Abstract
Background
Severe asthma may involve both innate and Type-2 cytokine associated adaptive immunity. While IL-27 has been reported to potentiate Th1 responses (including the chemokine CXCL9) and suppress Th2 responses, its function in asthma is unknown.
Objective
Evaluate IL-27 expression in human asthma, alone and in combination with Type-2 immunity to determine the relationship to disease severity and CXCL9 expression. Model these interactions in vitro in human bronchial epithelial cells (HBEC).
Methods
Bronchoalveolar lavage (BAL) cells from 87 participants were evaluated for IL-27 mRNA and protein, alone and in association with epithelial CCL26 (a marker of Type-2 activation) in relation to asthma severity and CXCL9 mRNA. HBECs cultured in air liquid interface (ALI) and stimulated with IL-27 (1–100 ng/ml) with/without IL-13 (1 ng/ml) were evaluated for CXCL9 expression by qRT-PCR and ELISA. Phosphorylated and total STAT1/3 were detected by western blot. siRNA knockdown of STAT1 or STAT3 was performed.
Results
BAL cell IL-27 mRNA and protein were increased in asthma. Patients with evidence for Type-2 pathway activation had higher IL-27 expression (p=0.02). Combined IL-27 and CCL26 expression associated with more severe asthma and higher CXCL9 expression (p=0.004, 0.007 respectively), while IL-27 alone was associated with milder disease. In vitro, IL-13 augmented IL-27 induced CXCL9 expression which appeared to be due to augmented STAT1 activation and reduced STAT3 activation.
Conclusions
IL-27, in combination with a Type-2/CCL26 signature identifies a more severe asthma phenotype, perhaps through combined effects of IL-27 and IL-13 on STAT signaling. Understanding these interactions could lead to new targets for asthma therapy.
Keywords: Asthma, IL-27, IL-13, CXCL9, Epithelial cells, Signal transducer and activator of transcription 1 (STAT1), Signal transducer and activator of transcription 3 (STAT3)
Introduction
Asthma is a heterogeneous disease consisting of multiple phenotypes. Although different phenotyping schemes have been suggested, the most prominent current schema relates to the presence or absence of a Type-2 cytokine signature.1, 2 However, a Type-2 signature can be present in patients with a range of disease severity and clinical characteristics suggesting that additional cytokine pathways also control the biologic and clinical presentation.1, 3, 4
IL-27, a novel cytokine sharing subunits with IL-6 families, is primarily produced by activated antigen-presenting cells, specifically dendritic cells, but also pulmonary macrophages.5, 6 Functionally, IL-27 is characterized by pleiotropic effects on T-helper cell function, differentiation, and development.7, 8 Recent studies have reported IL-27 to be increased in response to allergen, to be present in human eczematous skin lesions and to be genetically associated with asthma susceptibility.9–11 IL-27 has also been reported to induce steroid resistance, suggesting it could contribute to more complex asthma phenotypes.6 However, with its contradictory pro-inflammatory and anti-inflammatory effects,12–17 its biologic function in asthma is not clear.
A large body of evidence suggests that alterations in the respiratory epithelium play a crucial role in both development and persistence of asthma.18, 19 Indeed, pioneering studies to divide asthma into Th2-High vs. Low phenotypes evaluated the expression of three IL-13 induced genes in the airway epithelium of mild asthma patients as compared to healthy controls (HC).1 CCL26/eotaxin-3 similarly represents a gene highly induced by IL-13 which can be used to differentiate “Th2-High vs. Low”.1, 3 Like IL-13, IL-27 can also impact human bronchial epithelial cells (HBEC).20 Previous in vitro studies have suggested that STAT1 and STAT3 are the main signaling pathways controlling IL-27’s effects on epithelial cells.21 In myeloid cell, STAT3 activation appears to counterbalance STAT1 activation, limiting the overall expression of STAT1 dependent pathways.22 However, the functional impact of IL-27 on asthmatic HBEC and involved pathways remains poorly understood, particularly in the presence of a background Type-2 (IL-13) signature.
We therefore hypothesized that IL-27, in association with a Type-2 (IL-13) gene signature (high CCL26 expression) identifies a more severe asthma phenotype than the presence of either cytokine or its signature alone. We hypothesized that the mechanisms for this increased severity would include synergistic augmentation of epithelial expression of the Type-1 chemokine, monokine induced by gamma interferon (CXCL9), by IL-13 and IL-27, through alterations in the balance of STAT1 and STAT3. To evaluate this, bronchoscopic samples from a range of asthmatic and healthy control (HC) participants were analyzed for the presence of a Type-2 signature (based on epithelial CCL26 expression), alone and in association with bronchoalveolar lavage (BAL) cell IL-27 expression in association with asthma severity and CXCL9 expression. To determine potential mechanisms for these effects, the impact of IL-27, alone and in combination with IL-13, on primary HBEC activation of STAT1 and STAT3 was evaluated to determine their role in the synergistic increase in CXCL9 expression.
