ABSTRACT
Visceral leishmaniasis (VL) has a high fatality rate if not treated; nevertheless, the majority of human infections with the causative agent, Leishmania infantum chagasi, are asymptomatic. Although VL patients often present with increased levels of serum immunoglobulins, the contribution of antibodies to resistance or progression to disease remains unknown. Effector and regulatory functions of antibodies rely on their interactions with type I and II Fc receptors, and these interactions are tuned by the patterns of antibody Fc N-glycosylation. In view of these facts, we applied a robust method of IgG Fc N-glycopeptide profiling of serum samples from 187 patients with VL, 177 asymptomatic individuals, 116 endemic controls (individuals residing in areas where VL is endemic) and 43 nonendemic controls (individuals living in an area where VL is not endemic). We show that, in comparison to the overall IgG Fc N-glycan profiles of asymptomatic or uninfected healthy individuals, those of patients with VL are profoundly altered. These changes correlate with levels of serum cytokines and the inflammation marker C-reactive protein. We also fitted univariate and multivariate ordinal logistic regression models to demonstrate the ability of IgG Fc N-glycosylation features and immunity regulators present in serum to predict disease severity in VL patients. Importantly, we show that Fc N-glycosylation profiles change after treatment of VL. This study introduces important concepts contributing to the understanding of antibody responses in infections with Leishmania parasites and provides new insights into the pathology of human VL.
IMPORTANCE
Immunoglobulins (Ig) have been shown to present pro- and anti-inflammatory functions according to the profile of carbohydrates attached to their Fc region. Glycosylation features of serum IgG have been examined in relation to several autoimmune and infectious diseases and provide a mechanistic basis for the protective or pathogenic role of antibodies. Leishmania infantum chagasi is the causative agent of visceral leishmaniasis (VL) in South America, and we show that VL patients produce IgG with patterns of Fc glycans similar to those found in other inflammatory conditions. Specific Fc N-glycosylation features and levels of serum cytokines and C-reactive protein are significantly associated with the development of severe clinical symptoms and, notably, Fc glycosylation changes after treatment. The modifications detected in the N-glycosylation features of IgG Fc from VL patients raise new perspectives on the effector or regulatory role of antibodies in immune responses elicited by infection with Leishmania parasites.
INTRODUCTION
Visceral leishmaniasis (VL) is a vector-borne disease transmitted by sand flies, which inoculate the protozoan parasite Leishmania donovani, L. infantum, or L. infantum chagasi into the skin of a mammalian host. The parasites can evade the immune response, spread systemically, and propagate in macrophages mainly in the spleen, liver, bone marrow, and lymph nodes. Clinical manifestations generally include high fever, hepatosplenomegaly, weight loss, pancytopenia, and hypergammaglobulinemia that may progress with severe complications such as hemorrhage, sepsis, and ultimately death (1). The disease is characterized by the nonspecific release of several pro- and anti-inflammatory cytokines (described as a “cytokine storm”) (2–4) and by an inability of peripheral blood mononuclear cells (PBMCs) to respond to stimulation with leishmanial antigens that recovers after treatment (5). It is intriguing that while VL is highly lethal, the majority of human infections do not result in disease (6, 7). Although several factors of hosts, vectors, and parasites have been implicated as determinants of VL (reviewed in reference 8), the mechanisms that account for distinct outcomes after infection are not completely understood.
Elevated synthesis of immunoglobulins by patients with VL results from a polyclonal activation of B cells (9) with the production of parasite-specific and nonspecific antibodies (10, 11), as well as the formation of immune complexes (ICs) and rheumatoid factors (RF) (9, 12). The impact of B lymphocytes and antibodies on different outcomes of Leishmania infections remains poorly studied. While depletion of B cells rendered mice more resistant to infections with L. donovani or L. infantum (13, 14), these studies did not address the contributions of the various types of Fc receptors (FcRs) and of immunoglobulin subclasses to disease progression. Indeed, antibody effector functions, which range from proinflammatory to regulatory responses, rely heavily on interactions of an antibody class or subclass with specific type I and type II FcR expressed by innate and adaptive immune cells (15, 16). In this context, interaction of IgG1 with FcγRIII was shown to be detrimental in a mouse model of L. mexicana infection but not interactions of IgG2a/c or IgG3 (17). Moreover, uptake of IgG-opsonized L. major parasites by dendritic cells was mediated by FcγRI and FcγRIII and facilitated protective immunity in another mouse model (18), leading to contrasting conclusions about the role of IgG and FcγR in infections with Leishmania.
In addition to the variables within FcR and immunoglobulin classes and subclasses, interactions of IgG molecules with cellular receptors or the complement-activating protein mannan-binding lectin (MBL) are regulated by the nature of N-linked, biantennary glycan structures attached to Asn 297 of the IgG heavy chain (19, 20). These carbohydrate structures of Fc regions vary with regard to the presence of a core fucose or bisecting N-acetylglucosamine (GlcNAc) residues and to the degree of sialylation (N-acetylneuraminic acid) and galactosylation (Fig. 1A) (19). IgG Fc on which fucose is absent or bisecting GlcNAc residues are present increase their affinity for human FcγRIIIa and enhance IgG-mediated antibody-dependent cellular cytotoxicity (ADCC) (21, 22). A recent study with transgenic mice revealed that binding of sialylated IgG Fc to dendritic-cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) promoted the production of interleukin-33 (IL-33) by splenic macrophages, followed by an expansion of IL-4+ basophils and consequent upregulation of FcγRIIb (23). Additionally, terminal Fc galactose residues were shown to mediate a cooperative activity between FcγRIIb and dectin-1, which inhibited complement C5a-induced inflammation (24). Indeed, aberrant levels of agalactosylated IgG Fc glycoforms have been described in several inflammatory conditions (25–30). For instance, serum samples from patients with rheumatoid arthritis present elevated levels of IgG lacking Fc galactose residues, which are associated not only with markers of systemic inflammation (28, 29) but also with disease progression and severity (30).
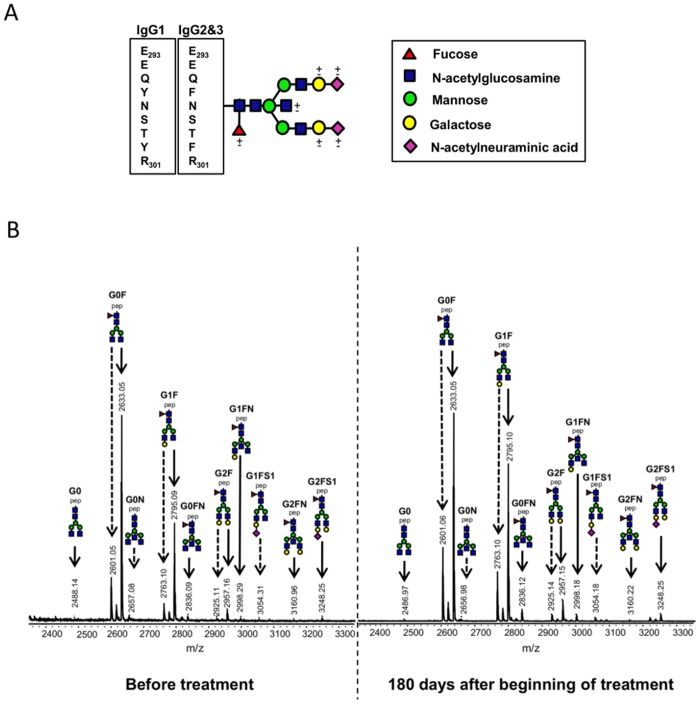
FIG 1 .
MALDI-TOF mass spectra of tryptic IgG Fc N-glycopeptides. (A) Schematic structure of a biantennary N-glycan of the IgG Fc region. IgG subclasses 1, 2, and 3 present peptide sequence differences. (B) Mass spectrometric profiles of Fc N-glycopeptides of IgG from the serum of an adult with VL before treatment (left) and 180 days after the beginning of treatment (right). Continued arrows represent IgG1 glycopeptides, and dashed arrows represent IgG2 and -3 glycopeptides. Glycan names indicate the presence of N-acetylneuraminic acid (S1), fucose (F), and bisecting N-acetylglucosamine (N) (linked to the central core mannose residue) and the number of galactoses (G0, G1, and G2). Structural schemes consist of pep (peptide moiety), blue squares (N-acetylglucosamine), red triangles (fucose), green circles (mannose), yellow circles (galactose), and purple diamonds (N-acetylneuraminic acid).
In view of the lack of information about the precise role of antibodies in different clinical outcomes of infection by L. infantum chagasi, we hypothesized that individuals who progress to disease could produce antibodies with impaired properties, such as IgG Fc N-glycosylation, which in turn could result in defective effector and regulatory functions. In the present work, we report for the first time that, compared with asymptomatic L. infantum chagasi-infected individuals and with healthy endemic and nonendemic controls (persons who live in areas where VL is endemic and in areas where VL is not endemic, respectively), VL patients present with a marked change in global IgG Fc N-glycosylation in serum. This change correlates with levels of serum cytokines and of the inflammation marker C-reactive protein (CRP). We also fitted univariate and multivariate ordinal logistic regression models that demonstrate the ability of intracorrelated serum mediators and IgG Fc N-glycosylation features to predict categories of disease severity among VL patients. Importantly, we observed that Fc N-glycosylation profiles change after treatment.
RESULTS
IgG Fc N-glycopeptide profiling by MALDI-TOF MS.
