Abstract
Preterm birth results in alterations in neural connectivity, but the impact of prematurity on the functional organization of the developing brain has yet to be explored. To test the hypothesis that preterm birth alters cortical organization during the late second and third trimesters of gestation, we interrogated cerebral lateralization at rest in 26 very preterm subjects (birth weight 500–1500 g) with no evidence of brain injury and 25 healthy term control subjects at term equivalent age. Employing an unbiased voxel-based measure of functional connectivity, these data demonstrated that cerebral lateralization is impaired in the prematurely-born. At term equivalent age, preterm neonates showed significantly less lateralization in regions subserving both receptive and expressive language, left Brodmann (BA) areas insula-BA22-BA21 and L BA45-BA47 (p<0.05 corrected for multiple comparisons for both). Exploratory region of interest analyses demonstrated significantly less inter-hemispheric connectivity from L BA22 to R BA22 in preterm infants compared to term controls (p<0.005) and from R BA22 to its homolog (p<0.005). L BA22, Wernicke’s area, was more strongly connected to R BA39, foreshadowing neural networks for language in preterm subjects at school age, adolescence and young adulthood. For these very preterm neonates born at less than 30 weeks PMA, the degree of prematurity had no influence on lateralization in these differential regions.
Keywords: Resting-state functional connectivity, Functional MRI, Intrinsic connectivity distribution, Cerebral lateralization, Preterm infant brain, Language development
1. INTRODUCTION
While it has been known for many years that prematurity results in disordered neural connectivity (Huppi et al., 1998; Inder et al., 2003; Nosarti et al., 2006; Smyser et al., 2010; White et al., 2014), emerging data suggest that preterm (PT) birth alters the fundamental functional organization of developing brain. Multiple studies across the past decade suggest that alterations in neural networks contribute to the cognitive and behavioral difficulties of the prematurely-born (Doesburg et al., 2011; Ment et al., 2009; Myers et al., 2010; Nosarti, 2013; Salvan et al., 2013), but recent reports demonstrate alterations in the genetically-determined process of cerebral lateralization in PT subjects at adolescence and young adulthood (Scheinost et al., 2014; Wilke et al., 2014). Lateralization implies localization of a cognitive task to a specific cerebral region, and lateralization of language is a critical characteristic of developing brain (Power et al., 2010; Renteria, 2012).
Functional MRI studies suggest that those regions known to constitute the neural network for language in adults, children and older infants are also activated in newborns in response to language stimulation (Dehaene-Lambertz et al., 2002; Dehaene-Lambertz et al., 2010; Dehaene-Lambertz et al., 2004). Consistent with neuropathologic studies (Chi et al., 1977), this network involves both frontal and temporal regions with a clear dominance of the left hemisphere (Pena et al., 2003; Perani et al., 2011), and those regions sub-serving language are functionally connected (Hickok and Poeppel, 2007).
Likewise, studies across the putative third trimester of gestation suggest that healthy PT neonates develop the structural basis for language during this time interval (Dubois et al., 2009; Leroy et al., 2011). Similarly, functional imaging demonstrates the emergence of auditory networks in PT subjects between 30–40 weeks of gestation (Doria et al., 2010; Omidvarnia et al., 2013; Smyser et al., 2011). However, relative to term controls, PT neonates exhibit altered discrimination of speech sounds and deficits in auditory memory at term equivalent age and long term deficits in language processing (Luu et al., 2009; Taylor et al., 2004; Therien et al., 2004).
These data suggest that PT birth results in both proximate and long-lasting changes in cerebral functional organization. To test the hypothesis that PT birth alters cortical organization during the late second and third trimesters of gestation, we interrogated cerebral lateralization at rest in very PT subjects and term control subjects at term-equivalent age employing a novel data-driven voxel-based connectivity analysis strategy. Secondary seed-based analyses provided information about neural networks for language in the preterm brain.
2. METHODS
This study was approved by the Yale University Human Investigation Committee.
2.1 Subjects
Preterm neonates with a birth weight between 500–1500 grams and healthy term controls born between 37–41 weeks’ postmenstrual age (PMA) were eligible for the protocol and prospectively enrolled between 9-01-10 and 4-30-14. All were inborn and appropriate for gestational age (AGA). Exclusion criteria included evidence of congenital infections, congenital malformations and/or chromosomal disorders, seizures, intraventricular hemorrhage, periventricular leukomalacia or focal abnormalities on any MRI.