Methods
Subjects
Participants were 18–65 years old enrolled in the Severe Asthma Research Program (SARP) or the Electrophilic Fatty Acid Derivatives in Asthma study.23 All studies were approved by the University of Pittsburgh Institutional Review Board and all participants provided informed consent. Subjects were nonsmokers in the last year and all had <5 pack-year smoking history. Severe asthma (SA) was defined using the 2001 American Thoracic Society (ATS) definition.24 Patients with mild asthma not receiving ICSs (Mild/no ICS group) had a prebronchodilator FEV1 of ≥80% predicted. Patients with mild-to-moderate asthma receiving low to moderate-dose ICSs (Mild-Mod/ICS group) had an FEV1 of greater than 60% predicted.25 HCs had no history of chronic respiratory disease and normal lung function, but could be atopic. All participants were extensively characterized as previously described, including clinical questionnaires, spirometric measures pre and post bronchodilator, fractional exhaled nitric oxide (FeNO), complete blood counts, allergy skin prick testing and IgE.25, 26
Bronchoscopy and sample processing
Epithelial brushings and bronchoalveolar lavage (BAL) cells were obtained bronchscopically from the 4–5th generation airways as previously published and the SARP manual of procedures.27, 28 Cells were placed in Qiazol (Qiagen) for extraction of RNA.
Quantitative real-time PCR
Epithelial and BAL cell RNA was extracted in Qiazol (Qiagen, Valencia, CA) and mRNA expression determined by quantitative real-time PCR (qRT-PCR). Primers and probes were purchased from Applied Biosystems (Foster City, CA; Assays on Demand: IL-27 p28, HS00377366_m1; CCL26, Hs00171146_m1; CXCL9, Hs00171065_m1). qRT-PCR was performed on the ABIPrism 7900 sequence detection system (Applied Biosystems) at core facilities at the University of Pittsburgh. The levels of each marker were determined relative to GAPDH using the delta-CT method.
Immunohistochemistry
BAL cell cytospins were fixed in 2% paraformaldehyde. Cytospins were rinsed, blocked and incubated overnight with goat polyclonal anti-human IL-27 antibody (R&D systems, Minneapolis, MN, USA) at a 1:50 dilution. Secondary antibody staining alone and isotype controls were used to confirm primary antibody specificity. Biotinylated secondary rabbit anti-goat antibody was added and the cytospins incubated with ABC reagent (Vector Laboratories, Burlingame, CA), developed with 3-amino-9-ethylcarbazole (AEC), counterstained with hematoxylin and overlaid with Crystal Mount (Electron Microscopy Sciences, Hatfield, PA). IL-27+ cells were counted blindly by two independent observers from 500 cells to obtain the percentage of IL-27+ cells.
Primary Air Liquid Interface Epithelial Cell Culture and siRNA Transfection
Primary HBEC obtained from bronchoscopic brushings were cultured under Air-liquid interface (ALI) as previously described.28, 29 From Day 0 of ALI, cells were stimulated with IL-13 (1ng/ml or 10ng/mL) (R&D systems, Minneapolis, MN, USA) or media alone every 48 hours for up to 8 days. IL-27 (1–100 ng/ml, R&D systems, Minneapolis, MN, USA) was added to media at Day 6. Cells were harvested for STAT proteins at 0, 30min, 1 and 6hrs after IL-27 stimulation and for CXCL9 mRNA at 12, 24, and 48hrs after IL-27 stimulation. Lower chamber media was harvested at 12, 24, and 48hrs after IL-27 stimulation for CXCL9 protein.
siRNA transfection were performed as previously described.29 300 μl siRNA mixture (50nM siRNA and 3 μl Mirus transfection reagent) was added to the upper chamber and transferred to the lower chamber 6 hours later. 48 hours later, IL-27 was added to the lower chamber. Cells and supernatants were harvested 24 hours after addition of IL-27.
SDS-PAGE and Western Blotting
Expression and activation of signal transducer and activator of transcription (STAT) in cultured epithelial cells were measured by Western blot on 4–12% SDS-PAGE gels using rabbit polyclonal antibodies for STAT1, p-STAT1, STAT3 and p-STAT3 (1:500; all from Cell Signaling Technology, Danvers, MA, USA).
CXCL9 ELISA
CXCL9 protein was measured in lower ALI supernatants by ELISA (detection limit 20 pg/ml) using R&D Systems antibody (Minneapolis, MN) by ELISAtech (Aurora, CO, USA). Undetectable CXCL9 protein was reported as 2 pg/ml.
Statistical Analysis
Data were analyzed for normality, with and without log transformation. Analysis of variance (ANOVA) (for normally distributed data) or Kruskal Wallis variation of ANOVA (for nonparametric data) was utilized to identify an overall level of significance among the groups or conditions. When overall p-value was <0.05, Tukey’s (for parametric), Wilcoxon or Chi-Square analysis (categorical data) of individual groups was performed. Bonferroni correction was applied for all intergroup comparisons. Spearman’s rho (rs) was used for correlations of nonparametric data and Pearson correlation for normally distributed data. Pearson’s Χ2 tests compared categorical values. Analysis of Covariance (ANCOVA) adjusted for age, body mass index and gender to evaluate the association of asthma severity with IL-27 expression. Blood eosinophils cut-off was ≥300 cells/μl.26 Median splits defined high vs. low levels of IL-27 mRNA (1.733 IL-27 indexed to GAPDH) and CCL26 mRNA (0.185 CCL26 indexed to GAPDH). Participants were subgrouped by IL-27 and Type-2 signature category into 4 groups, with epithelial CCL26 mRNA used as the biomarker for a Type-2 signature.3, 30 Groups were defined as Low IL-27 and Low Type-2 (CCL26), Low IL-27 and High Type-2, High IL-27 and Low Type-2 and High IL-27 and High Type-2. For in vitro studies, nonparametric signed-rank paired tests compared CXCL9 mRNA/protein in response to scramble or STAT1/3 siRNA. Statistical analysis was performed with JMP SAS software (SAS Institute, Cary, NC), and p-values <0.05 were considered significant except as noted.