To determine whether a given outcome after infection with L. infantum chagasi (VL, asymptomatic infection) correlates with distinct profiles of IgG Fc N-glycosylation, we performed high-throughput purification of IgG from serum samples from untreated patients with VL, asymptomatic individuals, and controls and from plasma of treated patients who were monitored for up to 180 days (Table 1). IgG was trypsinized, and the resulting Fc glycopeptides were registered by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (a typical spectrum is shown in Fig. 1B). The cleavage of IgG2 and IgG3 by trypsin results in identical Fc N-glycopeptides; therefore, these two subclasses were determined together, while IgG4 was not determined because of overlaps with other signals (26, 31). Sixteen IgG1 Fc glycoforms and 11 IgG2 and -3 Fc glycoforms could be detected (see Table S1 in the supplemental material), whereas structural assignment of the glycoforms detected was performed on the basis of the literature on IgG N-glycosylation (32–36). A standard IgG sample was added in triplicate to each sample plate in order to determine the intra- and interbatch variations of the analytical method for IgG1 and IgG2 and -3 Fc N-glycosylation features. The levels of galactosylation, sialylation, bisection (incidence of bisecting GlcNAc), and fucosylation were calculated (see Materials and Methods), and the relative standard deviation was <5% for each sample plate (intrabatch) and between plates (interbatch) for both IgG1 and IgG2 and -3 (see Fig. S1 in the supplemental material).
TABLE 1 .
Human sample groups used in this study
| Groupa | Untreated subject serum |
Treated VL patient plasmab |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total no. | Mean age, yr (SD) | No. (%) of: |
No. (%) with clinical severityc of: |
Total no. | Mean age, yr (SD) | No. (%) of: |
No. of days after beginning of treatment |
||||||||
| Males | Females | U-VL | C-VL | S-VL | Males | Females | 0 | 5 | 90 | 180 | |||||
| VL | 187 | 27.2 (18.0) | 128 (68) | 59 (32) | 43 (35) | 65 (52) | 16 (13) | 23 | 35.2 (12.1) | 13 (56) | 10 (44) | 23 | 23 | 19 | 12 |
| ASYMPd | 177 | 36.4 (17.8) | 62 (35) | 115 (65) | NAe | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ECf | 116 | 35.4 (19.6) | 36 (31) | 80 (69) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| NCg | 43 | 29.6 (10.1) | 20 (46) | 23 (54) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
VL patients (VL), asymptomatic individuals (ASYMP), and endemic controls (EC) were residing in Aracajú-SE or Teresina-PI (northeast), whereas nonendemic controls (NC) were from Ribeirão Preto-SP (southeast).
Plasma samples were collected at 0, 5, 90, and 180 days after the beginning of treatment.
Categories of clinical severity: U-VL, uncomplicated VL; C-VL, VL with complications (patients who required additional therapy, such as antibiotics or blood products); S-VL, severe VL (patients who had hemorrhagic complications and whose laboratory data indicated an increased risk of death).
Asymptomatic individuals were identified by a positive Montenegro skin test and/or positive antibody reactivity with L. infantum chagasi antigens.
NA, not applicable.
Endemic controls were identified by a negative Montenegro skin test and negative antibody reactivity with L. infantum chagasi antigens.
Nonendemic controls were identified by a negative Montenegro skin test and negative antibody reactivity with L. infantum chagasi antigens.
Patients with VL exhibit an altered global IgG Fc N-glycosylation phenotype.
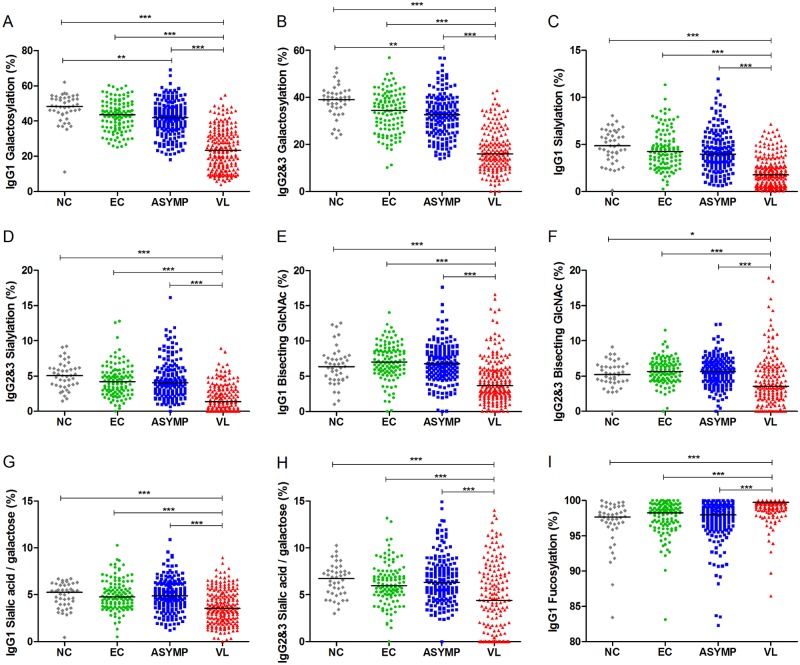
The calculation of the relative abundances of IgG1 and IgG2 and -3 Fc glycoforms bearing one or two terminal galactose residues revealed a significant reduction of the overall galactosylation of Fc in VL patients compared to that in asymptomatic individuals or controls (Fig. 2A and B; see the medians in Table S2 in the supplemental material). Moreover, asymptomatic individuals showed a significant decrease in the levels of Fc galactosylation from both subclasses compared to those of nonendemic controls but exhibited a profile similar to that of endemic controls (Fig. 2A and B; see Table S2). Next, we evaluated the levels of sialylation and bisection of IgG1 and IgG2 and -3 Fc regions, which were also significantly reduced in VL patients compared to those of the other clinical-epidemiological groups (Fig. 2C to F; see Table S2). The ratio of sialic acid to galactose residues demonstrates that Fc from VL patients also presented reduced sialylation of Fc galactoses (Fig. 2G and H; see Table S2). Conversely, IgG1 molecules from VL patients presented a significant increase in the prevalence of Fc fucose residues, while asymptomatic individuals and controls presented similar phenotypes (Fig. 2I; see Table S2).
FIG 2 .
Changes in IgG Fc N-glycosylation features with VL. Levels of galactosylation (A), sialylation (C), bisection (E), sialic acid/galactose ratio (G), and fucosylation (I) of IgG1, as well as levels of galactosylation (B), sialylation (D), bisection (F), and sialic acid/galactose ratio (H) of IgG2 and -3 are shown for nonendemic controls (NC), endemic controls (EC), asymptomatic individuals (ASYMP), and patients with VL. Statistically significant differences were evaluated by Kruskal-Wallis test, followed by Dunn’s post-hoc test; median values and significance levels are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
Age dependency of IgG Fc N-glycosylation features.
To address if the altered Fc N-glycan profiles observed in patients could be confounded by factors known to affect IgG Fc N-glycosylation patterns under physiological conditions (31), we evaluated the age dependency of IgG Fc N-glycosylation features. In accordance with previous reports, the overall levels of Fc galactosylation and sialylation presented a negative correlation with age for all groups (Fig. 3A to D), regardless of clinical-epidemiological status. An exception occurred in glycopeptides containing bisecting GlcNAc, where, surprisingly, the age correlation differed between patients and the other clinical-epidemiological groups: VL patients showed a negative correlation between age and bisection (Fig. 3E and F). Moreover, IgG1 Fc fucosylation increased with age in patients but decreased in asymptomatic individuals (Fig. 3G). Stratification of groups by sex resulted in similar age dependency of Fc N-glycosylation features (data not shown). Overall, these results demonstrate that the patterns of Fc glycosylation observed for VL patients do not simply reflect the age of individuals but are indeed induced with disease.
FIG 3 .
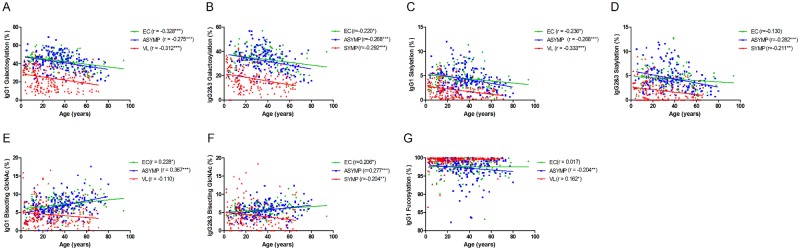
Age dependence of IgG Fc N-glycosylation in patients, asymptomatic individuals, and controls. Levels of galactosylation (A), sialylation (C), bisection (E), and fucosylation (G) of IgG1, as well as levels of galactosylation (B), sialylation (D), and bisection (F) of IgG2 and -3 are plotted versus age for endemic controls (EC; green circles), asymptomatic individuals (ASYMP; blue squares), and VL patients (VL; red triangles). Spearman’s rank correlation coefficients (r values), linear regression lines, and significance levels are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
Reactivity of IgG with L. infantum chagasi antigens and correlations with Fc N-glycosylation features.
Since antibody effector functions are also affected by features such as specificity, reactivity (37, 38), and affinity, we sought to evaluate the reactivity of IgG with soluble Leishmania antigens (SLA), regardless of whether they were induced by normal cognitive interactions between B and T cells or by means of polyclonal B cell activation (9). For this, we serially diluted serum samples (dilutions of 1:50, 1:100, and 1:400) and determined whether reactivity with SLA correlates with levels of Fc N-glycosylation. As expected, patient-derived IgG presented higher levels of SLA-specific antibodies, while asymptomatic individuals presented intermediate levels that were significantly higher than those of endemic controls (Fig. 4). Interestingly, Spearman’s rank correlations demonstrated that, in patients, the level of SLA-specific IgG was significantly associated with the overall decreases in levels of Fc galactosylation, sialylation, and bisection (see Fig. S2A to F in the supplemental material). In addition, fucosylation of the IgG1 Fc region from patients presented a weak but significant positive correlation with higher levels of SLA-specific IgG (see Fig. S2G). Levels of SLA-specific antibodies from asymptomatic individuals, while significantly higher than those of endemic controls, did not correlate with the levels of any Fc glycosylation feature.