2.2 Imaging Parameters
Subjects were scanned without sedation using a feed-and-wrap protocol in either a 3 T Siemens (Erlangen, Germany) TIM Trio or Verio MR system with a 32-channel parallel receiver head coil. Localizer images were acquired for prescribing the functional image volumes, aligning the seventh or eighth slice parallel to the plane transecting the anterior and posterior commissures. T1-weighted 2D anatomical images were collected (TR=300ms, TE=2.47ms, FoV=220mm, matrix size=256x256, slice thickness=4mm, Flip Angle=60º, Bandwidth=300Hz/pixel with 25 slices) with 25 AC-PC aligned axial-oblique slices in addition to 3D anatomical scans using Magnetization Prepared Rapid Gradient Echo (MPRAGE) (176 contiguous sagittal slices, slice thickness=1mm, matrix size=256x256, FoV=256mm, TR=2530ms, TE=2.77, Flip Angle=7º, Bandwidth=179Hz/pixel). After these structural images, acquisition of functional data began in the same slice locations as the axial-oblique T1-weighted data. Functional images were collected using an echo-planar image gradient echo pulse sequence (TR=1500ms, TE=27ms, FoV=220mm, matrix size=64x64, slice thickness=4mm, Flip Angle=60º, Bandwidth=2520Hz/pixel, 25 slices). Functional runs consisted of 186 volumes on the TIM Trio system or 235 volumes on the Verio system. The first 6 volumes were removed to allow the magnetization to reach the steady-state.
2.3 Connectivity Preprocessing
Data analyses were performed as previously described (Kwon et al., 2014). Briefly, data were converted from Digital Imaging and Communication in Medicine format to analyze format using XMedCon (http://xmedcon.sourceforge.net/). Images were slice-time-corrected and motion-corrected using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). All further analysis was performed using BioImage Suite (Joshi et al., 2011). Several covariates of no interest were regressed from the data including linear and quadratic drift, six rigid-body motion parameters, mean cerebral-spinal-fluid (CSF) signal, mean white-matter signal, and overall global signal. The data were temporally smoothed with a zero mean unit variance Gaussian filter (approximate cutoff frequency=0.12Hz). A gray matter mask was applied to the data so only voxels in the gray matter were used in the calculation. Finally, as motion has been shown to confound connectivity studies (Van Dijk et al., 2012), blocks of data with a displacement greater than 1.5mm or a rotation greater than 2 degrees of rotation were removed. There were no significant differences for motion when PT infants were compared to term controls (p=0.61). All subjects had at least 2.5 minutes of resting state data.
2.4 Determination of Connectivity Lateralization
After preprocessing, the connectivity lateralization of each voxel as measured by the cross-hemisphere intrinsic connectivity distribution (ch-ICD) was calculated for each individual subject as described previously (Scheinost et al., 2014). To calculate ch-ICD, the time course for a voxel was correlated with the time course for every other voxel in the ipsilateral hemisphere. ICD was used to model the distribution of these correlations as a Wiebull distribution (Scheinost et al., 2012). The ICD parameterization efficiently summarizes the greater than 10,000 connections to a voxel to a single summary parameter of interest. This procedure was repeated for every voxel resulting in a map representing each voxel’s connectivity to the same hemisphere. Similarly, a map representing a voxel’s connectivity to the contralateral hemisphere was calculated. Both the ipsilateral and contralateral maps were standardized by converting to z-scores such that maps across participants could be averaged and compared. This standardization does not change the connectivity patterns, but ensures all participants are equally scaled (Buckner et al., 2009). Finally, to detect patterns of between hemisphere connectivity, the ipsilateral and contralateral connectivity maps were subtracted creating our ch-ICD metric (Figure 1).
Figure 1. Cerebral Lateralization.
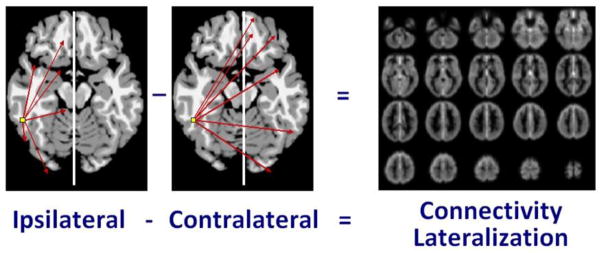
Ch-ICD, or cross-hemisphere Intrinsic Connectivity Distribution, is a measure of the cerebral lateralization of any voxel to a particular hemisphere. It independently compares the voxel’s connectivity to the intra-hemispheric (ipsilateral) and inter-hemispheric (contralateral) hemisphere and is calculated as ipsilateral connectivity minus contralateral connectivity.