Results
Demographics
Bronchial samples were obtained from 87 asthmatic and HC participants. The groups did not differ by race (Table I), although there were more females in all the asthmatic groups, as compared to the HCs. SAs were older (overall p <0.001) and had a higher body mass index compared with HCs (overall p=0.001) (Table I). All asthmatic groups had a higher percentage of females than the HC group and were more likely to be atopic with higher IgE and blood eosinophils (Table I). As expected, SA participants had the lowest FEV1% predicted despite 75% using systemic corticosteroids (CS) on regular basis.
Table I.
Baseline demographic characteristics (n=87)
| Subject group (n) | Healthy Controls (26) | Mild/no ICS (n=16) | Mild-Mod/ICS (n=16) | Severe Asthma (n=29) | Overall Difference (p-value) |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (y) | 27 (24–35) | 24 (20–31) | 31 (25–44) | 43 (34–55) | <0.001 |
| Sex (male/female) | 15/11 | 5/11 | 3/13 | 8/21 | 0.038 |
| Race (CA/AA/other) | 20/1/5 | 11/2/3 | 7/7/2 | 23/4/2 | 0.12 |
| BMI (kg/m2) | 24 (22–29) | 27 (24–31) | 27 (26–34) | 32 (26–37) | 0.001 |
| Exhaled NO (ppb) | 22 (15–40) | 40 (26–54) | 25 (16–35) | 38 (22–75) | 0.01 |
| Blood eosinophils (/ul) | 100 (100–200) | 200 (100–300) | 200 (100–300) | 300 (100–500) | 0.04 |
| Baseline FEV1 (%pred) | 95 (90–105) | 89 (85–97) | 89 (66–104) | 59 (45–71) | <0.001 |
| Serum IgE(kU/L) | 31 (15–83) | 98 (52–334) | 142 (42–430) | 172 (38–600) | <0.001 |
Definition of abbreviations: CA=Caucasians,; AA= African American; BMI= body mass index; %pred=percent predicted; IgE= immunoglobulin E; ppb= parts per billion. Categorical analyses were performed using Pearson chi-square tests. Continuous variables were analyzed using Kruskal-Wallis tests and presented as medians (25th–75th percentile).
Cells/samples for each outcome were not available from each participant. The online supplement contains tables for each of the substudies (Supplemental Tables I–II). Thirty-nine of 51 participants in the IHC subgroup overlapped and generally mirrored the larger mRNA group (Supplemental Table I). However, in contrast to the larger mRNA group, there were no differences in blood eosinophils.
IL-27 expression is increased in BAL cells from Type-2 asthmatic participants
IL-27 mRNA
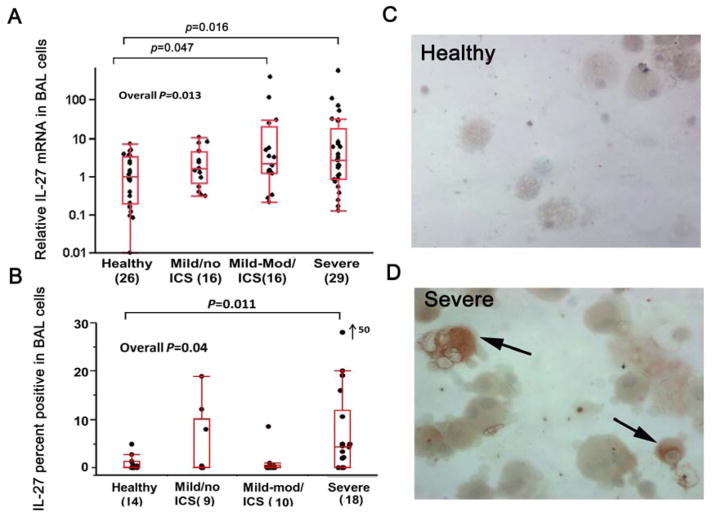
BAL cell IL-27 mRNA differed among the groups (overall p=0.013) and was higher in SA and Mild-Mod/ICS as compared with HCs, which remained significant when correcting for age, BMI and gender (Figure 1A).
Figure 1.
A. Relative IL-27 mRNA in BAL cells by qRT-PCR reference to GAPDH; B. Comparison of the percentage of IL-27 positive cells in BAL cytospins, as determined by means of IHC; C and D. Cytospin preparations in a Healthy (C) and a SA (D). Arrows=IL-27 positive monocyte-macrophages. Panels are shown at ×40 magnification
IL-27 protein (by IHC)
The percentage of IL-27 positive cells also differed by participant group, with SA participants having the highest overall percent, which remained significant when correcting for age, BMI and gender (p=0.011) (Figure 1B). The percent of IL-27 positive cells moderately correlated with BAL cell IL-27 mRNA (rs=0.38, p=0.017 for all; rs=0.44, p=0.018 for asthmatics). IL-27 protein appeared to be mainly expressed in monocyte-macrophages (Figure 1C and D).