FIG 4 .
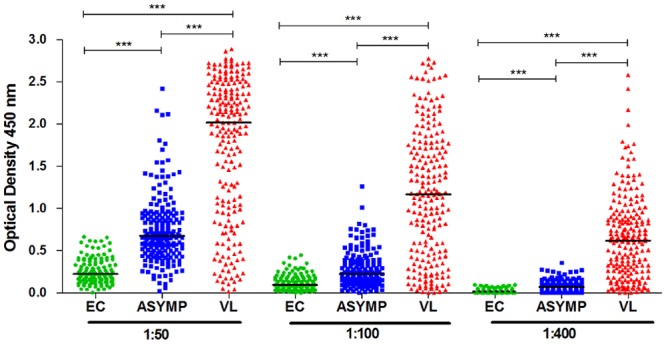
Levels of SLA-specific IgG. The reactivity of IgG with SLA in three different dilutions of serum from endemic controls (EC; green circles), asymptomatic individuals (ASYMP; blue squares), and patients with VL (VL; red triangles) is shown. Statistically significant differences were evaluated by Kruskal-Wallis test, followed by Dunn’s post-hoc test; median values and significance levels are shown (***, P ≤ 0.001).
Altered abundance of IgG Fc N-glycans is associated with levels of inflammatory and regulatory serum mediators.
We next determined whether the modified IgG Fc N-glycan phenotype found herein could also be associated with serum cytokine and CRP levels. First, compared to asymptomatic and control individuals and in agreement with other studies (2–4), we found increased concentrations of IL-1β, IL-6, tumor necrosis factor alpha (TNF-α), IL-12p70, gamma interferon (IFN-γ), IL-17, IL-10, IL-5, and CRP in serum samples from VL patients (see Fig. S3A to H and Table S2 in the supplemental material). Furthermore, asymptomatic individuals presented higher TNF-α levels than endemic controls (see Fig. S3C and Table S2). Serum samples from both patients and asymptomatic individuals showed a trend toward higher concentrations of IL-4 than endemic controls (see Fig. S3I and Table S2). Additional analyses demonstrated that in patients, but not in asymptomatic or uninfected individuals, the concentrations of serum mediators are associated with IgG1 and IgG2 and -3 Fc N-glycosylation features (Table 2; see Fig. S4). Whereas IgG1 Fc galactosylation and sialylation were correlated only with the levels of TNF-α and CRP, these same features in IgG2 and -3 Fc were correlated with the levels of several cytokines (Table 2; see Fig. S4A to H). Interestingly, IgG1 Fc bisection was significantly correlated with the levels of all of the serum mediators that were measured, showing the strongest correlation with IL-6 (Table 2; see Fig. S4I to L). In contrast, IgG2 and -3 Fc bisection presented weaker correlations with fewer mediators (Table 2). IgG1 Fc fucosylation presented weak associations with levels of IFN-γ, IL-1β, and IL-10 (Table 2).
TABLE 2 .
Correlations between Fc N-glycosylation features and concentrations of serum mediators from VL patientsa
| Glycosylation feature | IL-6 | IL-1β | TNF-α | IL-12p70 | IFN-γ | IL-17 | IL-10 | IL-5 | IL-4 | CRP |
|---|---|---|---|---|---|---|---|---|---|---|
| IgG1 | ||||||||||
| Gal | −0.07 | −0.11 | −0.27* | 0. 00 | −0.24 | −0.05 | −0.15 | 0.24 | −0.13 | −0.36* |
| Sial | 0.05 | −0.10 | −0.22† | 0.05 | 0.01 | −0.01 | −0.10 | 0.05 | −0.13 | −0.31* |
| BisGlcNAc | −0.43* | −0.23* | −0.23* | −0.35* | −0.38* | −0.27* | −0.37* | −0.16‡ | −0.32* | −0.15‡ |
| Core Fuc | 0.12 | 0.18† | 0.10 | 0.08 | 0.18† | 0.10 | 0.15‡ | 0.03 | 0.10 | 0.13 |
| IgG2 and -3 | ||||||||||
| Gal | −0.23† | −0.23† | −0.42* | −0.19† | −0.14 | −0.14 | −0.30* | −0.14 | −0.17‡ | −0.41* |
| Sial | −0.13 | −0.16‡ | −0.32* | −0.07 | −0.06 | −0.04 | −0.21† | −0.05 | −0.13 | −0.33* |
| BisGlcNAc | −0.26* | −0.10 | −0.17‡ | −0.08 | −0.26* | −0.12 | −0.24† | 0.08 | −0.16‡ | −0.13 |
Spearman’s rank correlation coefficients are shown for the levels of Fc galactosylation (Gal), sialylation (Sial), bisecting N-acetylglucosamine (BisGlcNAc), and core fucosylation (Core Fuc), and significance is shown as follows: *, P < 0.001; †, P< 0.01; ‡, P< 0.05.
Relationship between clinical severity and IgG Fc N-glycosylation features and serum mediators.
We hypothesized that severity of VL could be predicted by differential concentrations of serum biomarkers (4), concomitantly with the relative abundances of IgG Fc N-linked glycans. To determine which factors could be related to the severity of VL, 124 patients were classified into three categories of clinical severity (Table 1). Patients with severe disease presented significantly lower levels of IgG1 and IgG2 and -3 Fc galactosylation and sialylation (see Fig. S5A to D in the supplemental material) than patients with uncomplicated VL, while the levels of IL-6, TNF-α, IFN-γ, IL-17, IL-10, and CRP were significantly higher in patients with complicated and severe VL (see Fig. S5E to J). The relative abundances of IgG Fc N-glycosylation features and serum mediators were introduced as predictors of disease severity in univariate ordinal logistic regression models. Higher odds of increased IgG1 Fc galactosylation, sialylation, and IgG2 and -3 sialylation were found in less severe clinical outcomes of VL (Table 3). In contrast, the odds of increased concentrations of serum IL-6, TNF-α, IFN-γ, IL-17, IL-10, and CRP were higher in more severe clinical outcomes (Table 3). All of these mediators were highly intracorrelated among this group of patients (see Fig. S6A to L), and variables presenting colinearity were evaluated. Subsequently, a multivariate logistic regression model was fitted to determine the contribution of factors to disease severity. A stepwise backward elimination procedure was used to select variables that contribute the most to the model. The associations of the relative abundance of IgG1 Fc galactosylation, serum IL-6, and CRP with the categories of severity were retained as predictors of disease severity (Table 3).
TABLE 3 .
Relationship between (i) Fc N-glycosylation features and serum mediators and (ii) clinical severitya
| Predictors | OR | 95% CI | β | P value |
|---|---|---|---|---|
| Univariate models | ||||
| IgG1 Fc Gal | 0.11 | 0.02–0.52 | −2.24 | 0.006 |
| IgG1 Fc Sial | 0.35 | 0.16–0.78 | −1.05 | 0.010 |
| IgG2 and -3 Fc Sial | 0.24 | 0.09–0.64 | −1.43 | 0.005 |
| IL-6 | 2.98 | 1.74–5.12 | 1.09 | <0.001 |
| TNF-α | 3.11 | 1.77–5.45 | 1.13 | <0.001 |
| IFN-γ | 2.31 | 1.37–3.91 | 0.84 | 0.002 |
| IL-17 | 3.87 | 1.65–9.07 | 1.35 | 0.002 |
| IL-10 | 2.41 | 1.50–3.87 | 0.88 | <0.001 |
| CRP | 11.38 | 3. 23–40.0 | 2.43 | <0.001 |
| Multivariate model | ||||
| IgG1 Fc Gal | 0.12 | 0.02–0.63 | −2.12 | 0.012 |
| IL-6 | 2.60 | 1.49–4.56 | 0.96 | 0.001 |
| CRP | 9.05 | 2.45–33.5 | 2.20 | 0.001 |
Significant statistical associations of predictors with categories of severity were evaluated by using univariate and multivariate ordinal logistic regression models. CI, confidence interval; Gal, level of galactosylation; OR, odds ratio; Sial, level of sialylation; β, estimative coefficient.
IgG Fc N-glycosylation changes after treatment of patients with VL.
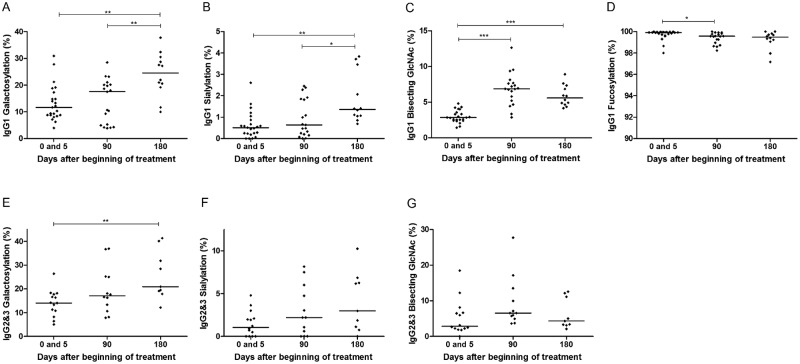
Since clinical severity was correlated with IgG1 and IgG2 and -3 Fc N-glycosylation profiles, in addition to the cross-sectional data, we analyzed the effect of treatment upon these features. For this, we evaluated plasma samples from an initial group of 23 patients, the majority of whom could be monitored for 90 days, whereas 12 patients were monitored for 180 days after the beginning of treatment (Table 1). Representative MALDI-TOF MS profiles of the same patient before and after treatment are shown in Fig. 1B. The relative abundances of Fc glycans before and 5 days after the beginning of treatment did not show significant differences (data not shown); therefore, we treated the mean value of the relative abundance of Fc glycosylation features at days 0 and 5 for each patient as one group (0 and 5). As a result, we found that the levels of IgG1 Fc galactosylation and sialylation increased significantly at day 180 after the beginning of treatment (Fig. 5A and B), while the incidence of bisecting GlcNAc increased at 90 days and was maintained 180 days after the beginning of treatment (Fig. 5C). IgG1 Fc fucosylation presented a significant decrease at 90 days; nevertheless, its incidence at 180 days after the beginning of treatment did not differ significantly from that at earlier stages (Fig. 5D). Of note, IgG2 and -3 Fc galactose residues differed significantly after the beginning of treatment but Fc sialylation or bisection did not (Fig. 5F and G). Overall, our results indicate that disease remission is accompanied by changes in subclass-specific IgG Fc N-glycosylation features.