2.5 Region of Interest Connectivity
Secondary region of interest (ROI) connectivity analysis was performed to explore (post-hoc) regions highlighted in our primary analysis (see Table 1 for seed regions). The ROIs were defined on the reference brain and transformed back (via the inverse of the transforms described below) into individual participant space. The time course of the reference region in a given participant was then computed as the average time course across all voxels in the reference region. This time course was correlated with the time course for every other voxel in the gray matter to create a map of r-values, reflecting ROI-to-whole-brain connectivity. These r-values were transformed to z-values using Fisher's transform yielding one map for each ROI and for each participant representing the strength of correlation to the ROI.
Table 1.
Characteristics of Seed Regions of Interest
| Seed ROI | Volume (mm3) | Talairach Coordinatesa | Origin of Seed Regions |
|---|---|---|---|
| L BA22 | 1018.51 | −42, −7, −7 | ch-ICD analysisb |
| R BA39 | 1402.82 | 29, −71, 31 | Seed-based connectivity analysis from L BA22b |
| R BA22 | 744.63 | 54, −36, 14 | Seed-based connectivity analysis from L BA22b |
| L Motor Cortex | 4098.75 | −44, −17, 42 | Control region |
Talairach coordinates are given for the center of the mass;
Seeds are p < 0.005 on original maps
2.6 Common Space Registration
To facilitate comparisons of imaging data, all single participant ch-ICD results were first spatially smoothed with a 6mm Gaussian filter and warped to a common template space through the concatenation of a series of linear and non-linear registrations. The functional series were linearly registered to the T1 axial-oblique (2D anatomical) image. The 2D anatomical image was linearly registered to the MPRAGE (3D anatomical) image. The 3D anatomical image was non-linearly registered to the template brain. All transformation pairs were calculated independently and combined into a single transform warping the single participant results into common space. This single transformation allows the single participant images to be transformed to common space with only one transformation, reducing interpolation error. All transformations were estimated using the intensity-only component of the method implemented by BioImage Suite as previously reported (Papademetris et al., 2004).
2.7 Brain Volumes
To control for possible differences in brain volumes, total brain volume (TBV) was calculated from each subject’s MPRAGE images. For each subject, the brain was manually traced and extracted from non-brain tissue using previously described tools and methods (Blumberg et al., 2005). TBV was calculated as volume of cerebral gray matter, white matter, ventricular CSF, cerebellum and brainstem. To validate our volumes, two raters each performed the manual segmentation of 5 randomly chosen structural MRIs. The correlation between raters for volume was r=0.978, the intra-class correlation was r=0.989, and the average Jaccard index between segmentation was 92.8%.
2.8 Statistical Analyses
Imaging data were analyzed using t-tests. Significance was assessed at a p<0.05 for between group ch-ICD comparisons (PT group minus term group), p<0.001 for within group seed composites (within PT and term groups), and p<0.005 for between group seed comparisons (PT group minus term group). All maps were corrected for multiple comparisons across gray matter via AFNI's AlphaSim program. All results were also localized in terms of the Brodmann areas (BA) and Talairach coordinates using BioImage Suite’s digital Brodmann atlas and Talairach atlas (Lacadie et al., 2008).
Demographic data were analyzed either using standard chi-squared test statistics or using Fisher’s exact test for categorical data. Continuous-valued data were analyzed either using t-tests or using Mann-Whitney u-tests when a normal distribution could not be assumed to compare groups. Finally, Pearson’s correlations and general linear model (GLM) analyses were performed to assess the relationship between perinatal variables and ch-ICD. The comparison of ch-ICD between PT and term group status was performed after adjusting for five biologically or clinically relevant covariates including gender, PMA at scan, scanner type, TBV and a motion variable (mean frame-to-frame displacement). PMA at birth was indicative of a subject’s group status (PT vs. term), therefore we did not include it in the regression analyses.
All analyses were performed using SAS version 9.4 (Cary, NC). P-value of less than 0.05 was considered to be statistically significant.
3. RESULTS
Thirty PT infants and 32 term controls met all inclusion criteria, and data from 26 PT and 25 term infants were analyzed after exclusion of 11 infants due to motion artifact. There was no significant difference in motion between the PT and term groups (p=0.51). Although there was no gender difference between the two groups, PMA at scan and TBV were significantly less for PT subjects compared to term controls (p<0.001 for both; Table 2).
Table 2.