Relation of IL-27 expression to markers of Type-2 inflammation
IL-27 mRNA and protein (IHC) were higher in participants with high blood eosinophils (identified as >300 cells/μl)26 (p<0.001 and p=0.002, respectively). IL-27 mRNA was also higher (2.67(1.10–17.69) vs. 1.34(0.40–3.32), p=0.02) in participants with high epithelial CCL26 mRNA (as defined by median split), but there was no difference in IL-27 protein.
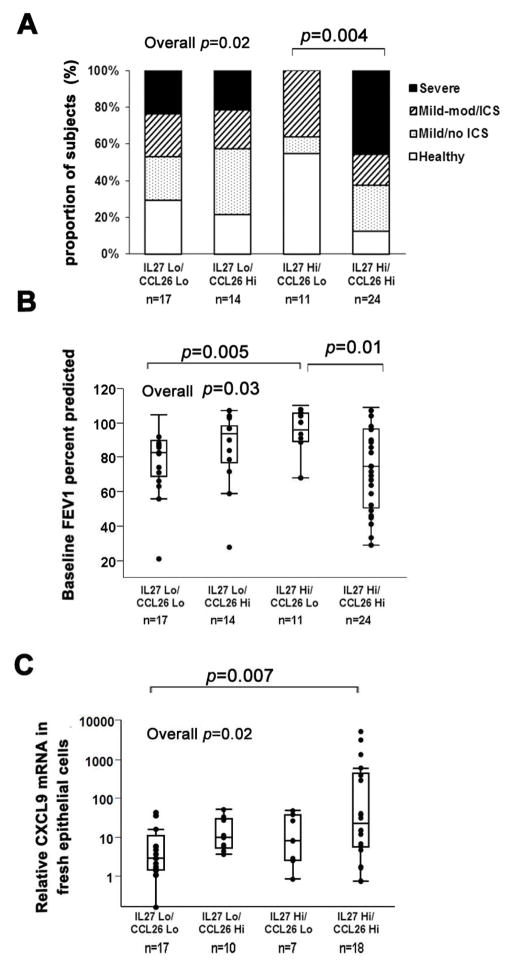
IL-27 in combination with an epithelial Type-2 cytokine gene signature (High CCL26) is associated with more severe asthma
As described in the methods, participants were divided into 4 subgroups: Low IL-27/Low Type-2, Low IL-27/High Type-2, High IL-27/Low Type-2 and High IL-27/High Type-2.3 Sixty-six of the 87 participants with IL-27 mRNA data had epithelial CCL26 mRNA data available and demographics of this subgroup did not differ from the larger parent group (Table II). There was nearly a 2-fold increase in the percent of traditionally defined SA when both IL-27 and CCL26 were high (IL-27-Hi/Type-2-Hi subgroup) compared to all other groups (40% compared to 24% in the IL-27-Lo/Type-2-Lo group and 21% in the IL-27-Lo/Type-2-Hi group). Interestingly, there were no SA participants in the IL-27-Hi/Type-2-Lo group (overall p=0.02) (Figure 2A), and this group had the best FEV1 % predicted, while the IL-27-Hi/Type-2-Hi group had the lowest one (overall p=0.03) (Figure 2B). The IL-27-Hi/Type-2-Hi group was more likely to chronically use oral CS (47.6%) compared to none in the IL-27-Hi/Type-2-Lo group (overall p=0.01, IL-27-Hi/Type-2-Lo vs. IL-27-Hi/Type-2-Hi p=0.002). The IL-27 Hi/Type-2-Hi subgroup also expressed higher amounts of the Type-1 associated chemokine CXCL9 in freshly brushed epithelial cells (overall p=0.02) (Figure 2C).
Table II.
Subject demographic characteristics by BAL IL-27 mRNA and epithelial CCL26 mRNA (n=66)
| Subject group (n) | low IL-27/low CCL26 (17) | low IL-27/high CCL26 (14) | high IL-27/low CCL26 (11) | high IL-27/high CCL26 (24) | Overall Difference (p-value) |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (y) | 35 (21–47) | 33 (27–41) | 26 (24–31) | 37 (27–57) | 0.23 |
| Sex (male/female) | 7/10 | 5/8 | 6/5 | 4/18 | 0.16 |
| Race (CA/AA/other) | 12/4/1 | 6/3/4 | 10/0/1 | 16/5/1 | 0.13 |
| BMI (kg/m2) | 31 (22–39) | 28 (25–32) | 25 (23–28) | 28 (24–34) | 0.36 |
| Exhaled NO (ppb) | 25 (19–38) | 32 (15–42) | 21 (15–46) | 37 (31–76) | 0.03 |
| Blood eosinophils/μl | 200 (100–275) | 150 (100–275) | 200 (100–300) | 300 (175–500) | 0.15 |
| Serum IgE (kU/L)* | 90 (23–479) | 206 (81–359) | 42 (27–113) | 69 (25–461) | 0.14 |
| OCS (% yes) | 18 | 18 | 0 | 48 | 0.01 |
Definition of abbreviations: CA=Caucasians; AA= African American; BMI= body mass index; OCS = inhaled corticosteroids; IgE= immunoglobulin E; ppb= parts per billion. Categorical analyses were performed using Pearson chi-square tests. Continuous variables analyzed using Kruskal/Wallis tests and presented as medians (25th–75th percentile).
Figure 2.