FIG 5 .
Changes in IgG Fc N-glycosylation features with treatment. (A, B, C, and D) IgG1 levels of galactosylation, sialylation, bisection, and fucosylation, respectively. (E, F, and G) IgG2 and -3 levels of galactosylation, sialylation, and bisection, respectively. The mean glycosylation levels on days 0 and 5 after the beginning of treatment are shown (0 and 5) next to the glycosylation levels at 90 days (90) and 180 days (180) after the beginning of treatment. Statistically significant differences were evaluated by Kruskal-Wallis test, followed by Dunn’s post-hoc test, and significance levels are indicated (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
DISCUSSION
The contribution of B cells and antibody responses to the different outcomes after infection with L. infantum chagasi has yet to be established. In addition, hypergammaglobulinemia is a hallmark of VL. Knowledge is lacking about particular properties of antibodies produced during active disease or asymptomatic infection. Using a robust method of IgG Fc N-glycopeptide profiling (39), we demonstrate that the overall IgG Fc N-glycan profiles are subject to profound alterations and obtained insights into the role of antibodies in potentially protective and pathogenic immune responses. Our results can be discussed in terms of studies of Fc N-glycans in infectious diseases; their roles in B cell activation and regulation, in inflammation, and in antibody-mediated effector functions; the processes that result in different Fc N-glycan profiles; and the mechanisms through which Fc N-glycan profiles can affect the severity of VL or assist in treatment.
Association of IgG Fc N-linked glycan profiles with outcomes of infectious diseases.
In this study, distinct glycosylation features of IgG1 and IgG2 and -3 that determine the inflammatory and regulatory functions of IgG molecules and possibly cognate functions of antibodies were associated with outcomes of infections with L. infantum chagasi. Several studies have addressed the composition and role of IgG Fc N-glycans in human infectious diseases; e.g., global IgG galactosylation is significantly perturbed in patients with tuberculosis (25); patients with hepatitis C virus who develop cirrhosis present increased levels of agalactosylated IgG specific for alpha-Gal epitope [Gal-1-3Gal1-(3)4GlcNAc-R] (40); antiviral activity is modulated by natural variations in Fc glycosylation of HIV-specific antibodies (27); the opsonizing capacity of IgG for hepatitis B virus depends on the profile of Fc glycosylation, which is also associated with clinical outcomes of infection with this virus (41); and individuals asymptomatically infected with Wuchereria bancrofti had significantly lower levels of disialylated IgG than endemic controls and patients with pathology (42). However, the analytical techniques applied in those studies did not discriminate between IgG subclasses and/or registered overall glycosylation but not Fc-specific glycosylation or did not detail specific Fc glycosylation profiles.
Role of IgG Fc N-linked glycan profiles in B cell functions, in inflammation, and in effector immune mechanisms that may limit infections with L. infantum chagasi.
Collectively, our data are in line with defective activation of B lymphocytes in VL patients, which affects not only the synthesis of immunoglobulins but also important processes such as posttranslational modifications. Asymptomatic individuals examined in this study presented significantly higher levels of SLA-specific antibodies than uninfected controls; however, they did not present any correlation between antibody titers and Fc N-glycan profiles, indicating an appropriate B cell activation and regulation of antibody responses. Our data also suggest that, conversely, IgG-mediated regulation of B cell proliferation and activation is disrupted in VL patients. The observed reduced levels of IgG Fc sialylation in VL may have an impact upon inhibition of B cell proliferation (43). In addition, the increased abundance of IgG1 Fc fucose residues may affect the tonicity of the engagement of IgG with the inhibitory FcγRIIb (44) in such a manner as to impair the regulatory mechanisms of B cell activation that this receptor mediates (45). Although the hyperactive synthesis of immunoglobulins by B cells could be implicated in the changes of IgG Fc N-glycosylation features of VL patients, previous reports did not find significant correlations between the total serum IgG concentration and an altered Fc N-glycosylation phenotype in other conditions (25, 46).
These findings suggest that in order for a humoral response to be effective against VL development (i.e., disease), antibodies must present a range of glycosylation profiles without a bias toward certain types of glycans. This balance could achieve destruction of parasites with minimal inflammatory damage. Recent studies demonstrated the development of an antigen-dependent shift in IgG Fc N-glycosylation and consequent antibody function (27, 47). In this context, it is also important to evaluate whether Leishmania-specific antibodies and autoantibodies of the IgG subclasses from patients and asymptomatic individuals present different patterns of Fc N-glycosylation such as those found in this study for total IgG1 and IgG2 and -3. Our data show that VL patients present both an increased frequency of IgG1 Fc fucosylation and a reduced incidence of IgG1 and IgG2 and -3 Fc bisection, changes that are known to impact the effectiveness of ADCC (21, 22). IgG-mediated ADCC was shown to be an effective mechanism to control Trypanosoma cruzi (48), a trypanosomatid protozoan like L. infantum chagasi; however, the importance of this mechanism in outcomes of infections with Leishmania parasites has not been thoroughly evaluated. Recently, an in vitro model of enhanced killing of L. amazonensis was shown to require a synergistic response of IFN-γ, parasite antigens and nonspecific soluble IgG2a ICs. The presence of ICs activated the FcγR common chain in mice macrophages, which culminated in the generation of NADPH oxidase-dependent superoxide, an important mediator against L. amazonensis (49).
Notably, L. infantum chagasi opsonized with serum of patients with VL induced the production of intracellular IL-10 by human monocytes in vitro (50), which may play a role in disease progression. However, the effects of serum samples from asymptomatic individuals on IL-10 production by monocytes were not examined. The contribution of Fc N-glycosylation profiles should also be evaluated with specific subsets of monocytes that are responsible for IgG-dependent effector functions in vivo (51). Since the uptake of L. major by dendritic cells and the production of IL-12 and of protective responses required parasite-reactive IgG and interactions with FcγRI and FcγRIII in a mouse model (18), the effect of Fc N-glycosylation upon these phenomena should also be examined in cells from humans and experimental models of VL. Furthermore, the significant correlation between high reactivity to SLA and an altered Fc glycosylation profile in patients suggests that immune complexes composed of L. infantum chagasi-specific antibodies could induce responses that differ from those seen in asymptomatic individuals, depending on the patterns of IgG Fc N-glycans and also on the type of innate immune cells that ICs interact with.
A further perspective is given by recent studies that show that changes in the constant region of antibodies can also affect the secondary structure and thus the recognition capacity of antibodies (37, 52). Indeed, the conformation and degree of flexibility of Fc CH2 domains depend on the N-glycans attached to the Fc at Asn 297 (53), so it is reasonable to speculate that secondary structure of antigen-binding regions might depended on Fc N-glycosylation profiles. No differences were observed between the binding affinities of a platelet-specific monoclonal antibody enriched or nonenriched for sialic acid (54); however, other specificities were not examined and neither was the effect of the other carbohydrate residues that are part of the Fc N-glycosylation profiles.
Mechanisms that affect IgG Fc N-linked glycan profiles.
A recent genome-wide association study with an IgG glycome found nine gene loci to be significantly associated with the profiles of glycans of this protein. Four of those loci contained genes coding for glycosyltransferases (ST6GAL1, B4GALT1, FUT8, and MGAT3) (55). Currently, there is little knowledge of mechanisms that regulate the production and activity of glycosyltransferases, and our data indicate that profiles of IgG Fc N-linked glycans in VL patients could be influenced or influence the production of cytokines via activating FcRs (12). Consistent with our findings, Jeddi and colleagues (46) showed that mice overexpressing IL-6 produce lower levels of galactosylated IgG, presumably because of a defective interaction between galactosyltransferases and IgG. Also consistent with our findings, humans suffering from inflammatory arthritis and undergoing treatment with anti-TNF antibodies present with increased levels of galactosylated IgG (56). Asymptomatic infected individuals, who presented intermediate levels of TNF-α relative to those of controls and VL patients, presented higher levels of sialylated IgG than VL patients. In addition, signaling by cytokines was able to modulate the composition of IgG1 Fc N-linked glycans in an in vitro B cell microenvironment (57). These findings suggest that the balance of pro- and anti-inflammatory IgG Fc N-glycan profiles is complex and depends on or affects the balance between a set of cytokines.
To date, no studies have verified the association of circulating levels of cytokines with IgG Fc N-glycan profiles in humans or experimental animals infected with L. infantum chagasi. Similar to this study, several others have shown that several cytokines, including those with proinflammatory effects, are abnormally higher in persons with VL than in healthy, uninfected individuals (2–4). However, only the present study and another, by Peruhype-Magalhães and colleagues (2), examined levels of serum cytokines in infected asymptomatic individuals. Both studies found that in asymptomatic individuals, the levels of all of the cytokines measured were similar to those of uninfected healthy controls. The exception was TNF-α levels, which in the present study, but not in that by Peruhype-Magalhães and colleagues, were significantly lower than those in VL patients but significantly higher than the levels seen in controls, as pointed out above. The present study, however, examined almost 10-fold more individuals presenting with symptomatic and asymptomatic infections with L. infantum chagasi than that conducted by Peruhype-Magalhães and colleagues and therefore may have achieved greater statistical significance.
Association of IgG Fc N-linked glycan profiles with severity of VL.
