Abstract
Background:
Formaldehyde is used in medicine and industry, and it is known to have detrimental effects on various systems including the nervous system, by increasing oxidative stress. However, data are scarce related to substances that can protect against the neurotoxicity induced by formaldehyde. Therefore, this study was designed to assess the protective effects of selenium against the toxic effect of this compound.
Methods:
A total of 48 adult male mice were divided randomly into six groups, i.e., (1) control, (2) treated with formaldehyde, (3) treated with formaldehyde plus 0.1 mg/kg selenium, (4) treated with formaldehyde plus 0.2 mg/kg selenium, (5) treated with formaldehyde plus 0.4 mg/kg selenium, and (6) treated with formaldehyde plus 0.8 mg/kg selenium. At the end of 14 days, the cerebellums were removed for histological evaluation. Morphological changes were examined using Image J software. The data were analyzed using SPSS software version 20.0 and analysis of variance (ANOVA).
Results:
Formaldehyde caused a reduction in the numbers and sizes of Purkinje cells and granular cells; in addition, the thickness of the granular layer was thinner than that in the control mice (P < 0.05). Treatment with 0.1 mg/kg selenium resulted in an increase in the number of Purkinje cells as well as the area of the gray matter compared to those of the control mice.
Conclusion:
Formaldehyde-induced neuronal damage was prevented by the administration of 0.1 mg/kg selenium, hence this treatment shows therapeutic potential for the treatment of neurotoxicity
Keywords: formaldehyde, cerebellum, morphometric, mice, neurotoxicity, selenium
1. Introduction
Formaldehyde is an environmental pollutant that is extensively soluble in water and has a sharp odor (1). The liquid form of formaldehyde, which usually is a 37% solution of formaldehyde in distilled water, is called formaldehyde, whereas the solid form is known as paraformaldehyde (2). As estimated by the National Institute for Occupational Safety and Health (NIOSH), the permissible exposure limit for formaldehyde in all workplaces is in the range of 1.5–3 mg/m3 (3,4). However, many studies have demonstrated that the formaldehyde levels in occupational sites are higher than this limit. Formaldehyde has a high propensity for reacting with RNA, DNA, and protein, which leads to health problems (5, 6). Many people are exposed to formaldehyde in industrial laboratories, such as those in which wood, plaster, textiles, paper, ink, and leather are utilized. In addition, anatomists and pathologists are affected by formaldehyde as a fixative (2). Formaldehyde also is found in cigarette smoke, domestic air, deodorants, home cleaning agents, toothpaste, and cosmetic products. Thus, exposure to formaldehyde for everyone is possible at any time (2). Formaldehyde has toxic effects on the skin, eyes, respiratory tract, urogenital system, and the nervous system (2, 7– 9). Some studies have been shown that formaldehyde can cause a high level of reactive oxygen species (ROS). Selenium is a well-known antioxidant that prevents the production of free radicals. Many reports have shown that selenium treatment protects against ROS (10–12). However, there are very few studies concerning the effect of antioxidant treatment on histopathological changes of the cerebellum caused by formaldehyde toxicity. Therefore, this study was designed to assess the protective effects of selenium against the neuronal damage caused by formaldehyde.
2. Material and Methods
Experiments were conducted using adult male Naval Medical Research Institute (NMRI) mice. Regarding our current state of knowledge, there is very little information in the literature concerning the effects of formaldehyde on the cerebellum or the protective effects of antioxidants. Also, females’ hormones have an effect on the results, so adult male mice were used for this study. The mice were maintained according to the guidelines of the Institutional Animal Ethics Committee. They were kept in polypropylene cages at the temperature 24°C, 45% relative humidity, and 12-h light and dark cycles with free access to drinking water and food. Possible confounding factors in this study were the weight, age, environment, water, and food of the mice. However, these conditions were identical for all of the mice, and we controlled any confounding factors.
A total of 48 male mice were divided randomly into six groups. The control group received an intraperitoneal injection of normal saline every day. The mice in Group II received 10 mg/kg of formaldehyde intraperitoneally (ip) once daily for 14 days. Groups III–VI were injected ip with selenium (0.1, 0.2, 0.4 and 0.8 mg/kg) plus formaldehyde. At the end of the two-week experimental period, all of the mice were killed, and their brains were obtained for histological examination. The cerebellum specimens were embedded in paraffin wax, sectioned with 5-μ thicknesses, and stained with haematoxylin–eosin (H&E). The images were captured by an Olympus (Japan) light microscope under a 40X objective lens. Digital images of the cerebellum were analyzed using Image J software. The software was used to investigate the following parameters, i.e., the thickness of the granular, molecular, and Purkinje layers, the number cells in the gray matter of the cerebellum, the thickness of the white matter, and comparative information concerning the gray matter and white matter in the mice’s cerebellums. The results were presented as mean ± S.D., and all data were analyzed using SPPS software version 20.0 and one-way analysis of variance (ANOVA). A value of P < 0.05 was considered to be significant.