Characteristics of study subjectsa
| Preterm (n=26) | Term (n=25) | P-value | |
|---|---|---|---|
| Postmenstrual age at birth (weeks) | 27 ± 1.5 (24 – 30) | 40 ± 1 (38 – 41) | < 0.001 |
| Birth weight, range (grams) | 970 ± 280 (550 – 1500) | 3450 ± 460 (2850 – 4000) | < 0.001 |
| Postmenstrual age at scan (weeks) | 39.6 ± 2.9 (36 – 43) | 42.3 ± 1.0 (39 – 44) | < 0.001 |
| Male gender | 8 (31%) | 15 (60%) | 0.05 |
| Bronchopulmonary dysplasia | 11 (42%) | ||
| Retinopathy of prematurity | 6 (23%) | ||
| Total Brain Volume (mm3) | 386,355 ± 82,518 (213,387 – 508,223) | 457,260 ± 52,543 (352,775 – 553,518) | < 0.001 |
Values are mean ± SD and (ranges: min – max)
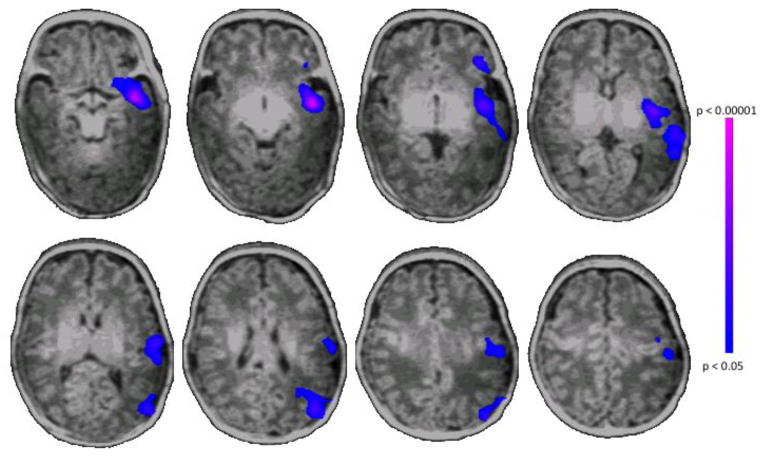
As shown in Figure 2, voxel-wise, whole brain analyses revealed significant differences in cerebral lateralization in the left hemisphere language regions, including lateral BA45 and 47 (regions contributing to Broca’s area), insula, BA22 (Wernicke’s area) and BA21 (the lateral temporal cortex), as well as portions of BA39 and 40 (p<0.05 for all) for PT subjects compared to term controls. As shown in Table 3, after adjusting for five covariates including gender, PMA at scan, scanner type, TBV and the motion variable (mean frame-to-frame displacement), group status (i.e., PT or term control) was shown to be an independent and statistically significant predictor of cerebral lateralization for both L IFG (lateral BA45-BA47) [beta (standard error): -0.162 (0.054), p=0.005] and the perisylvian region including insula-BA22-BA21 (beta (standard error): -0.125 (0.045), p=0.008). The negative regression coefficients (i.e., beta values) suggested that a PT subject had less lateralization than a term subject who otherwise had the same characteristics and these negative differences were statistically significant in insula-BA22-BA21 and BA45-BA47 regions.
Figure 2. Group comparison of cerebral lateralization.
ch-ICD analysis showed preterm subjects have decreased cerebral lateralization in L BA45, 47, 21, 22, lateral temporal cortex, insula, 39 and 40 compared to term subjects (p<0.05 for between group comparisons, corrected for multiple comparisons).
Table 3.
Association between group status and lateralization in left hemisphere language regions and motor region
| Group (PT vs. Term) | ||
|---|---|---|
| Regions | Beta (standard error) | P-value |
| Insula-BA22-BA21 | −0.125 (0.045) | 0.008 |
| BA19-BA39 | −0.106 (0.084) | 0.21 |
| Lateral BA45-BA47 | −0.162 (0.054) | 0.005 |
| Medial BA47 | −0.123 (0.084) | 0.15 |
| BA40 | −0.083 (0.050) | 0.11 |
| BA21 | −0.108 (0.075) | 0.16 |
| Motor Region | 0.035 (0.048) | 0.48 |
Note: Gender, PMA at scan, scanner type, TBV and motion variable (mean frame-to-frame displacement) were adjusted in the regression analyses.
In contrast, there was no statistically significant association between group status and lateralization in the motor region (p=0.477).
In addition, a regression analysis of PT subjects did not identify bronchopulmonary dysplasia as a significant predictor of lateralization (p=0.076 for insula-BA22-BA21; p-values>0.1 for all other regions), although there was a trend to significance for insula-BA22-BA21. There was no correlation between PMA at birth and cerebral lateralization within the PT group for either of these differential regions (e.g., lateral BA45-BA47: Pearson r=0.04, p=0.85; insula-BA22-BA21: r=0.18, p=0.37).