The abundance of terminal Fc galactose residues strongly influences the interaction of IgG with cellular receptors and MBL (20, 24, 58), which is thought to be a disease-enhancing opsonin for intracellular pathogens (59, 60). We have previously shown the association of genetic polymorphisms causing high levels of MBL with severe disease in human VL (60). Therefore, the interaction of MBL with IgG molecules in patients with VL would be facilitated because of a low abundance of terminal Fc galactose residues and could be involved in the exacerbation of disease. However, we emphasize that the activity of Fc hypogalactosylated IgG was unimpaired in MBL-null mice but was dependent on FcγR (58). Interestingly, previous studies have found large amounts of circulating RF in individuals with VL (12). The presence of RF, together with the observed high abundance of Fc-agalactosylated IgG, may contribute to a detrimental nonspecific and systemic inflammatory response (61).
Antibodies were implicated in complement cascade activation with further generation of C5a and exacerbation of disease in a mouse model of VL (14). In view of this fact, investigations using experimental models of VL should verify if phenotypes of IgG Fc N-glycosylation similar to those found herein occur and whether generation of C5a is affected by Fc N-glycan profiles. This hypothesis is supported by the demonstration that ICs composed by highly galactosylated IgG1 promoted cooperation between FcγRIIb and dectin-1 on neutrophils and inhibited C5a-mediated inflammation (24). Also of interest, another study reported that B-cell-deficient mice were resistant to infection with L. donovani but developed neutrophil-mediated destruction of the liver (13). Transfer of normal or chronic-infection serum was able to abrogate this tissue pathology and minimally impacted the resistance to infection, thus suggesting a regulatory role for antibodies in this model (13).
The overall low abundance of Fc galactosylation and/or sialylation that we describe in VL patients, as well as the high levels of proinflammatory and regulatory serum mediators reported herein and by others (2–4), indicates that severe VL should be regarded as a systemic inflammatory response syndrome (62). However, it remains to be determined whether the altered IgG Fc N-glycosylation phenotype of individuals with VL is a cause or consequence of imbalanced responses.
The evaluation and stratification of clinically characterized patients according to requirement of additional therapy or presence of severe clinical symptoms retrieved significant associations with levels of IgG Fc galactosylation and sialylation, as well as inflammatory and regulatory serum mediators that have been previously associated with the progression and severity of VL (4, 63). One can speculate that the levels of proinflammatory and regulatory serum mediators, together with the composition of IgG Fc N-glycans, may influence systemic and/or site-specific molecular mechanisms. For example, IL-10 was significantly correlated with the incidence of IgG1 Fc fucosylation and bisecting GlcNAc and all IgG2 and -3 Fc N-glycosylation features in VL patients (Table 2). This cytokine has been implicated in the pathogenesis of VL (63), and it is a growth and differentiation factor for activated B lymphocytes (64) and therefore could also contribute to the maintenance of differentiated plasma cells that produce IgG with aberrant Fc N-glycosylation features. Strong correlations between the serum IL-10 and IFN-γ levels of patients concur with findings that show that Th1 cells are the main source of IL-10 during VL; induction of IL-10 is believed to promote the regulation of excessive damage caused by inflammatory mediators (65, 66). In patients with rheumatoid arthritis, levels of CRP and IL-6 were found to be correlated with a higher abundance of agalactosylated IgG, as well with MBL2 polymorphisms (28). We found that levels of IL-6 correlate with IgG1 Fc bisecting GlcNAc and IgG2 and -3 Fc galactosylation and bisection, while CRP levels were associated mostly with overall levels of IgG Fc galactosylation and sialylation (Table 2). In a more detailed analysis, IgG1 Fc galactosylation, IL-6, and CRP were significantly associated with the degree of severity (Table 3), thus indicating that a chronic inflammatory response might account for systemic damage in more severe stages of the disease. Of interest, in comparison with healthy controls, ICs isolated from VL patients induced increased production of pro- and anti-inflammatory cytokines, such as IL-6 and IL-10, by PBMCs. However, the effect of such ICs was more proinflammatory when the levels of individual cytokines and their natural inhibitors were compared (67).
Recently, IL-6 was implicated in the alternative activation of macrophages by inducing an increased response to IL-4 (68), the signaling pathways of which lead to STAT6 phosphorylation (69). Indeed, L. donovani has been shown to induce STAT6-dependent expression of host arginase 1. Furthermore, alternative activation of macrophages, amplified by the addition of IL-4, contributed to impaired control of infection in a hamster model (70). Interestingly, differential glycosylation of CRP activates complement-mediated hemolysis of erythrocytes in patients with VL and tuberculosis (71). The extent to which the whole serum glycome of patients with VL might be altered and how these differences could be associated with the evolution and outcome of infection and with the severity of the disease is intriguing and merits investigation. Our data suggest that tightly regulated responses should cover parasite control concomitantly with containment of immunopathology caused by strong inflammatory responses. This concept is further supported by the intermediate TNF-α levels seen in the serum of asymptomatic individuals, which were higher than those of endemic controls but lower than those of VL patients.
IgG Fc N-linked glycan profiles as biomarkers of active VL and new treatment options.
The evaluation of serum mediators has been useful for identifying biomarkers of active VL or remission after treatment (3), and IgG Fc N-glycosylation may help with further stratification of patients. Our analysis demonstrated that the relative abundances of IgG1 Fc galactosylation, sialylation, and bisecting GlcNAc and IgG2 and -3 Fc galactosylation are significantly modified in patients after successful therapy, although levels of Fc glycosylation features did not reach the levels observed in controls within the 6-month observation period after initiation of treatment. Differences in the activity of glycosyltransferases due to polymorphisms in genes coding for these enzymes may explain this and also contribute to the development of VL. Therefore, the IgG N-glycome of these individuals may depend on their genotypes, as well on their pathophysiologic status. Overall, these observations raise new perspectives for the treatment of patients, at least those with severe VL. For instance, therapy with intravenous immunoglobulin enriched with sialic acid (72) might provide an efficient tool with which to overcome the complications that result from an excessive nonspecific inflammatory response in VL.
Future perspectives and conclusion.
Previous studies with mice have demonstrated that IgG-mediated effects are significantly dependent on the genetic background (18, 50). Therefore, the role of antibodies among different experimental models of VL could also depend on the genetic background of the host. Accordingly, the determination of IgG Fc N-glycosylation features in experimental mouse models of VL should be carried out with several inbred mouse strains with variable resistance or susceptibility to the disease. Treatment of purified IgG from normal and chronic-infection serum with glycosidases prior to transfer to infected hosts could reveal whether Fc N-glycans indeed influence the regulatory or inflammatory functions of IgG in experimental models of VL. Overall, this study introduces a new concept contributing to an improved understanding of antibody responses in infections with Leishmania parasites and provides new insights into the pathology of human VL.
MATERIALS AND METHODS
Study groups and ethics statement.
Serum samples were collected from patients with symptoms of VL admitted to the University Hospital, UFS, Aracajú-SE, or the Natan Portella Institute of Tropical Diseases, UFPI, Teresina-PI, Brazil. Diagnosis was confirmed by identification of Leishmania amastigotes in Giemsa-stained smears of bone marrow aspirate, and patients diagnosed with VL received therapy with pentavalent antimony and/or amphotericin B according to Brazilian guidelines (73). One hundred twenty-four patients with VL were further classified into three categories of disease severity (Table 1) as reported previously (60). Additional study subjects included healthy individuals living in the same areas and considered to be infected with L. infantum chagasi (asymptomatic individuals), who were identified by a positive Montenegro skin test (MST+) and the presence of specific antibodies (Table 1). Controls included a group of individuals residing in the same areas where VL is not endemic (endemic controls) and individuals living in Ribeirão Preto-SP, an area where VL is not endemic (nonendemic controls), who were MST− and negative for the presence of specific antibodies (Table 1). Plasma samples were collected from a cohort of patients before and up to 180 days after the beginning of treatment according to Brazilian guidelines (73) (Table 1). This study was approved by the Research Ethics Committee of the Clinics Hospital of the Ribeirão Preto School of Medicine—USP (protocol 2347/2012), and written informed consent was obtained from all of the participants or their parents or legal guardians.
Parasite and antigens.
Promastigotes of the L. infantum chagasi reference strain (MHOM/BR/74/PP75) were cultured in Schneider’s medium supplemented with 10% inactive fetal bovine serum, 2% human urine, 2 mM l-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. Late-log-phase promastigotes were enriched on the basis of negative agglutination by peanut agglutinin, and SLA were extracted. Briefly, promastigotes were washed with sterile phosphate-buffered saline, resuspended in Tris-HCl (pH 7.5) supplemented with a protease inhibitor cocktail (Roche Diagnostics), and submitted to five cycles of freeze-thawing with a liquid nitrogen bath. The lysate was sonicated, homogenized, and centrifuged at 14,000 × g for 5 min at 4°C.
ELISA for antibody reactivity with Leishmania antigens.
Enzyme-linked immunosorbent assay (ELISA) plates (Corning) were sensitized with 20 µg/ml SLA (2 µg/well) diluted in 0.5 M carbonate/bicarbonate buffer, pH 9.6. Plates were blocked with 5% bovine serum albumin for 2 h and treated successively with 1:50, 1:100, and 1:400 dilutions of serum for 1 h at 37°C. Following two washes with Tris-buffered saline and Tween 20, plates were incubated with recombinant protein G-peroxidase conjugate (Thermo Scientific Pierce) diluted 1:15,000 for 1 h at 37°C. After another washing step, the reactions were revealed by addition of the substrate 3,3′,5,5′-tetramethylbenzidine and stopped with 0.2 N H2SO4. Optical densities at 450 nm were registered.
Quantification of serum cytokines and CRP.