3. Results
The results of morphometric study are shown in Table 1. The morphology of the neurons in the cerebellum tissue of the control group had a normal appearance (Figure 1A). After formaldehyde treatment, the number of Purkinje cells, the number of granular cells, and the thickness of the granular layer were less than those of the control mice (P < 0.05). In addition, the mice in the group treated with formaldehyde had dissociation of the Purkinje cells from the granular layer, which was not observed in the control mice. In selenium treatment group (0.1 mg/kg), the thickness of the molecular layers was significantly greater than those of the control mice, i.e., 642.75 ± 198.25 µ vs. 528.59 ± 111.45 µ. Also, the thickness of granular layer of cerebellum was higher in this group than in the control group (556.24 ± 187.92 µ vs. 452.71 ± 127.35 µ). Treatments with selenium at dose of 0.1 mg/kg resulted in a greater number of Purkinje cells than existed in the controls (Fig. 1). However, selenium at the dose of 0.1 mg/kg resulted in a decrease in the height of the Purkinje cells.
Table 1.
Mean values of the thickness and number of the cerebellum layers in all groups
| Group1 | Group2 | Group3 | Group4 | Group5 | Group6 | |
|---|---|---|---|---|---|---|
| Molecular layer thickness (μ) | 528.59 ± 111.45 | 554.21 ± 104.62 | 597.72 ± 215.10 | 522.73 ± 153.74 | 534.71 ± 141.33 | 592.51 ± 236.19 |
| Granular layer thickness (μ) | 452.71 ± 127.35 | 400.2 ± 78.39 | 451.21 ± 176.8 | 299.01 ± 73.12 | 440.94 ± 127.57 | 523.46 ± 213.45 |
| Gray layer thickness (μ) | 1072.77 ± 119.4 | 1014.48 ± 91.50* | 1134.66 ± 195.9* | 1052.33 ± 113.4* | 1060.78 ± 134.45* | 1211.43 ± 224.82* |
| White matter thickness (μ) | 69.39 ± 26.52 | 80.64 ± 29.09 | 181.08 ± 120.54* | 147.51 ± 82.90 | 67.24 ± 11.59 | 113.2 ± 106.63 |
| Gray to white matter ratio | 14.81 ± 1.31 | 12.58 ± 0.40* | 6.26 ± 2.95* | 7.13 ± 1.2* | 15.77 ± 1.5* | 10.69 ± 1.60* |
| Number of molecular cell (n/mm2) | 12.86 ± 5.03 | 11.83 ± 1.16 | 15.92 ± 4.87* | 10.4 ± 3.37 | 10.0 ± 2.95* | 13.53 ± 3.79 |
| Number of granular cell (n/mm2) | 99.6 ± 22.52 | 43.77 ± 6.41* | 100 ± 32.42 | 49.71 ± 8.67* | 63.44 ± 22.48 | 141.45 ± 70.74* |
| Number of purkinje cell (n/mm2) | 5.82 ± 1.41 | 3.14 ± 0.36* | 8.47 ± 3.01* | 6.13 ± 1.84 | 6.15 ± 1.72 | 6.57 ± 1.80 |
| Height of purkinje cell (μ) | 91.47 ± 16.45 | 60.07 ± 8.07* | 85.73 ± 12.13 | 83.43 ± 12.18* | 85.13 ± 11.38 | 95.46 ± 23.73 |
Figure 1.