A. Proportion of traditional disease severities by molecular subgroups defined by BAL IL-27 mRNA and epithelial CCL26 mRNA. B. Baseline FEV1 percent predicted among groups by BAL IL-27 and epithelial CCL26. C, Relative CXCL9 mRNA on log scale in freshly brushed epithelial cells among groups by BAL IL-27 and epithelial CCL26.
IL-13 augments IL-27 induced production of CXCL9 by HBEC in vitro
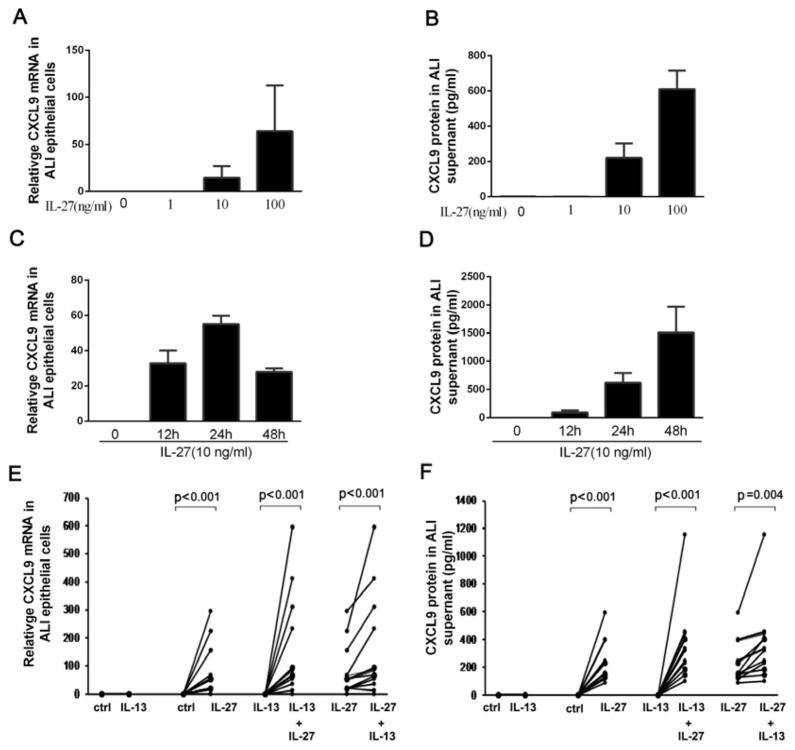
As participants with a combined IL-27 and Type-2 airway signature manifested the most severe asthma, in association with the highest expression of airway CXCL9, we hypothesized that IL-13 and IL-27 would synergistically increase CXCL9 expression by primary human airway epithelial cells. IL-27 alone dose dependently induced CXCL9 mRNA and protein from HBEC (in ALI) (n=6, p<0.001) (Figure 3A, 3B), consistent with a previous report.31 IL-27 induced CXCL9 mRNA production peaked at 24 hours (n=4, p<0.001) (Figure 3C), later than previously reported in epithelial cell lines.32 IL-13 alone did not generate CXCL9 expression. However, pretreatment of primary epithelial cells with low dose IL-13 (1ng/ml) for eight days synergistically increased CXCL9 mRNA and protein at 24h over that of either cytokine alone (mRNA p<0.001 n=13 and protein p=0.004 n=13) (Figure 3E, 3F). In contrast when epithelial cells were treated with high dose IL-13 (10ng/ml), CXCL9 mRNA/protein were not augmented as compared to IL-27 alone (n=4) (supplemental Figure 1).
Figure 3.
A. Dose response for IL-27 induced CXCL9 mRNA and B. protein in primary epithelial cells (n=6). C. Time course for IL-27 (10 ng/ml) induced CXCL9 mRNA production, CXCL9 mRNA peaked at 24 hours and D. CXCL9 protein was continuously generated through 48 hours (n=4). E. Pretreatment with IL-13 (for 8 days) did not induce CXCL9 expression. However, IL-13 pretreatment (1 ng/ml for 8 days) in combination with IL-27 (10 ng/ml for 24 hours) increased CXCL9 mRNA and F. protein compared to IL-27 or IL-13 alone in ALI cultured epithelial cells (n=13).