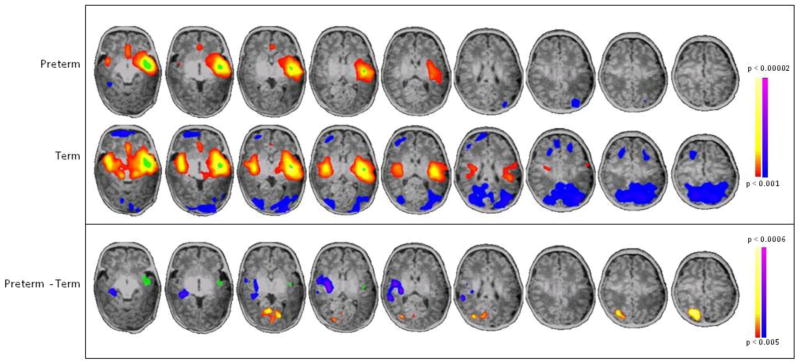
Comparing seed-based connectivity from L BA22, the region of greatest differential lateralization in PT and term controls, PT infants showed decreased connectivity from L BA22 to its R BA22 homolog and increased connectivity from L BA22 to R BA39 (Figure 3). Seed locations and volumes are given in Table 1. Similarly, when a seed was placed in R BA22, there was decreased connectivity from R BA22 to its L BA22 homolog in PT infants compared to term controls (Figure 4). In addition, a seed placed in R BA39 demonstrated that PT infants showed both decreased local connectivity within this region and greater cross-hemispheric connections from R BA39 to the contralateral hemisphere (L BA22, insula, putamen and amygdala) compared to term controls (Figure 5).
Figure 3. Seed-based connectivity analysis of L BA22.
Seed placed in L BA22 (green) showed that preterm subjects have decreased connectivity to its homologue, R BA22 (blue/purple), and increased connectivity to R BA39 (yellow/red) compared to term controls (p<0.001 for individual group composites and p<0.005 for between group comparisons, both corrected for multiple comparisons).
Figure 4. Seed-based connectivity analysis of R BA22.
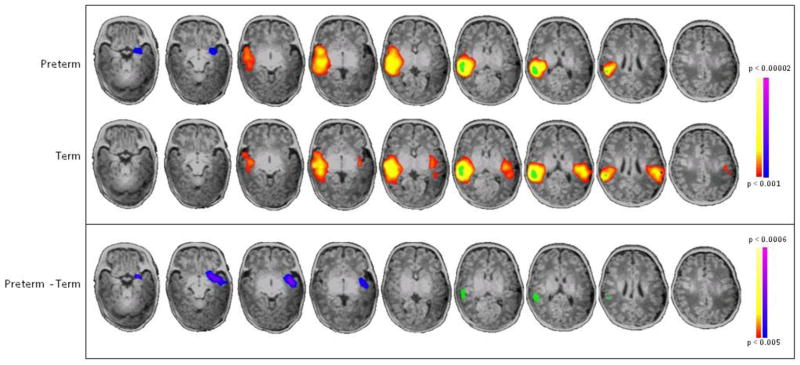
Seed placed in R BA22 showed preterm subjects have decreased connectivity to its homologue, L BA22 (blue/purple) in preterm subjects compared to term controls (p<0.001 for individual group composites and p<0.005 for between group comparisons, both corrected for multiple comparisons).
Figure 5. Seed-based connectivity analysis of R BA39.
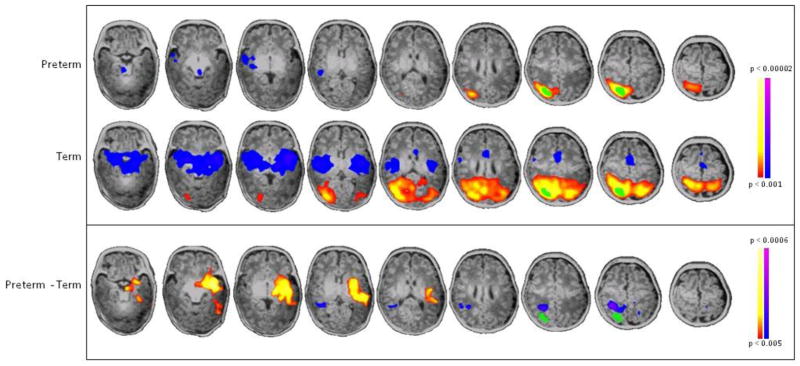
Seed placed in R BA39 showed preterm subjects have decreased intra-hemispheric connectivity within this region and increased inter-hemispheric connectivity to L BA22, insula, putamen and amygdala (yellow/red) compared to term controls (p<0.001 for individual group composites and p<0.005 for between group comparisons, both corrected for multiple comparisons).