Serum cytokine measurements were performed with the MILLIPLEX MAP Human Cytokine Magnetic Bead Assay (EMD Millipore). IFN-γ, IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17A, and TNF-α, were quantified via a MAGPIX platform and calculated by MILLIPLEX Analyst software (EMD Millipore). A range of 3.2 to 10,000 pg/ml recombinant cytokines was used to establish standard curves and the sensitivity of the assay. To measure CRP levels, a turbidimetric assay was performed according to the manufacturer’s (Wiener Lab) instructions.
IgG Fc N-glycosylation analysis.
IgG isolation from serum or plasma, digestion with trypsin, and glycopeptide purification were performed as described elsewhere (26, 31). The resultant tryptic IgG Fc N-glycopeptides were analyzed by MALDI-TOF MS. Briefly, IgG Fc N-glycopeptide samples were spotted onto an MTP 384 polished steel target plate (Bruker Daltonics) and allowed to dry at room temperature. Subsequently, 4-chloro-α-cyanocinnamic acid (5 mg/ml in 70% acetonitrile; Bionet Research) was added to each sample spot and allowed to dry. Glycopeptides were analyzed on an UltrafleXtreme MALDI-TOF/TOF mass spectrometer (Bruker Daltonics) operated in negative-ion reflectron mode, and ions between m/z 1,000 and 3,800 were recorded. Mass spectra were internally calibrated by using a peptide calibration standard (Bruker Daltonics). Data processing and evaluation were performed with FlexAnalysis software (Bruker Daltonics) and Microsoft Excel. Relative intensities of IgG Fc glycopeptides were obtained by integrating and summing four isotopic peaks; this was followed by normalization to the total subclass-specific glycopeptide intensities. The degrees of Fc galactosylation (G), sialylation (S), bisection (N), and fucosylation (F) of IgG1 and IgG2 and -3 were calculated as previously described (28). The following formulas were used: Fc galactosylation, (G1 + G1F + G1FN + G1N + G1S1 + G1FS1) × 0.5 + G2 + G2F + G2FN + G2N + G2S1 + G2FS1 for the IgG1 subclass and (G1F + G1FN + G1S1 + G1FS1) × 0.5 + G2F + G2FN + G2FS1 for the IgG2 and -3 subclasses; Fc sialylation, (G1S1+ G1FS1+ G2S1 and G2FS1 for the IgG1 subclass and G1S1+ G1FS1+ and G2FS1 for the IgG2 and -3 subclasses). The percentage of sialic acid residues present on galactose moieties (sialic acid/galactose ratio) was calculated by dividing the prevalence of Fc sialylation by twice the level of galactosylation; bisection, G0N + G1N + G2N + G0FN + G1FN and G2FN for IgG1 and G0N + G0FN + G1FN + and G2FN for IgG2 and -3; fucosylation, G0F + G0FN + G1F + G1FN + G1FS1 + G2F + G2FN + G2FS1. The incidence of IgG2 and -3 fucosylation was not evaluated, as a large portion of the afucosylated IgG2 and -3 glycoforms could not be determined because of mass overlap with isomeric IgG4 glycoforms (31).
Statistical analysis.
Data analysis was performed with IBM SPSS Statistics V.20.0 and GraphPad Prism V 5.0. The Kruskal-Wallis test with Dunn’s multiple-comparison test was used to evaluate differences among independent groups, and Spearman’s rank correlation was applied to assess nonparametric associations. Variables were checked for colinearity by examining tolerance and the variance inflation factor. Ordinal logistic regression models were used to identify the relationship between (i) IgG Fc N-glycosylation features and serum mediators and (ii) clinical severity. P values of less than 0.05 were considered significant.
SUPPLEMENTAL MATERIAL
Reproducibility of the IgG Fc N-glycosylation profiling method. (A) IgG1 Fc N-glycosylation features obtained for the standard serum sample added in triplicate for each sample plate in a total of eight plates. (B) IgG2 and -3 Fc N-glycosylation features obtained for the standard serum sample added in triplicate for each sample plate in a total of eight plates. Download
Correlations between levels of L. infantum chagasi-specific IgG and of Fc N-glycosylation features. (A, C, E, and G) IgG reactivity to SLA (dilution of 1:100) correlated with IgG1 levels of galactosylation, sialylation, bisection, and fucosylation, respectively. (B, D, and F) IgG reactivity to SLA (dilution of 1:100) correlated with IgG2 and -3 levels of galactosylation, sialylation, and bisection, respectively. Spearman’s rank correlation coefficients (r values), linear regression lines, and significance levels are depicted (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Download
Changes in serum cytokines with VL. Levels of IL-1β (A), IL-6 (B), TNF-α (C), IL-12p70 (D), IFN-γ (E), IL-17 (F), IL-10 (G), IL-5 (H), and IL-4 (I) are shown for endemic controls (EC), asymptomatic individuals (ASYMP), and patients with VL. Log10-transformed concentrations (pg/ml) are depicted. Significant changes were evaluated by Kruskal-Wallis test, followed by Dunn’s post-hoc test; median values and significance levels are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Download
Correlations between IgG Fc N-glycosylation features and serum mediators of VL patients. Log2-transformed concentrations of serum mediators are depicted. Spearman’s rank correlation coefficients (r values) and significance levels are shown (***, P ≤ 0.001). Download
Changes in IgG Fc N-glycosylation features and serum mediators with clinical severity. Relative abundances of Fc galactosylation (A, B) and sialylation (C, D) of IgG1 and IgG2 and -3 and levels of IL-6 (E), TNF-α (F), IFN-γ (G), IL-17 (H), IL-10 (I), and CRP (J) are shown for patients with uncomplicated VL (U-VL), complicated VL (C-VL), and severe VL (S-VL). Log10-transformed concentrations (pg/ml) are depicted. Significant changes were evaluated by Kruskal-Wallis test, followed by Dunn’s post-hoc test; median values and significance levels are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Download
Intracorrelation among IgG Fc N-glycosylation or serum mediators of patients characterized according to severity. Log2-transformed concentrations of serum mediators are depicted. Spearman’s rank correlation coefficients (r values) and significance levels are shown (**, P ≤ 0.01; ***, P ≤ 0.001). Download
Descriptives of IgG1 and IgG2 and -3 Fc N-glycopeptides among distinct clinical-epidemiological populations. Statistically significant differences between (i) nonendemic controls, endemic controls, and asymptomatic individuals and (ii) patients were evaluated by Kruskal-Wallis test and are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
Descriptives of Fc N-glycosylation features and serum mediators among distinct clinical-epidemiological populations. Statistically significant differences between (i) nonendemic controls, endemic controls, and asymptomatic individuals and (ii) patients were evaluated by Kruskal-Wallis test and are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
ACKNOWLEDGMENTS
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant 559603/2009-6), the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grants 2009/53645-3, 2012/06708-2, and 2012/06708-2), and by the European Union’s Seventh Framework Program (FP7-Health-F5-2011) under grant agreement 278535 (HighGlycan). L.G.G. and G.R.G. were supported by FAPESP (scholarships 2011/23819-0 and 2013/00382-0, respectively).
We thank João S. Silva at the University of São Paulo for the support of this work, which was partially performed in his laboratory. We are grateful to Carolien A. M. Koeleman at the Leiden University Medical Center for technical assistance. We thank Rodrigo P. Soares at FIOCRUZ, Belo Horizonte, for providing the parasite strain.
Footnotes
Citation Gardinassi LG, Dotz V, Hipgrave Ederveen A, de Almeida RP, Nery Costa CH, Costa DL, de Jesus AR, Mayboroda OA, Garcia GR, Wuhrer M, de Miranda Santos IKF. 2014. Clinical severity of visceral leishmaniasis is associated with changes in immunoglobulin G Fc N-glycosylation. mBio 5(6):e01844-14. doi:10.1128/mBio.01844-14.
