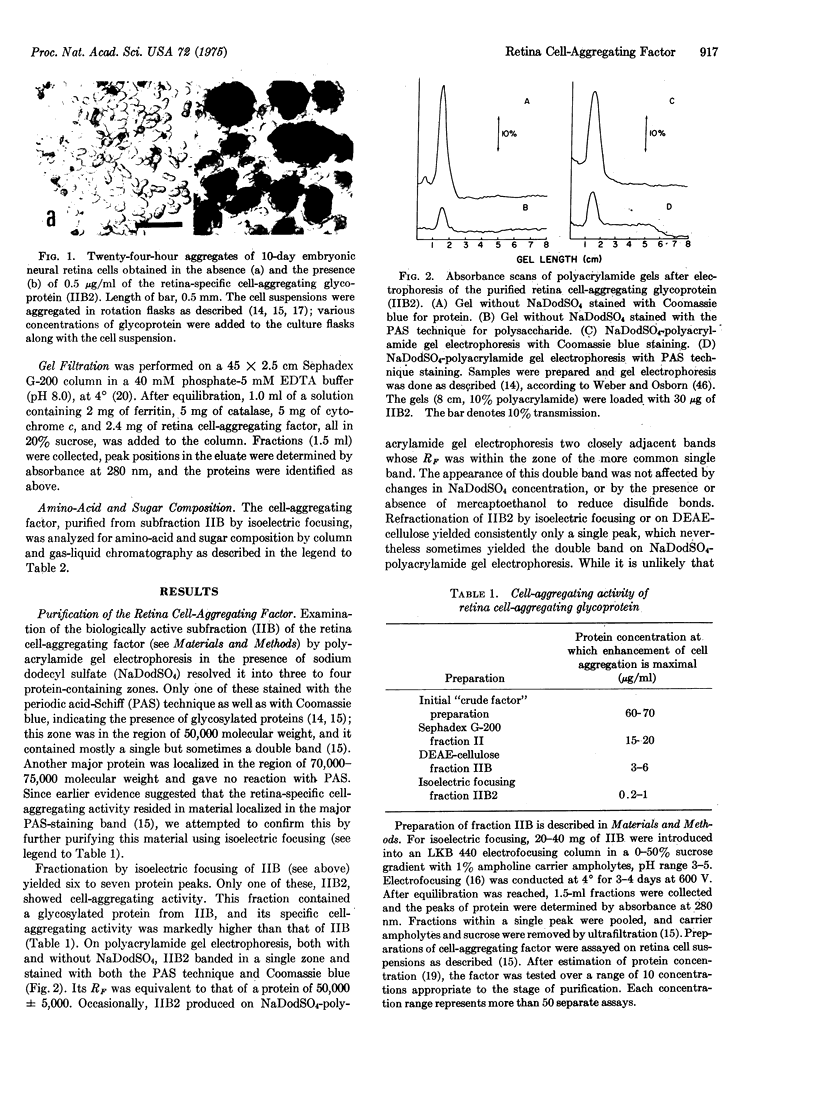
Abstract
The tissue-specific, cell-aggregating component of embryonic neural retina cells was purified from the retina cell-aggregating factor and characterized as a glycoprotein. Its molecular weight in solution is in the range of 50,000, and it contains 10-15% carbohydrate. The amino-acid and carbohydrate compositions have been determined. The glycoprotein is produced by embryonic neural retina cells in primary monolayered cultures and is released into the medium. Its tissue-specific, cell-aggregating effect requires integrity of the polypeptide portion, but not of the carbohydrate portion. We suggest that the isolated molecule is a specific determinant of the embryonic retina cell-surface and that it is involved in mediating self-recognition and selective adhesiveness of these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H. The major histocompatibility complex in transplantation immunology. Transplant Proc. 1973 Mar;5(1):23–29. [PubMed] [Google Scholar]
- Balsamo J., Lilien J. Embryonic cell aggregation: kinetics and specificity of binding of enhancing factors. Proc Natl Acad Sci U S A. 1974 Mar;71(3):727–731. doi: 10.1073/pnas.71.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F. Evolutionary significance of the HL-A system. Nature. 1972 May 19;237(5351):139–passim. doi: 10.1038/237139a0. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Edelman G. M. The genetic control of immunoglobulin synthesis. Annu Rev Genet. 1972;6:1–46. doi: 10.1146/annurev.ge.06.120172.000245. [DOI] [PubMed] [Google Scholar]
- Garber B. B., Moscona A. A. Reconstruction of brain tissue from cell suspensions. 2. Specific enhancement of aggregation of embryonic cerebral cells by supernatant from homologous cell cultures. Dev Biol. 1972 Feb;27(2):235–243. doi: 10.1016/0012-1606(72)90100-5. [DOI] [PubMed] [Google Scholar]
- Garfield S., Hausman R. E., Moscona A. A. Embryonic cell aggregation: absence of galactosyltransferase activity in retina-specific cell-aggregating factor. Cell Differ. 1974 Nov;3(4):215–219. doi: 10.1016/0045-6039(74)90004-9. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Moscona A. A. Tissue-specific cell-surface antigens in embryonic cells. J Cell Biol. 1972 May;53(2):435–449. doi: 10.1083/jcb.53.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund H. Isoelectric focusing in pH gradients--a technique for fractionation and characterization of ampholytes. Methods Biochem Anal. 1971;19:1–104. doi: 10.1002/9780470110386.ch1. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Cell-surface interactions: differential inhibition by proflavine of embryonic cell aggregation and production of specific cell-aggregating factor. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3111–3114. doi: 10.1073/pnas.70.11.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P., Humphreys S., Humphreys T. Characterization of sponge aggregation factor. A unique proteoglycan complex. Biochemistry. 1973 Jul 31;12(16):3045–3050. doi: 10.1021/bi00740a016. [DOI] [PubMed] [Google Scholar]