In contrast, a seed-connectivity analysis was performed for the L motor area as a control region; as shown in Figure 6, there were no statistically significant differences in connectivity from the L motor seed between the two groups.
Figure 6. Seed-based connectivity analysis of L motor cortex.
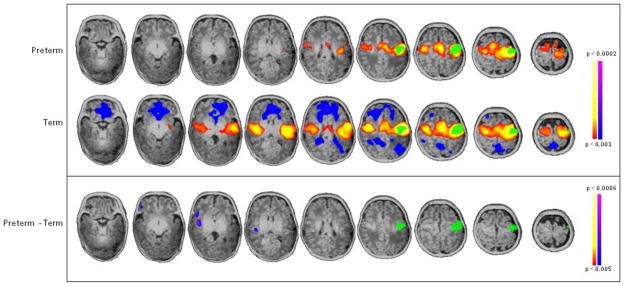
Seed placed in L motor cortex (green) showed no statistically significant difference in connectivity throughout the whole brain between preterm and term subjects (p<0.001 for individual group composites and p<0.005 for between group comparisons, both corrected for multiple comparisons).
4. DISCUSSION
Lateralization of language is an essential characteristic of the developing human brain, and these data suggest that this most fundamental process is impaired in the prematurely-born. At term equivalent age, PT neonates show significant lack of lateralization to regions subserving both receptive and expressive language, L insula-BA22-BA21 and L lateral BA45-BA47. ROI analyses demonstrate a significant decrease in inter-hemispheric connectivity from L BA22 to its homolog – and back again from R BA22 to L Wernicke’s for the PT group. Furthermore, L BA22 is more strongly connected to R BA39, angular gyrus, in preterm infants than term controls, foreshadowing neural networks for language in PT subjects at school age, adolescence and young adulthood. Also noteworthy is the lack of correlation of the degree of prematurity (PMA at birth) with lateralization in language regions at term equivalent age in the PT group. Likewise for the PT group, PMA at scan shows no association with lateralization, suggesting the lack of late postnatal influence on lateralization.
Emerging research suggests a strong coupling between functional connectivity, blood supply, and metabolic demand (Liang et al., 2013; Vaishnavi et al., 2010), suggesting that regions with greater connectivity have greater blood flow and metabolism to support the additional connections. As such, a decrease in voxel-wise connectivity or lateralization may reflect an underlying decrease in blood flow or metabolism. This decrease in functional connectivity may be seen in injured regions of the brain, as well as in regions with altered development secondary to epigenetic changes related to prematurity, and may result in the reduced capacity to establish long-range connections.
Prior functional imaging studies of PT neonates at term equivalent age have examined the emergence of inter-hemispheric neural networks (Doria et al., 2010; Fransson et al., 2007; Smyser et al., 2010). They have begun to describe those rich club nodes destined to serves as hubs for the efficient long-range transfer of information (Ball et al., 2014; van den Heuvel et al., 2014), but none has yet described the functional organization of developing brain at a time before the onset of language.
Nonetheless, the fetus may provide the appropriate control for PT neonates. Recent studies demonstrate the emergence of inter-hemispheric connectivity for language regions including both Wernicke’s and Broca’s regions during the late second and third trimester of gestation in typically developing fetuses (Anderson and Thomason, 2013; Thomason et al., 2013). In addition, graph theory analyses suggest that a ventral-frontal-temporal cortex module present in both younger and older fetuses (i.e., those < 31 weeks of gestation and those > 31 weeks of gestation) becomes more left-lateralized and integrated with frontal and parietal language regions with increasing postmenstrual age (Thomason et al., 2014). Taken together, these data present evidence for fetal functional lateralization of language mimicking that of older infants, children and adults. It also provides confirming evidence for the alterations in functional lateralization of language regions we report in the prematurely-born.
The strengths of this study are the innovative imaging strategies, prospective data collection and moderately robust sample size. The weaknesses include the as yet unavailable language testing data and longitudinal imaging studies targeting both critical periods and regions of functional alteration.
5. CONCLUSIONS
Preterm birth is a major pediatric public health problem in the world today (Blencowe et al., 2013a; Blencowe et al., 2013b), and almost 40% of preterm subjects experience language disorders (Northam et al., 2012; Pritchard et al., 2014). If a major aim of perinatal intensive care is to promote typical development in the prematurely born (Bauer and Msall, 2010), then interrogation of molecular mechanisms responsible for language in the developing brain is critically important for this goal (Johnson et al., 2009; Tebbenkamp et al., 2014). Future studies should identify the critical periods and regions in which lateralization are perturbed in preterm neonates and the epigenetic events which drive these findings.