Amplified STAT1 signaling augments CXCL9 production by IL-27 on Type-2 cytokine background
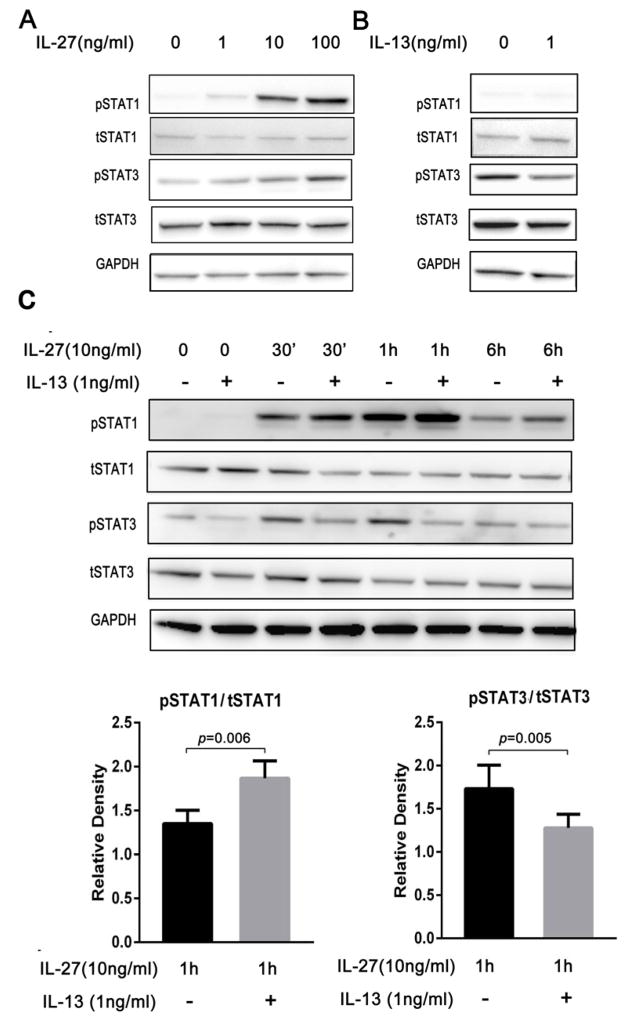
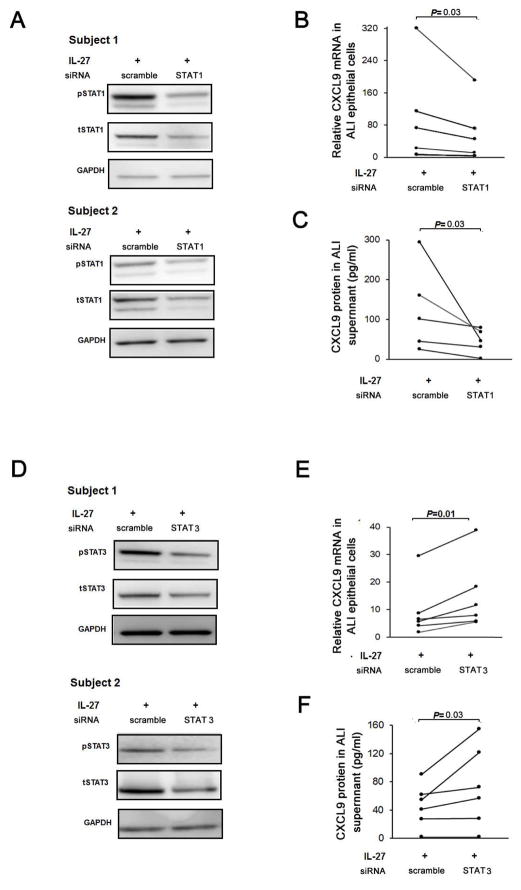
STAT1 signaling is believed critical for CXCL9 production.33 IL-27 significantly increased STAT1 phosphorylation in primary HBECs, with the greatest activation 1 hr post stimulation, later than that reported in cell lines (Figure 4A, 4C).21 IL-27 induced phosphorylation of STAT1 expression was higher in HBECs pretreated with low dose IL-13 as compared to those treated with IL-27 alone (Figure 4C). STAT1 siRNA knockdown decreased IL-27 induced CXCL9 mRNA and protein in HBECs confirming its importance for IL-27 induced CXCL9 (Figure 5). Thus, enhanced activation of STAT1 in the presence of IL-13 and IL-27 contributes to the synergistic increase in CXCL9.
Figure 4.
A. IL-27 activated both p-STAT1 and p-STAT3 in primary epithelial cells in a dose response manner 1 hr after stimulation (n=6). B. IL-13 decreased p-STAT3 activation, while having no effect on STAT1 (n=6). C. IL-27(10ng/ml) in combination with IL-13(1ng/ml) augmented p-STAT1 activation, while p-STAT3 decreased compared with IL-27 alone (n=10).
Figure 5.
A. Epithelial cells in ALI transfected with STAT1 siRNA and stimulated by IL-27 10ng/ml for 24 hours demonstrate knockdown of both phospho and total STAT1. B. siRNA for STAT1 decreased CXCL9 mRNA (n=5) and C. protein (n=5), nonparametric signed-rank paired tests. D. Epithelial cells in ALI transfected with STAT3 siRNA and stimulated by IL-27 10ng/ml for 24 hours demonstrate knockdown of both phospho and total STAT3. E. siRNA for STAT3 decreased CXCL9 mRNA (n=6) and F. protein (n=6). All data analyzed using nonparametric signed-rank paired tests.
Decreased STAT3 activation contributes to the IL-13 induced synergistic increase in IL-27 stimulated CXCL9 production
In addition to STAT1 activation, IL-27 increased activation/phosphorylation of STAT3. In contrast, IL-13 decreased activation of STAT3 compared to media alone (Figure 4B). Unlike the enhanced activation of STAT1 by IL-13 in combination with IL-27, IL-13 decreased IL-27 induced phosphorylation of STAT3 (Figure 4C), suggesting that increased activation of STAT1 in the presence of reduced activation of STAT3 contributes to the synergistic increase in CXCL9 expression in response to IL-13 and IL-27. In support of this hypothesis, STAT3 siRNA knockdown of STAT-3 in the presence of IL-27 alone also increased CXCL9 expression (Figure 5).