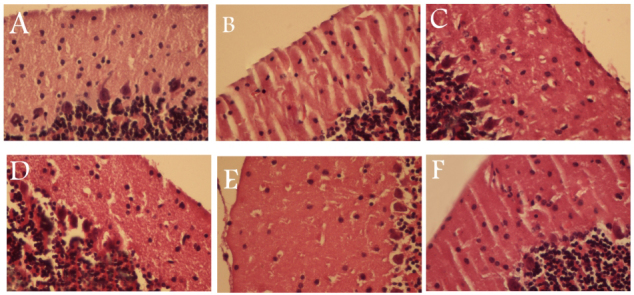
Sections of the mice’s cerebellums for all six groups: A, the control group, shows the normal appearance of the three layers of the cerebellum, i.e., the molecular layer, Purkinje layer, and granular cell layer; B, the formaldehyde group, indicates a loss of Purkinje cells and dissociation of the Purkinje cells from the granular layer; C, the formaldehyde + 0.1 mg/kg group, shows an improvement in the number of Purkinje cells and the thickness of the gray matter; D, the formaldehyde + 0.2 mg/kg Se group; E, the formaldehyde + 0.4 mg/kg Se group; F, the formaldehyde + 0.8 mg/kg Se group H&E X 400, which shows the absence of Purkinje cells
4. Discussion
Our results indicated that treatment with selenium at the optimum dose (0.1 mg/kg) can protect against the neuronal damage induced by formaldehyde in the cerebellum tissue. Formaldehyde has adverse effects on several different systems, and this is especially true for the central nervous system. Various reports have indicated that exposure to formaldehyde can cause headaches as well as mental, memory, and sleep disorders. Also, it has been shown to cause sensory-emotional and balance disorders in exposed employees in industries and hospitals (13). It has been shown that formaldehyde is a substrate for the cytochrome P-450 monooxygenase system’s 2E1 isozyme, which can activate such enzymes as peroxidase, aldehyde oxidase, and xanthine oxidase, resulting in increased formation of reactive oxygen species. The increased production of ROS results in oxidative stress in the brain. Antioxidants have a critical role in scavenging free oxygen radicals in the tissues of the body. Many studies have reported that antioxidant treatment can prevent oxidative stress in the tissues. For example, Zararsiz et al. reported that the daily treatment of rats with 400 mg/kg of omega Ɯ-3 fatty acids decreased cellular damage in the hippocampus and prefrontal tissue. In addition, the administration of omega-3 fatty acids increased superoxide dismutase and reduced malondealdehyde levels (13).
Several studies have shown that low concentration of formaldehyde can stimulate neurons, whereas high levels act as a depressant on the nervous system (13). In a study by Kabuto and colleagues, it was found that the administration of melatonin, a well-known antioxidant, reversed the negative effects that were observed in rats that were exposed to formaldehyde (14). Also, Gurelet et al. reported that 300 mg/kg of vitamin E significantly reduced the neuronal damage caused by exposure to formaldehyde (15). Degenerative damage and dark neurons were observed in the mice that were exposed to formaldehyde. These damages were significantly less in the antioxidant-treated group than those in the formaldehyde group. In addition, the number of the neurons was greater in the antioxidant-treated group than in the formaldehyde group (15). In our study, the formaldehyde-induced changes in the cerebellum were reversed by selenium treatment as an antioxidant. The administration of selenium in the amount of 0.1 mg/kg resulted in a significant increase in the number of neurons in the molecular layer and increased the thicknesses of the granular and molecular layers. One limitation of this was study was that we did not have sufficient financial support to acquire biochemical findings in parallel with the histopathological evaluations.
5. Conclusion
The results of this study showed that formaldehyde caused a reduction in the numbers and sizes of Purkinje cells and granular cells; in addition, the thickness of the granular layer was thinner than that in the control mice. The administration of selenium at a level of 0.1 mg/kg resulted in an increases number of Purkinje cells and an increase in the area of the gray matter. This means that there was a protective effect of N-Acetyle Cysteine against formaldehyde-induced damage, which demonstrated its potential for clinical use. Our results indicated that the administration of selenium at the level of 0.1 mg/kg for 14 days prevented formaldehyde-induced neural damage in mice. Also, we recommend that the mechanisms by which N-Acetyle Cysteine prevents neuronal damage be assessed in a future study.
Acknowledgments
The author thanks Mr. Mohammadi and Mrs. Dorophki for their technical assistance. This work was supported by the Office of the Vice-Chancellor for Research Affairs at Gonabad University of Medical Sciences.
Footnotes
Conflict of Interest: There is no conflict of interest to be declared.
