected that the BDG assay would perform better in CSF compared with serum in cases of cryptococcal meningitis. Cryptococcus is thought to release lower concentrations of BDG compared with other pathogenic yeasts [1], which may explain the loss of sensitivity of the assay at lower fungal burdens. A lower assay cutoff in CSF, however, could theoretically overcome some of the loss in sensitivity.
When we considered indeterminate values as positive rather than negative, the overall sensitivity of the assay in CSF increased without any loss in specificity. This was not the case in serum, where sensitivity improved at the expense of poorer specificity after manipulation of the cutoff. This result could reflect the fact that assay performance was standardized using serum. The relatively poor specificity (62% at ≥80 pg/mL threshold) in serum among this population with advanced acquired immune deficiency syndrome (AIDS) with suspected meningitis is in and of itself interesting. Because persons living with AIDS have increased microbial translocation, which is best studied in relation to lipopolysaccharide [21], this poor serum specificity may potentially reflect that there is also yeast product translocation. Its significance requires further investigation.
Until now, BDG has been inadequately evaluated in individuals with HIV-associated cryptococcal meningitis. Persons with HIV-associated cryptococcal meningitis have higher fungal burdens and more disseminated disease compared with non-HIV-infected persons with cryptococcosis [22, 23]. Patients infected with HIV might therefore be expected to have higher concentrations of BDG. For this reason, our findings cannot be broadly generalized. We would expect the sensitivity to be lower in persons who are HIV seronegative. Our cohort of hospitalized African patients represents an extreme of typical disease progression, with a median fungal burden of ≥100 000 CFU/mL. Ugandan patients had higher fungal burdens relative to those from South Africa, which may at least partially explain the higher sensitivity of the BDG assay observed in Uganda.
High initial quantitative cryptococcal cultures have previously been shown to be predictive of adverse outcomes [24]. In our analysis, higher BDG levels were associated with both higher fungal burdens and increased mortality independent of fungal burden assessed by qualitative culture within 1 and 10 weeks of cryptococcal meningitis diagnosis. These associations suggest a potential prognostic role for BDG, which, perhaps combined with CRAG titers [25], could be used to help guide treatment decisions in patients with HIV-associated cryptococcal meningitis, particularly because labor- and time-intensive quantitative fungal cultures are unavailable in most settings.
In contrast to CRAG titers, which can remain positive for months to years [26], BDG concentrations appear to rapidly normalize with effective treatment. This rapid normalization could have at least 2 important clinical implications. First, a reliable marker for CSF clearance could lead to more tailored duration of induction antifungal therapy, minimizing drug toxicity while ensuring adequate resolution of infection [27]. Second, BDG might be useful in cases of symptomatic relapse, where the presence of live Cryptococcus is of central diagnostic significance [28, 29], helping to differentiate cases of immune reconstitution inflammatory syndrome (IRIS) from true culture-positive relapse without the time-dependent reliance on culture. Further studies investigating BDG clearance over time are required to ascertain the clinical utility of BDG.
Finally, our analysis of immune biomarkers in CSF has demonstrated a possible role for BDG in the host immune response to C neoformans. Our findings support other studies that have observed elevated levels of IL-8, MIP-1β (CCL4), MCP-1 (CCL2), and TNF-α either in response to fungal pathogens [30, 31] or purified BDG itself [32, 33]. The observation that BDG levels are associated with proinflammatory chemokines suggests a perhaps underappreciated role for BDG in provoking an immune response in the CNS of patients with cryptococcal meningitis. In particular, the independent association with the monocyte chemokine MCP-1 suggests a role in the activation of macrophages, which has previously been observed to be an integral part of the CNS immune response to C neoformans [34, 35]. Although the scope of our study precludes drawing a causal relationship from these observations, future studies should consider investigating the importance of BDG in the innate immune response to Cryptococcus.
Limitations of BDG testing include an inability to distinguish between fungal species, false-positive results linked to certain antibiotics such as amoxicillin-clavulanate, and cross-reactivity with some bacteria, which also produce BDG [36, 37]. In resource-limited settings such as ours, the availability of definitive fungal diagnostics for organisms other than Cryptococcus is limited. An inability to determine the cause of false-positive BDG results in our study was limited by these challenges, as well as the retrospective design or our study. Randomized prospective cost-effectiveness evaluations of the BDG assay would need to assess the role of BDG in monitoring patients with cryptococcal meningitis.