HIGHLIGHTS.
We compare cerebral lateralization in preterm and term neonates at term age.
Preterm neonates have altered lateralization in left hemisphere language areas.
L BA22, Wernicke’s area, is more strongly connected to R BA39 in preterm neonates.
Results foreshadow findings in preterm children, adolescents and young adults.
Preterm birth at very low postmenstrual age alters corticogenesis.
Acknowledgments
Supported by NIH NS074022, T32 HD07094, T32 DA022975, UL1 TR000142
We thank Drs. Richard Ehrenkranz and Deborah Hirtz for their scientific expertise; Hedy Sarofin and Terry Hickey for their technical assistance; and the families who participated in this study.
ABBREVIATIONS
- BA
Brodmann area
- Ch-ICD
Cross-hemisphere intrinsic connectivity distribution (i.e., lateralization)
- GLM
General linear model
- ICD
Intrinsic connectivity distribution
- PMA
Postmenstrual age
- PT
Preterm
- TBV
Total brain volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AL, Thomason ME. Functional plasticity before the cradle: a review of neural functional imaging in the human fetus. Neurosci Biobehav Rev. 2013;37:2220–2232. doi: 10.1016/j.neubiorev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Ball G, Aljabar P, Zebari S, Tusor N, Arichi T, Merchant N, Robinson EC, Ogundipe E, Rueckert D, Edwards AD, Counsell SJ. Rich-club organization of the newborn human brain. Proc Natl Acad Sci U S A. 2014;111:7456–7461. doi: 10.1073/pnas.1324118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer SC, Msall ME. Optimizing neurodevelopmental outcomes after prematurity: lessons in neuroprotection and early intervention. Minerva Pediatr. 2010;62:485–497. [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, Lawn J Born Too Soon Preterm Birth Action Group. Born too soon: The global epidemiology of 15 million preterm births. Reprod Health. 2013a;10(Suppl 1):S2. doi: 10.1186/1742-4755-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, Chou D, Say L, Modi N, Katz J, Vos T, Marlow N, Lawn JE. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. 2013b;74(Suppl 1):17–34. doi: 10.1038/pr.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, Pittman B, Martin A, Peterson BS, Fulbright RK, Krystal JH. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Montavont A, Jobert A, Allirol L, Dubois J, Hertz-Pannier L, Dehaene S. Language or music, mother or Mozart? Structural and environmental influences on infants' language networks. Brain Lang. 2010;114:53–65. doi: 10.1016/j.bandl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Pena M, Christophe A, Landrieu P. Phoneme perception in a neonate with a left sylvian infarct. Brain Lang. 2004;88:26–38. doi: 10.1016/s0093-934x(03)00284-0. [DOI] [PubMed] [Google Scholar]
- Doesburg SM, Ribary U, Herdman AT, Miller SP, Poskitt KJ, Moiseev A, Whitfield MF, Synnes A, Grunau RE. Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. Neuroimage. 2011;54:2330–2339. doi: 10.1016/j.neuroimage.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, Larkman DJ, Rees G, Edwards AD. Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A. 2010;107:20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 2009;19:414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U. Resting-state networks in the infant brain. PNAS. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Warfield S, Kikinis R, Barnes P, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Inder TE, Anderson AW, Neil JJ, Ment LR. Yearbook of Perinatal and Neonatal Medicine. 2003. Imaging the high risk developing brain. [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, Papademetris X. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 2011;9:69–84. doi: 10.1007/s12021-010-9092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Scheinost D, Lacadie C, Benjamin J, Myers EH, Qiu M, Schneider KC, Rothman DL, Constable RT, Ment LR. GABA, Resting-State Connectivity and the Developing Brain. Neonatology. 2014;106:149–155. doi: 10.1159/000362433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, Glasel H, Dubois J, Hertz-Pannier L, Thirion B, Mangin JF, Dehaene-Lambertz G. Early maturation of the linguistic dorsal pathway in human infants. J Neurosci. 2011;31:1500–1506. doi: 10.1523/JNEUROSCI.4141-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A. 2013;110:1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123:1037–1044. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Huppi PS. Microstructural markers of outcome in developing brain. The Lancet Neurology. 2009;8:1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Myers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, Katz KH, Schneider KC, Makuch RW, Constable RT, Ment LR. Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage. 2010;51:1445–1452. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam GB, Liegeois F, Tournier JD, Croft LJ, Johns PN, Chong WK, Wyatt JS, Baldeweg T. Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain. 2012;135:3781–3798. doi: 10.1093/brain/aws276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C. Structural and functional brain correlates of behavioral outcomes during adolescence. Early Hum Dev. 2013;89:221–227. doi: 10.1016/j.earlhumdev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rubia K, Smith A, Frearson S, Williams SC, Rifkin L, Murray RM. Altered functional neuroanatomy of reponse inhibition in adolescent males who were born very preterm. Devel Med Child Neurol. 2006;48:265–271. doi: 10.1017/S0012162206000582. [DOI] [PubMed] [Google Scholar]