Discussion
Asthma is increasingly recognized as encompassing various complex phenotypes and, at least in a subset, includes the presence of a Type-2 cytokine signature. In this study, IL-27, an innate cytokine with diverse effects on adaptive immune pathways, was demonstrated to be increased in severe asthma, as well as in participants with an enhanced epithelial Type-2 signature. Asthma patients with high levels of IL-27 in the presence of a Type-2 signature (as manifested by epithelial eotaxin-3/CCL26 expression), represent a subtype of asthma manifested by the worst FEV1 and a greater likelihood of chronic oral CS use. The combination of high levels of IL-27 and CCL26 expression ex vivo was also associated with concomitant increases in expression of the Type-1 associated chemokine, CXCL9. These ex vivo findings were then recapitulated in vitro in primary HBEC. The upstream Type-2 cytokine, IL-13, in combination with IL-27 augmented expression of CXCL9 through a combination of effects on STAT1 and STAT3 activation. These findings suggest that Type-2 asthma phenotypes can by altered and even worsened by interactions with additional immune pathways.
Type-2 associated inflammation appears to identify approximately 50% of asthma patients.1 Several biomarkers are being linked to this phenotype, including eosinophils (blood and lung), fractional exhaled NO (FeNO), eotaxin-3/CCL26, CLCA1, periostin and others.1, 30 CCL26, a potent eosinophilic chemokine exclusive to humans, is strongly induced by Type-2 cytokines in vitro in epithelial cells. 1, 3, 34 Although epithelial CCL26 has been associated with Type-2 asthma, it is present across a range of asthma severities.1, 3, 4, 30 This suggests that additional immune-inflammatory processes influence development of severe asthma, including recently reported elements of Type-1 immunity.35 The data reported here add to that by showing that IL-27 mRNA, which has been associated with Type-1 immunity, is also increased in Type-2 asthma. However, importantly, this study went on to show that only when high levels of IL-27 were present in combination with a Type-2 signature (epithelial CCL26) was there an association with increasing severity of disease.
Direct comparison of the molecular phenotypes presented here with previously described clinical phenotypes/clusters is difficult. However, the increased severity, low lung function eosinophilia and high systemic CS use in the IL-27-Hi/Type-2-Hi cluster suggests overlap with Cluster 5 as defined by Moore et al36 and Cluster 6 by Wu et al. 37 Future unbiased clustering approaches incorporating molecular characteristics such as those reported here are needed.
The mechanisms and implications for the co-existence of IL-27, a Th1-like regulatory chemokine with Type-2 airway inflammation are unknown. As IL-27 has been reported to be increased by allergen stimulation,9 it is conceivable that IL-27 may be stimulated as a counter-regulatory cytokine to restrict Th2 inflammation.16,38 On the other hand, IL-27, perhaps triggered by viral infection, pollutants or even autoimmunity could contribute to triggering Type-1 immune processes adding complexity to an ongoing Type-2 process. In support of that hypothesis, participants in this study with elevations in IL-27 only (and a low Type-2 signature), had the mildest asthma severity including the best lung function and the least oral CS use. In contrast when associated with a high Type-2/CCL26 signature, the combined subgroup had the worst asthma severity. This association with worsening severity could be explained by high Type-2 inflammation impairing IL-27 mediated suppression of CD4+ cells, perhaps through diminished IL-10 production. 39, 40 Importantly, however, we also observed that participants with high IL-27 and CCL26 had evidence for increased levels of the Type-1 chemokine, CXCL9. This more complicated immune response, involving elements of Type-1, Type-2 immunity and IL-27 could also contribute to impaired CS responses and accompanying loss of asthma control.6
Given the association of IL-27 with Type-1 immunity it is not surprising that CXCL9, a CXC chemokine,41 was increased by IL-27 stimulation. CXCL9 has been reported to be increased during asthma exacerbations as well as during the late-phase of allergen challenge.41–43 While one may have hypothesized that in the presence of the CC chemokine CCL26, CXCL9 levels would be lower, we observed IL-27 in combination with the Type-2 signature gene CCL26 to be associated with higher CXCL9 levels. In contrast, participants with IL-27 high alone did not have high CXCL9 mRNA levels. While the mechanisms for these differences are not certain, previous studies suggest IL-27 behaves primarily as a regulatory cytokine, enhancing, rather than driving Th1 inflammation. Thus, the effects of IL-27 on CXCL9 in the absence of additional stimuli (Type 1 or 2) may be self-limited or countered by other immune pathways.8
This surprising ex vivo observation of high CXCL9 levels in the presence of IL-27 and CCL26 led us to evaluate the impact of IL-13 and IL-27 on human airway epithelial cells in vitro. Intriguingly, IL-27 has been reported to act synergistically with other cytokines, particularly TNF-α, such that synergistic effects with IL-13 may not be surprising.20,45,46 As expected, IL-27 stimulated CXCL9 secretion, while IL-13 (at low or high dose) had no impact on CXCL9. However, when epithelial cells were treated with IL-13 at low dose (1 ng/ml) and IL-27 added, there was a surprising synergistic increase in CXCL9 mRNA and protein. These low doses of IL-13 are likely closer to physiologically relevant levels, compared the typically used in vitro dose.47–49 Intriguingly, the high (as opposed to low) dose of IL-13 did not augment CXCL9 expression confirming the complexity of these pathway interactions.