REFERENCES
- 1. Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. 2007. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 5:873–882. 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 2. Peruhype-Magalhães V, Martins-Filho OA, Prata A, Silva LdeA, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimarães-Carvalho SF, Ferrari TC, Van Weyenbergh J, Correa-Oliveira R. 2006. Mixed inflammatory /regulatory cytokine profile marked by simultaneous raise of interferon-gamma and interleukin-10 and low frequency of tumour necrosis factor-alpha(+) monocytes are hallmarks of active human visceral leishmaniasis due to Leishmania chagasi infection. Clin. Exp. Immunol. 146:124–132. 10.1111/j.1365-2249.2006.03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duthie MS, Guderian J, Vallur A, Bhatia A, Lima Dos Santos P, Vieira de Melo E, Ribeiro de Jesus A, Todt M, Mondal D, Almeida R, Reed SG. 2014. Alteration of the serum biomarker profiles of visceral leishmaniasis during treatment. Eur. J. Clin. Microbiol. Infect. Dis. 33:639–649. 10.1007/s10096-013-1999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costa DL, Rocha RL, Carvalho RMA, Lima-Neto AS, Harhay MO, Costa CHN, Barral-Neto M, Barral AP. 2013. Serum cytokines associated with severity and complications of kala-azar. Pathog. Glob. Health 107:78–87. 10.1179/2047773213Y.0000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White AC, Castes M, Garcia L, Trujillo D, Zambrano L. 1992. Leishmania chagasi antigens recognized in cured visceral leishmaniasis and asymptomatic infection. Am. J. Trop. Med. Hyg. 46:123–131. [DOI] [PubMed] [Google Scholar]
- 6. Zijlstra EE, el-Hassan AM, Ismael A, Ghalib HW. 1994. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. Am. J. Trop. Med. Hyg. 51:826–836. [DOI] [PubMed] [Google Scholar]
- 7. Badaró R, Jones TC, Lorenço R, Cerf BJ, Sampaio D, Carvalho EM, Rocha H, Teixeira R, Johnson WD., Jr. 1986. A prospective study of visceral leishmaniasis in an endemic area of Brazil. J. Infect. Dis. 154:639–649. 10.1093/infdis/154.4.639. [DOI] [PubMed] [Google Scholar]
- 8. McCall LI, Zhang WW, Matlashewski G. 2013. Determinants for the development of visceral leishmaniasis disease. PLoS Pathog. 9:e1003053. 10.1371/journal.ppat.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galvão-Castro B, Sá Ferreira JA, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. 1984. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human American visceral leishmaniasis. Clin. Exp. Immunol. 56:58–66. [PMC free article] [PubMed] [Google Scholar]
- 10. Evans TG, Krug EC, Wilson ME, Vasconcelos AW, de Alencar JE, Pearson RD. 1989. Evaluation of antibody responses in American visceral leishmaniasis by ELISA and immunoblot. Mem. Inst. Oswaldo Cruz 84:157–166. 10.1590/S0074-02761989000800031. [DOI] [PubMed] [Google Scholar]
- 11. Ryan JR, Smithyman AM, Rajasekariah GH, Hochberg L, Stiteler JM, Martin SK. 2002. Enzyme-linked immunosorbent assay based on soluble promastigote antigen detects immunoglobulin M (IgM) and IgG antibodies in sera from cases of visceral and cutaneous leishmaniasis. J. Clin. Microbiol. 40:1037–1043. 10.1128/JCM.40.3.1037-1043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearson RD, de Alencar JE, Romito R, Naidu TG, Young AC, Davis JS., IV 1983. Circulating immune complexes and rheumatoid factors in visceral leishmaniasis. J. Infect. Dis. 147:1102. 10.1093/infdis/147.6.1102. [DOI] [PubMed] [Google Scholar]
- 13. Smelt SC, Cotterell SE, Engwerda CR, Kaye PM. 2000. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J. Immunol. 164:3681–3688. 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 14. Deak E, Jayakumar A, Cho KW, Goldsmith-Pestana K, Dondji B, Lambris JD, McMahon-Pratt D. 2010. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur. J. Immunol. 40:1355–1368. 10.1002/eji.200939455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nimmerjahn F, Ravetch JV. 2005. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310:1510–1512. 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 16. Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV. 2014. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat. Immunol. 15:707–716. 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chu N, Thomas BN, Patel SR, Buxbaum LU. 2010. IgG1 is pathogenic in Leishmania mexicana infection. J. Immunol. 185:6939–6946. 10.4049/jimmunol.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. 2006. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J. Exp. Med. 203:177–188. 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zauner G, Selman MHJ, Bondt A, Rombouts Y, Blank D, Deelder AM, Wuhrer M. 2013. Glycoproteomic analysis of antibodies. Mol. Cell. Proteomics 12:856–865. 10.1074/mcp.R112.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. 1995. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1:237–243. 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 21. Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. 2002. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277:26733–26740. 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 22. Zou G, Ochiai H, Huang W, Yang Q, Li C, Wang LX. 2011. Chemoenzymatic synthesis and Fcγ receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcγIIIa receptor. J. Am. Chem. Soc. 133:18975–18991. 10.1021/ja208390n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. 2011. Intravenous gamma globulin suppresses inflammation through a novel T(H)2 pathway. Nature 475:110–113. 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, Winkler A, Hess C, Reid DM, Majoul IV, Strait RT, Harris NL, Köhl G, Wex E, Ludwig R, Zillikens D, Nimmerjahn F, Finkelman FD, Brown GD, Ehlers M, Köhl J. 2012. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 18:1401–1406. 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parekh R, Isenberg D, Rook G, Roitt I, Dwek R, Rademacher T. 1989. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J. Autoimmun. 2:101–114. 10.1016/0896-8411(89)90121-2. [DOI] [PubMed] [Google Scholar]
- 26. Selman MH, Niks EH, Titulaer MJ, Verschuuren JJ, Wuhrer M, Deelder AM. 2011. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J. Proteome Res. 10:143–152. 10.1021/pr1004373. [DOI] [PubMed] [Google Scholar]
- 27. Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. 2013. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 123:2183–2192. 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Troelsen LN, Jacobsen S, Abrahams JL, Royle L, Rudd PM, Narvestad E, Heegaard NH, Garred P. 2012. IgG glycosylation changes and MBL2 polymorphisms: associations with markers of systemic inflammation and joint destruction in rheumatoid arthritis. J. Rheumatol. 39:463–469. 10.3899/jrheum.110584. [DOI] [PubMed] [Google Scholar]
- 29. Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM, Huizinga TW, Wuhrer M, van Schaardenburg D, Toes RE, Scherer HU. 8 October 2013. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 30. van Zeben D, Rook GA, Hazes JM, Zwinderman AH, Zhang Y, Ghelani S, Rademacher TW, Breedveld FC. 1994. Early agalactosylation of IgG is associated with a more progressive disease course in patients with rheumatoid arthritis: results of a follow-up study. Br. J. Rheumatol. 33:36–43. 10.1093/rheumatology/33.suppl_2.36. [DOI] [PubMed] [Google Scholar]
- 31. Baković MP, Selman MH, Hoffmann M, Rudan I, Campbell H, Deelder AM, Lauc G, Wuhrer M. 2013. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 12:821–831. 10.1021/pr300887z. [DOI] [PubMed] [Google Scholar]
- 32. Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F. 2008. Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics 8:2858–2871. 10.1002/pmic.200700968. [DOI] [PubMed] [Google Scholar]
- 33. Stadlmann J, Weber A, Pabst M, Anderle H, Kunert R, Ehrlich HJ, Peter Schwarz H, Altmann F. 2009. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics 9:4143–4153. 10.1002/pmic.200800931. [DOI] [PubMed] [Google Scholar]
- 34. Wuhrer M, Stam JC, van de Geijn FE, Koeleman CA, Verrips CT, Dolhain RJ, Hokke CH, Deelder AM. 2007. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics 7:4070–4081. 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- 35. Yamada E, Tsukamoto Y, Sasaki R, Yagyu K, Takahashi N. 1997. Structural changes of immunoglobulin G oligosaccharides with age in healthy human serum. Glycoconj. J 14:401–405. 10.1023/A:1018582930906. [DOI] [PubMed] [Google Scholar]
- 36. Shikata K, Yasuda T, Takeuchi F, Konishi T, Nakata M, Mizuochi T. 1998. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj. J 15:683–689. 10.1023/A:1006936431276. [DOI] [PubMed] [Google Scholar]
- 37. Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, Lopalco L, Tuffery P, Bomsel M. 2012. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc. Natl. Acad. Sci. U. S. A. 109:12680–12685. 10.1073/pnas.1200024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sela-Culang I, Kunik V, Ofran Y. 2013. The structural basis of antibody-antigen recognition. Front. Immunol. 4:302. 10.3389/fimmu.2013.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huffman JE, Pučić-Baković M, Klarić L, Hennig R, Selman MHJ, Vučković F, Novokmet M, Krištić J, Borowiak M, Muth T, Polašek O, Razdorov G, Gornik O, Plomp R, Theodoratou E, Wright AF, Rudan I, Hayward C, Campbell H, Deelder AM, Reichl U, Aulchenko YS, Rapp E, Wuhrer M, Lauc G. 2014. Comparative performance of four methods for high-throughput glycosylation analysis of immunoglobulin G in genetic and epidemiological research. Mol. Cell. Proteomics 13:1598–1610. 10.1074/mcp.M113.037465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehta AS, Long RE, Comunale MA, Wang M, Rodemich L, Krakover J, Philip R, Marrero JA, Dwek RA, Block TM. 2008. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J. Virol. 82:1259–1270. 10.1128/JVI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ho CH, Chien RN, Cheng PN, Liu JH, Liu CK, Su CS, Wu IC, Li IC, Tsai HW, Wu SL, Liu WC, Chen SH, Chang TT. 10 July 2014. Aberrant serum IgG glycosylation in chronic hepatitis B is associated with histological liver damage and is reversible by antiviral therapy. J. Infect. Dis. 10.1093/infdis/jiu388. [DOI] [PubMed] [Google Scholar]
- 42. O’Regan NL, Steinfelder S, Schwedler C, Rao GB, Srikantam A, Blanchard V, Hartmann S. 13 August 2014. Filariasis asymptomatically infected donors have lower levels of disialylated IgG compared to endemic normals. Parasite Immunol. 10.1111/pim.12137. [DOI] [PubMed] [Google Scholar]