In summary, we report that BDG can be detected in the CSF of persons with AIDS and cryptococcal meningitis. Not surprisingly, the sensitivity of the BDG assay in CSF increases with increasing initial fungal burden, suggesting a potential role in prognosis and for the tailoring of antifungal duration [27]. (1→3)-β-d-Glucan is not a replacement for CRAG due to inferior sensitivity of BDG. Yet unlike CRAG, CSF BDG rapidly normalizes with effective cryptococcal meningitis treatment. (1→3)-β-d-Glucan may be useful as a diagnostic adjunct for evaluating treatment efficacy or in rapidly differentiating cryptococcal relapse from IRIS, while awaiting CSF culture results. Cryptococcal meningitis should be considered in all immunosuppressed patients with a positive BDG test result. Additional studies are necessary to confirm our observations.
Supplementary Material
Acknowledgments
Disclaimer. The grantholder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by the National Research Foundation (NRF)-supported research are that of the author and that the NRF accepts no liability whatsoever in this regard.
Financial support. This research was supported by the Fogarty International Center and National Institute of Neurologic Diseases and Stroke (R01NS086312, R25TW009345) and the National Institute of Allergy and Infectious Diseases (U01AI089244, T32AI055433, K23AI073192). Graeme Meintjes was supported by a Wellcome Trust fellowship (098316) and supported in part by the National Research Foundation of South Africa (Grant UID 85858).
Potential conflicts of interest. Y. Z. and M. F. are employees of Associates of Cape Cod.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Odabasi Z, Paetznick VL, Rodriguez JR, et al. Differences in beta-glucan levels in culture supernatants of a variety of fungi. Med Mycol. 2006;44:267–72. doi: 10.1080/13693780500474327. [DOI] [PubMed] [Google Scholar]
- 2.Tsoni SV, Brown GD. beta-Glucans and dectin-1. Ann N Y Acad Sci. 2008;1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- 3.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander BD, Smith PB, Davis RD, et al. The (1,3){beta}-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J Clin Microbiol. 2010;48:4083–8. doi: 10.1128/JCM.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litvintseva AP, Lindsley MD, Gade L, et al. Utility of (1–3)-beta-D-glucan testing for diagnostics and monitoring response to treatment during the multistate outbreak of fungal meningitis and other infections. Clin Infect Dis. 2014;58:622–30. doi: 10.1093/cid/cit808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikulska M, Furfaro E, Del Bono V, et al. (1–3)-beta-D-glucan in cerebrospinal fluid is useful for the diagnosis of central nervous system fungal infections. Clin Infect Dis. 2013;56:1511–2. doi: 10.1093/cid/cit073. [DOI] [PubMed] [Google Scholar]
- 7.Petraitiene R, Petraitis V, Hope WW, et al. Cerebrospinal fluid and plasma (1-->3)-beta-D-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother. 2008;52:4121–9. doi: 10.1128/AAC.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki T, Kohno S, Mitsutake K, et al. Plasma (1-->3)-beta-D-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J Clin Microbiol. 1995;33:3115–8. doi: 10.1128/jcm.33.12.3115-3118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–9. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 10.Obayashi T, Negishi K, Suzuki T, et al. Reappraisal of the serum (1-->3)-beta-D-glucan assay for the diagnosis of invasive fungal infections--a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864–70. doi: 10.1086/588295. [DOI] [PubMed] [Google Scholar]
- 11.Bellanger AP, Grenouillet F, Henon T, et al. Retrospective assessment of beta-D-(1,3)-glucan for presumptive diagnosis of fungal infections. APMIS. 2011;119:280–6. doi: 10.1111/j.1600-0463.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 12.Akamatsu N, Sugawara Y, Kaneko J, et al. Preemptive treatment of fungal infection based on plasma (1 --> 3)beta-D-glucan levels after liver transplantation. Infection. 2007;35:346–51. doi: 10.1007/s15010-007-6240-7. [DOI] [PubMed] [Google Scholar]