- Omidvarnia A, Fransson P, Metsaranta M, Vanhatalo S. Functional bimodality in the brain networks of preterm and term human newborns. Cereb Cortex. 2013 doi: 10.1093/cercor/bht120. [DOI] [PubMed] [Google Scholar]
- Papademetris X, Jackowski A, Schultz R, Staib L, Duncan J, Barillot C, Haynor D, Hellier P. Medical Image Computing and Computer-Assisted Intervention-MICCAI 2004. Springer; Berlin / Heidelberg: 2004. Integrated intensity and point-feature nonrigid registration; pp. 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena M, Maki A, Kovacic D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci U S A. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Spada D, Baldoli C, Poloniato A, Lohmann G, Friederici AD. Neural language networks at birth. Proc Natl Acad Sci U S A. 2011;108:16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard VE, Bora S, Austin NC, Levin KJ, Woodward LJ. Identifying very preterm children at educational risk using a school readiness framework. Pediatrics. 2014;134(3):e825–32. doi: 10.1542/peds.2013-3865. [DOI] [PubMed] [Google Scholar]
- Renteria ME. Cerebral asymmetry: a quantitative, multifactorial, and plastic brain phenotype. Twin Res Hum Genet. 2012;15:401–413. doi: 10.1017/thg.2012.13. [DOI] [PubMed] [Google Scholar]
- Salvan P, Froudist Walsh S, Allin MP, Walshe M, Murray RM, Bhattacharyya S, McGuire PK, Williams SC, Nosarti C. Road work on memory lane-functional and structural alterations to the learning and memory circuit in adults born very preterm. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Benjamin J, Lacadie CM, Vohr B, Schneider KC, Ment LR, Papademetris X, Constable RT. The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. Neuroimage. 2012;62:1510–1519. doi: 10.1016/j.neuroimage.2012.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Lacadie C, Vohr BR, Schneider KC, Papademetris X, Constable RT, Ment LR. Cerebral lateralization is protective in the very prematurely born. Cereb Cortex. 2014 doi: 10.1093/cercor/bht430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Snyder AZ, Neil JJ. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 2011;56:1437–1452. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Minich N, Bangert B, Filipek PA, Hack M. Long-term neuropsychological outcomes of very low birth weight: associations with early risks for periventricular brain insults. J Int Neuropsychol Soc. 2004;10:987–1004. doi: 10.1017/s1355617704107078. [DOI] [PubMed] [Google Scholar]
- Tebbenkamp AT, Willsey AJ, State MW, Sestan N. The developmental transcriptome of the human brain: implications for neurodevelopmental disorders. Curr Opin Neurol. 2014;27:149–156. doi: 10.1097/WCO.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therien JM, Worwa CT, Mattia FR, deRegnier RA. Altered pathways for auditory discrimination and recognition memory in preterm infants. Dev Med Child Neurol. 2004;46:816–824. doi: 10.1017/s0012162204001434. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Brown JA, Dassanayake MT, Shastri R, Marusak HA, Hernandez-Andrade E, Yeo L, Mody S, Berman S, Hassan SS, Romero R. Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS One. 2014;9:e94423. doi: 10.1371/journal.pone.0094423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, Anderson AL, Yeo L, Mody S, Hernandez-Andrade E, Hassan SS, Studholme C, Jeong JW, Romero R. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 2013;5:173ra124. doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Kersbergen KJ, de Reus MA, Keunen K, Kahn RS, Groenendaal F, de Vries LS, Benders MJ. The neonatal connectome during preterm brain development. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TP, Symington I, Castellanos NP, Brittain PJ, Froudist Walsh S, Nam KW, Sato JR, Allin MP, Shergill SS, Murray RM, Williams SC, Nosarti C. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin. 2014;4:352–365. doi: 10.1016/j.nicl.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Hauser TK, Krageloh-Mann I, Lidzba K. Specific impairment of functional connectivity between language regions in former early preterms. Hum Brain Mapp. 2014;35:3372–3384. doi: 10.1002/hbm.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]