- 43. Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, Schoen AL, Bitterling J, Stoehr AD, Petzold D, Schommartz T, Mertes MM, Schoen CT, Tiburzy B, Herrmann A, Köhl J, Manz RA, Madaio MP, Berger M, Wardemann H, Ehlers M. 2013. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J. Clin. Invest. 123:3788–3796. 10.1172/JCI65938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sibéril S, de Romeuf C, Bihoreau N, Fernandez N, Meterreau JL, Regenman A, Nony E, Gaucher C, Glacet A, Jorieux S, Klein P, Hogarth MP, Fridman WH, Bourel D, Béliard R, Teillaud JL. 2006. Selection of a human anti-RhD monoclonal antibody for therapeutic use: impact of IgG glycosylation on activating and inhibitory Fc gamma R functions. Clin. Immunol. 118:170–179. 10.1016/j.clim.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 45. Nimmerjahn F, Ravetch JV. 2008. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34–47. 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 46. Jeddi P, Keusch J, Lydyard PM, Bodman-Smith KB, Chesnutt MS, Wofsy D, Hirota H, Taga T, Delves PJ. 1999. The effect on immunoglobulin glycosylation of altering in vivo production of immunoglobulin G. Immunology 98:475–480. 10.1046/j.1365-2567.1999.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selman MH, de Jong SE, Soonawala D, Kroon FP, Adegnika AA, Deelder AM, Hokke CH, Yazdanbakhsh M, Wuhrer M. 2012. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol. Cell. Proteomics 11:M111.014563. 10.1074/mcp.M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Velge P, Kusnierz JP, Ouaissi A, Marty B, Pham BN, Capron A. 1991. Trypanosoma cruzi: infection of T lymphocytes and their destruction by antibody-dependent cell-mediated cytotoxicity. Eur. J. Immunol. 21:2145–2152. 10.1002/eji.1830210924. [DOI] [PubMed] [Google Scholar]
- 49. Gibson-Corley KN, Bockenstedt MM, Li H, Boggiatto PM, Phanse Y, Petersen CA, Bellaire BH, Jones DE. 2014. An in vitro model of antibody-enhanced killing of the intracellular parasite Leishmania amazonensis. PLoS One 9:e106426. 10.1371/journal.pone.0106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. 2005. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 201:747–754. 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Biburger M, Aschermann S, Schwab I, Lux A, Albert H, Danzer H, Woigk M, Dudziak D, Nimmerjahn F. 2011. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity 35:932–944. 10.1016/j.immuni.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 52. Xia Y, Janda A, Eryilmaz E, Casadevall A, Putterman C. 2013. The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol. Immunol. 56:28–37. 10.1016/j.molimm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ahmed AA, Giddens J, Pincetic A, Lomino JV, Ravetch JV, Wang LX, Bjorkman PJ. 2014. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J. Mol. Biol. 426:3166–3179. 10.1016/j.jmb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaneko Y, Nimmerjahn F, Ravetch JV. 2006. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313:670–673. 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 55. Lauc G, Huffman JE, Pučić M, Zgaga L, Adamczyk B, Mužinić A, Novokmet M, Polašek O, Gornik O, Krištić J, Keser T, Vitart V, Scheijen B, Uh HW, Molokhia M, Patrick AL, McKeigue P, Kolčić I, Lukić IK, Swann O, van Leeuwen FN, Ruhaak LR, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, de Craen AJ, Deelder AM, Zeng Q, Wang W, Hastie ND, Gyllensten U, Wilson JF, Wuhrer M, Wright AF, Rudd PM, Hayward C, Aulchenko Y, Campbell H, Rudan I. 2013. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 9:e1003225. 10.1371/journal.pgen.1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Collins ES, Galligan MC, Saldova R, Adamczyk B, Abrahams JL, Campbell MP, Ng CT, Veale DJ, Murphy TB, Rudd PM, Fitzgerald O. 2013. Glycosylation status of serum in inflammatory arthritis in response to anti-TNF treatment. Rheumatology (Oxford) 52:1572–1582. 10.1093/rheumatology/ket189. [DOI] [PubMed] [Google Scholar]
- 57. Wang J, Balog CIA, Stavenhagen K, Koeleman CAM, Scherer HU, Selman MHJ, Deelder AM, Huizinga TWJ, Toes REM, Wuhrer M. 2011. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol. Cell. Proteomics 10:M110.004655. 10.1074/mcp.M110.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nimmerjahn F, Anthony RM, Ravetch JV. 2007. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. U. S. A. 104:8433–8437. 10.1073/pnas.0702936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Santos IK, Costa CH, Krieger H, Feitosa MF, Zurakowski D, Fardin B, Gomes RB, Weiner DL, Harn DA, Ezekowitz RA, Epstein JE. 2001. Mannan-binding lectin enhances susceptibility to visceral leishmaniasis. Infect. Immun. 69:5212–5215. 10.1128/IAI.69.8.5212-5215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alonso DP, Ferreira AF, Ribolla PE, de Miranda Santos IK, do Socorro Pires e Cruz M, Aécio de Carvalho F, Abatepaulo AR, Lamounier Costa D, Werneck GL, Farias TJ, Soares MJ, Costa CH. 2007. Genotypes of the mannan-binding lectin gene and susceptibility to visceral leishmaniasis and clinical complications. J. Infect. Dis. 195:1212–1217. 10.1086/512683. [DOI] [PubMed] [Google Scholar]
- 61. Imafuku Y, Yoshida H, Yamada Y. 2003. Reactivity of agalactosyl IgG with rheumatoid factor. Clin. Chim. Acta 334:217–223. 10.1016/S0009-8981(03)00245-6. [DOI] [PubMed] [Google Scholar]
- 62. Costa CH, Werneck GL, Costa DL, Holanda TA, Aguiar GB, Carvalho AS, Cavalcanti JC, Santos LS. 2010. Is severe visceral leishmaniasis a systemic inflammatory response syndrome? A case control study. Rev. Soc. Bras. Med. Trop. 43:386–392. 10.1590/S0037-86822010000400010. [DOI] [PubMed] [Google Scholar]
- 63. Gautam S, Kumar R, Maurya R, Nylén S, Ansari N, Rai M, Sundar S, Sacks D. 2011. IL-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. J. Infect. Dis. 204:1134–1137. 10.1093/infdis/jir461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. 1992. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 89:1890–1893. 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nylén S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. 2007. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J. Exp. Med. 204:805–817. 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Owens BMJ, Beattie L, Moore JWJ, Brown N, Mann JL, Dalton JE, Maroof A, Kaye PM. 2012. IL-10-producing Th1 cells and disease progression are regulated by distinct CD11c+ cell populations during visceral leishmaniasis. PLoS Pathog. 8:e1002827. 10.1371/journal.ppat.1002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elshafie AI, Ahlin E, Mathsson L, ElGhazali G, Rönnelid J. 2007. Circulating immune complexes (IC) and IC-induced levels of GM-CSF are increased in Sudanese patients with acute visceral Leishmania donovani infection undergoing sodium stibogluconate treatment: implications for disease pathogenesis. J. Immunol. 178:5383–5389. 10.4049/jimmunol.178.8.5383. [DOI] [PubMed] [Google Scholar]
- 68. Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Brönneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Brüning JC. 2014. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15:423–430. 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. 1996. Essential role of Stat6 in IL-4 signalling. Nature 380:627–630. 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 70. Osorio EY, Zhao W, Espitia C, Saldarriaga O, Hawel L, Byus CV, Travi BL, Melby PC. 2012. Progressive visceral leishmaniasis is driven by dominant parasite-induced. STAT6 activation and STAT6-dependent host arginase 1 expression. PLoS Pathog. 8:e1002417. 10.1371/journal.ppat.1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ansar W, Mukhopadhyay S, Habib SK, Basu S, Saha B, Sen AK, Mandal CN, Mandal C. 2009. Disease-associated glycosylated molecular variants of human C-reactive protein activate complement-mediated hemolysis of erythrocytes in tuberculosis and Indian visceral leishmaniasis. Glycoconj. J 26:1151–1169. 10.1007/s10719-009-9236-y. [DOI] [PubMed] [Google Scholar]
- 72. Samuelsson A, Towers TL, Ravetch JV. 2001. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 291:484–486. 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 73. Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica 2006. Manual de vigilância e controle da leishmaniose visceral.. Ministério da Sáude, Brasília, Brazil. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reproducibility of the IgG Fc N-glycosylation profiling method. (A) IgG1 Fc N-glycosylation features obtained for the standard serum sample added in triplicate for each sample plate in a total of eight plates. (B) IgG2 and -3 Fc N-glycosylation features obtained for the standard serum sample added in triplicate for each sample plate in a total of eight plates. Download
Correlations between levels of L. infantum chagasi-specific IgG and of Fc N-glycosylation features. (A, C, E, and G) IgG reactivity to SLA (dilution of 1:100) correlated with IgG1 levels of galactosylation, sialylation, bisection, and fucosylation, respectively. (B, D, and F) IgG reactivity to SLA (dilution of 1:100) correlated with IgG2 and -3 levels of galactosylation, sialylation, and bisection, respectively. Spearman’s rank correlation coefficients (r values), linear regression lines, and significance levels are depicted (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Download
Changes in serum cytokines with VL. Levels of IL-1β (A), IL-6 (B), TNF-α (C), IL-12p70 (D), IFN-γ (E), IL-17 (F), IL-10 (G), IL-5 (H), and IL-4 (I) are shown for endemic controls (EC), asymptomatic individuals (ASYMP), and patients with VL. Log10-transformed concentrations (pg/ml) are depicted. Significant changes were evaluated by Kruskal-Wallis test, followed by Dunn’s post-hoc test; median values and significance levels are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Download
Correlations between IgG Fc N-glycosylation features and serum mediators of VL patients. Log2-transformed concentrations of serum mediators are depicted. Spearman’s rank correlation coefficients (r values) and significance levels are shown (***, P ≤ 0.001). Download
Changes in IgG Fc N-glycosylation features and serum mediators with clinical severity. Relative abundances of Fc galactosylation (A, B) and sialylation (C, D) of IgG1 and IgG2 and -3 and levels of IL-6 (E), TNF-α (F), IFN-γ (G), IL-17 (H), IL-10 (I), and CRP (J) are shown for patients with uncomplicated VL (U-VL), complicated VL (C-VL), and severe VL (S-VL). Log10-transformed concentrations (pg/ml) are depicted. Significant changes were evaluated by Kruskal-Wallis test, followed by Dunn’s post-hoc test; median values and significance levels are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001). Download
Intracorrelation among IgG Fc N-glycosylation or serum mediators of patients characterized according to severity. Log2-transformed concentrations of serum mediators are depicted. Spearman’s rank correlation coefficients (r values) and significance levels are shown (**, P ≤ 0.01; ***, P ≤ 0.001). Download
Descriptives of IgG1 and IgG2 and -3 Fc N-glycopeptides among distinct clinical-epidemiological populations. Statistically significant differences between (i) nonendemic controls, endemic controls, and asymptomatic individuals and (ii) patients were evaluated by Kruskal-Wallis test and are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
Descriptives of Fc N-glycosylation features and serum mediators among distinct clinical-epidemiological populations. Statistically significant differences between (i) nonendemic controls, endemic controls, and asymptomatic individuals and (ii) patients were evaluated by Kruskal-Wallis test and are shown (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).