- Hunt R. C., Brown J. C. Surface glycoproteins of mouse L cells. Biochemistry. 1974 Jan 1;13(1):22–28. doi: 10.1021/bi00698a004. [DOI] [PubMed] [Google Scholar]
- KATCHALSKY A., DANON D., NEVO A. Interactions of basic polyelectrolytes with the red blood cell. II. Agglutination of red blood cells by polymeric bases. Biochim Biophys Acta. 1959 May;33(1):120–138. doi: 10.1016/0006-3002(59)90505-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lilien J. E., Moscona A. A. Cell aggregation: its enhancement by a supernatant from cultures of homologous cells. Science. 1967 Jul 7;157(3784):70–72. doi: 10.1126/science.157.3784.70. [DOI] [PubMed] [Google Scholar]
- Lilien J. E. Specific enhancement of cell aggregation in vitro. Dev Biol. 1968 Jun;17(6):657–678. doi: 10.1016/0012-1606(68)90012-2. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolaish E., Schenck J. R., Hargie M. P., Burokas S., Richter W. R., Barlow G. H., Moscona A. A. Characterization of specific cell aggregating materials from sponge cells. Biochem Biophys Res Commun. 1965 Aug 16;20(4):383–388. doi: 10.1016/0006-291x(65)90587-5. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Moscona A. A. Purification of the specific cell-aggregating factor from embryonic neural retina cells. Exp Cell Res. 1974 Aug;87(2):438–443. doi: 10.1016/0014-4827(74)90514-x. [DOI] [PubMed] [Google Scholar]
- Moscona A. A. Cell aggregation: properties of specific cell-ligands and their role in the formation of multicellular systems. Dev Biol. 1968 Sep;18(3):250–277. doi: 10.1016/0012-1606(68)90035-3. [DOI] [PubMed] [Google Scholar]
- Moscona A. A. STUDIES ON CELL AGGREGATION: DEMONSTRATION OF MATERIALS WITH SELECTIVE CELL-BINDING ACTIVITY. Proc Natl Acad Sci U S A. 1963 May;49(5):742–747. doi: 10.1073/pnas.49.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S. B., Edidin M., Orr C. W., Roseman S. An L-glutamine requirement for intercellular adhesion. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1395–1402. doi: 10.1073/pnas.63.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S. B., Humphreys T. Isolation of specific macromolecules required for adhesion of mouse tumour cells. Nature. 1971 Jul 9;232(5306):125–127. doi: 10.1038/232125a0. [DOI] [PubMed] [Google Scholar]
- Pessac B., Defendi V. Cell aggregation: role of acid mucopolysaccharides. Science. 1972 Feb 25;175(4024):898–900. doi: 10.1126/science.175.4024.898. [DOI] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971 Nov;51(21):536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. Studies on intercellular adhesive selectivity. Dev Biol. 1968 Dec;18(6):602–631. doi: 10.1016/0012-1606(68)90029-8. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Turner R. S., Burger M. M. Involvement of a carbohydrate group in the active site for surface guided reassociation of animal cells. Nature. 1973 Aug 24;244(5417):509–510. doi: 10.1038/244509a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Winzler R. J. Carbohydrates in cell surfaces. Int Rev Cytol. 1970;29:77–125. doi: 10.1016/s0074-7696(08)60033-9. [DOI] [PubMed] [Google Scholar]