References
- 1.Usanmaz SE, Akarsu ES, Vural N. Neurotoxic effects of acute and subacute formaldehyde exposures in mice. Envir Toxicol Pharmacol. 2002;11:93–100. doi: 10.1016/s1382-6689(01)00109-0. http://dx.doi.org/10.1016/S1382-6689(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 2.İnci Mehmet, Zararsız İsmail, Davarcı Mürsel, Görür Sadık. Toxic effects of formaldehyde on the urinary system. Turkish Journal of Urology. 2013;39(1):48–52. doi: 10.5152/tud.2013.010. http://dx.doi.org/10.5152/tud.2013.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong EY, Ray R, Gao DL, Wernli KJ, Li W, Fitzgibbons ED, et al. Reproductive history, occupational exposures, and thyroid cancer risk among women textile workers in Shanghai, China. Int Arch Occup Environ Health. 2006;79:251–8. doi: 10.1007/s00420-005-0036-9. http://dx.doi.org/10.1007/s00420-005-0036-9. [DOI] [PubMed] [Google Scholar]
- 4.Yu LQ, Jiang SF, Leng SG, He FS, Zheng YX. Early genetic effects on workers occupationally exposed to formaldehyde. Zhonghua Yu Fang Yi Xue Za Zhi. 2005;39:392–5. [PubMed] [Google Scholar]
- 5.Cheng G, Shi Y, Sturla SJ, Jalas JR, McIntee EJ, Villalta PW, et al. Reactions of formaldehyde plus acetaldehyde with deoxyguanosine and DNA: formation of cyclic deoxyguanosine adducts and formaldehyde cross-links. Chem Res Toxicol. 2003;16:145–52. doi: 10.1021/tx025614r. http://dx.doi.org/10.1021/tx025614r. [DOI] [PubMed] [Google Scholar]
- 6.Metz B, Kersten GF, Hoogerhout P, Brugghe HF, Timmermans HA, de Jong A, et al. Identification of formaldehyde-induced modifications in proteins: reactions with model peptides. J Biol Chem. 2004;279:6235–43. doi: 10.1074/jbc.M310752200. http://dx.doi.org/10.1074/jbc.M310752200. [DOI] [PubMed] [Google Scholar]
- 7.Kriebel D, Myers D, Cheng M, Woskie S, Cocanour B. Short term effect of formaldehyde on peak expiratory flow and irritant symptoms. Arch Environ Health. 2001;56:11–18. doi: 10.1080/00039890109604049. http://dx.doi.org/10.1080/00039890109604049. [DOI] [PubMed] [Google Scholar]
- 8.Sarsilmaz M, Kaplan S, Songur A, Colakoglu S, Aslan H, Tunc AT, Ozen OA, Turgut M, Bas O. Effects of postnatal formaldehyde exposure on pyramidal cell number, volume of cell layer in hippocampus and hemisphere in the rat: a stereological study. Brain Res. 2007;1145:157–167. doi: 10.1016/j.brainres.2007.01.139. http://dx.doi.org/10.1016/j.brainres.2007.01.139. [DOI] [PubMed] [Google Scholar]
- 9.Ozen OA, Akpolat N, Songur A, Kuş I, Zararsiz I, Ozaçmak VH, et al. Effect of formaldehyde inhalation on Hsp70 in seminiferous tubules of rat testes: an immunohistochemical study. Toxicol Ind Health. 2005;21:249–54. doi: 10.1191/0748233705th235oa. http://dx.doi.org/10.1191/0748233705th235oa. [DOI] [PubMed] [Google Scholar]
- 10.Gurel A, Coskun O, Armutcu F, Kanter M, Ozen OA. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroanat. 2005;29:173–8. doi: 10.1016/j.jchemneu.2005.01.001. http://dx.doi.org/10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Saito Y, Nishio K, Yoshida Y, Niki E. Cytotoxic effect of formaldehyde with free radicals via increment of cellular reactive oxygen species. Toxicology. 2005;210:235–45. doi: 10.1016/j.tox.2005.02.006. http://dx.doi.org/10.1016/j.tox.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Mohammadi S, Movahedin M, Mowla SJ. Up-regulation of CatSper genes family by selenium. Reprod Biol Endocrinol. 2009;7:126, 1–6. doi: 10.1186/1477-7827-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zararsiz Ismail, Kus Ilter, Ogeturk Murat, Akpolat Nusret, Kose Evren, Meydan Sedat, Sarsilmaz Mustafa. Melatonin prevents formaldehyde-induced neurotoxicity in prefrontal cortex of rats: an immunohistochemical and biochemical study. Cell Biochem Funct. 2007;25:413–418. doi: 10.1002/cbf.1315. http://dx.doi.org/10.1002/cbf.1315. [DOI] [PubMed] [Google Scholar]
- 14.Kabuto H, Yokoi I, Ogawa N. Melatonin inhibits iron-induced epileptic discharges in rats by suppressing peroxidation. Epilepsia. 1998;39:237–243. doi: 10.1111/j.1528-1157.1998.tb01367.x. http://dx.doi.org/10.1111/j.1528-1157.1998.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 15.Gurel Ahmet, Coskun Omer, Armutcu Ferah, Kanter Mehmet, Ozen Oguz Aslan. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: Biochemical and histological studies. Journal of Chemical Neuroanatomy. 2005;29:173–178. doi: 10.1016/j.jchemneu.200501.001. [DOI] [PubMed] [Google Scholar]