- 13.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370:2487–98. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulware DR, Rolfes MA, Rajasingham R, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg Infect Dis. 2014;20:45–53. doi: 10.3201/eid2001.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764–7. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 16.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 17.Wiesner DL, Moskalenko O, Corcoran JM, et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. MBio. 2012;3:pii: e00196–12. doi: 10.1128/mBio.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Wyk M, Govender NP, Mitchell TG, et al. Multilocus sequence typing of serially collected isolates of Cryptococcus from HIV-infected patients in South Africa. J Clin Microbiol. 2014;52:1921–31. doi: 10.1128/JCM.03177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obayashi T, Yoshida M, Mori T, et al. Plasma (1-->3)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet. 1995;345:17–20. doi: 10.1016/s0140-6736(95)91152-9. [DOI] [PubMed] [Google Scholar]
- 20.Mitsutake K, Miyazaki T, Tashiro T, et al. Enolase antigen, mannan antigen, Cand-Tec antigen, and beta-glucan in patients with candidemia. J Clin Microbiol. 1996;34:1918–21. doi: 10.1128/jcm.34.8.1918-1921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee PI, Ciccone EJ, Read SW, et al. Evidence for translocation of microbial products in patients with idiopathic CD4 lymphocytopenia. J Infect Dis. 2009;199:1664–70. doi: 10.1086/598953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YC, Wang JT, Sun HY, Chen YC. Comparisons of clinical features and mortality of cryptococcal meningitis between patients with and without human immunodeficiency virus infection. J Microbiol Immunol Infect. 2011;44:338–45. doi: 10.1016/j.jmii.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Klock C, Cerski M, Goldani LZ. Histopathological aspects of neurocryptococcosis in HIV-infected patients: autopsy report of 45 patients. Int J Surg Pathol. 2009;17:444–8. doi: 10.1177/1066896908320550. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. 2014;58:736–45. doi: 10.1093/cid/cit794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabanda T, Siedner MJ, Klausner JD, et al. Point-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluid. Clin Infect Dis. 2014;58:113–6. doi: 10.1093/cid/cit641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powderly WG, Cloud GA, Dismukes WE, et al. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: value in the management of AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1994;18:789–92. doi: 10.1093/clinids/18.5.789. [DOI] [PubMed] [Google Scholar]
- 27.Rhein J, Boulware DR. Prognosis and management of cryptococcal meningitis in patients with human immunodeficiency virus infection. Neurobehav HIV Med. 2012;4:45–61. [Google Scholar]
- 28.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musubire AK, Boulware DR, Meya DB, et al. Diagnosis and management of cryptococcal relapse. J AIDS Clin Res. 2013;(Suppl 3) doi: 10.4172/2155-6113.s3-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Levitz SM. Stimulation of macrophage inflammatory protein-1alpha, macrophage inflammatory protein-1beta, and RANTES by Candida albicans and Cryptococcus neoformans in peripheral blood mononuclear cells from persons with and without human immunodeficiency virus infection. J Infect Dis. 2000;181:791–4. doi: 10.1086/315250. [DOI] [PubMed] [Google Scholar]
- 31.Sironi M, Milanese C, Vecchi A, et al. Benzydamine inhibits the release of tumor necrosis factor-alpha and monocyte chemotactic protein-1 by Candida albicans-stimulated human peripheral blood cells. Int J Clin Lab Res. 1997;27:118–22. doi: 10.1007/BF02912445. [DOI] [PubMed] [Google Scholar]
- 32.Torosantucci A, Chiani P, Cassone A. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the beta-1,6 glucan of the fungal cell wall. J Leukoc Biol. 2000;68:923–32. [PubMed] [Google Scholar]
- 33.Notarnicola S, Madden H, Browning J, et al. Investigation of a Process Related Contaminant in a Clinical Drug Product. Poster presented at Recovery of Biological Products XI. Banff, Alberta, Canada: 2003. [Google Scholar]
- 34.Siddiqui AA, Brouwer AE, Wuthiekanun V, et al. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol. 2005;174:1746–50. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 35.Perfect JR, Hobbs MM, Granger DL, Durack DT. Cerebrospinal fluid macrophage response to experimental cryptococcal meningitis: relationship between in vivo and in vitro measurements of cytotoxicity. Infect Immun. 1988;56:849–54. doi: 10.1128/iai.56.4.849-854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mennink-Kersten MA, Ruegebrink D, Verweij PE. Pseudomonas aeruginosa as a cause of 1,3-beta-D-glucan assay reactivity. Clin Infect Dis. 2008;46:1930–1. doi: 10.1086/588563. [DOI] [PubMed] [Google Scholar]
- 37.Mennink-Kersten MA, Warris A, Verweij PE. 1,3-beta-D-glucan in patients receiving intravenous amoxicillin-clavulanic acid. N Engl J Med. 2006;354:2834–5. doi: 10.1056/NEJMc053340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.