This surprising augmentation of CXCL9 expression led to further investigations to understand its mechanisms. An siRNA approach confirmed that IL-27 induced CXCL9 through effects on the STAT1 signaling pathway. Interestingly, the presence of IL-13 enhanced the IL-27 induced phosphorylation of STAT1, which would increase CXCL9 production. On the other hand, IL-27 also activated STAT3 50, which has been suggested to serve as a counterbalance to STAT1.51–54 STAT3 may restrict STAT1 to avoid over activation of Type-1 signaling induced by IL-27. In the case of CXCL9 this could limit its expression. In the experiments reported here, IL-13 alone (compared to media) or in combination with IL-27 (compared to IL-27 alone) decreased STAT3 phosphorylation, suggesting IL-13 may limit the ability of STAT3 to inhibit CXCL9 expression. The ability of STAT3 to regulate CXCL9 expression was then confirmed by STAT3 siRNA knockdown. Similar to the combination of IL-13 and IL-27, IL-27 stimulation (alone), in the presence of STAT3 siRNA knockdown, further enhanced expression of CXCL9.
This is the first report of which we are aware that demonstrates IL-13 inhibition of STAT3 activation in primary HBEC. In contrast to our findings, several previous studies reported increased STAT3 phosphorylation in the presence of IL-13.41, 55–57 However, in human Th17 cells, IL-13 reportedly attenuated STAT3 activation.58 Therefore we speculate that the STAT3 response is cell and environment specific.59 In the case of HBECs, IL-13’s inhibition of STAT3 activation could serve to augment production of cytokines/chemokine either alone 60 or in combination with IL-27. The mechanisms for this ability of STAT3 activation to limit CXCL9 expression are not yet clear, but could include competition of the STAT3:STAT1 heterodimer with the STAT1 homodimer for STAT1 signaling or STAT1-indpendent mechanisms.61 STAT1 and 3 are in the same signaling family as STAT6, widely believed to be the primary signaling pathway activated by IL-13. Whether STAT6 interferes with STAT3 activation remains to be determined. Indeed, it might be helpful to model the interactions of these pathways under IL-27 and Type-2 cytokine stimulation in other systems, including transgenic mice.
Limitations of this study include the unavailability of all samples for all experiments, due to considerable demands whilst very small sample quantities. However, in each of the additional ex vivo substudies, over 75% of participants overlapped with the BAL cell IL-27 mRNA participants and the demographics of the substudy groups differed only marginally. In this report, we used IL-27 mRNA based on its more quantitative (and less subjective measures than IHC) to define high/low IL-27 levels, but protein levels are ultimately also likely to be more important. The ability of siRNA transfection to completely knockdown targets in fully differentiated primary epithelial cells remains limited. Therefore, siRNA results are based on moderate and variable effects. However, consistent decreases in target mRNA/protein expression were still observed. Finally, we used CCL26 mRNA to identify a Type-2 signature ex vivo., as opposed to directly measuring the upstream IL-13, given the low expression levels of IL-13 in CS-treated patients.
In summary, this study presents evidence for increased IL-27 in asthma. Particularly when its expression is associated with a Type-2/IL-13 gene signature, it identifies a subtype of asthma with more severe asthma characteristics, associated with increased Type-1 chemokine/CXCL9 expression. This surprising association with increased CXCL9 could be recapitulated in vitro involving enhanced STAT1 activation with associated reduction in STAT3 activation. These novel interactions of innate immune (IL-27) and Type-2 immunity (IL-13) could contribute to the increasingly complex inflammatory processes associated with severe asthma. Further investigation into mechanisms regulating the enhanced IL-27 production and the interactions with both Type 2 and Type 1 immunity could lead to novel targets for severe asthma therapy.
Supplementary Material
Key messages.
IL-27 expression is increased in BAL cells of asthma patients, identifying a more severe asthma phenotype when combined with a Type-2 (IL-13 associated) gene signature as represented by CCL26 expression.
These ex vivo findings were recapitulated in vitro in primary HBECs where IL-13 in combination with IL-27 augmented expression of the Th1-associated chemokine, CXCL9.
The synergistic increase in CXCL9 appears to involve enhanced STAT1 signaling, with diminished counter-regulation by STAT3.
Acknowledgments
Declaration of all sources of funding: National Institute of Health, National Heart Lung and Blood Institute, HL109152, HL064937, AI040600 and RR024153.
We thank Xiuxia Zhou, Jinming Zhao, Russell Traister, Milosevic Jadranka and Merritt Fajt for their helpful contributions and Crystal E. Uvalle for her excellent technical assistance.
Abbreviations used
- ALI
Air-liquid interface
- ATS
American Thoracic Society
- FeNO
Fractional exhaled nitric oxide
- FEV1
Forced expiratory volume in 1 second
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HBEC
Human bronchial epithelial cells
- BAL
Bronchoalveolar lavage
- HC
Healthy control
- SA
Severe asthma
- CS
corticosteroid
- ICS
Inhaled corticosteroid
- OCS
Oral corticosteroid
- STAT1
signal transducer and activator of transcription 1
- STAT3
signal transducer and activator of transcription 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–25. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 3.Coleman JM, Naik C, Holguin F, Ray A, Ray P, Trudeau JB, et al. Epithelial eotaxin-2 and eotaxin-3 expression: relation to asthma severity, luminal eosinophilia and age at onset. Thorax. 2012;67:1061–6. doi: 10.1136/thoraxjnl-2012-201634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto M, Tochino Y, Chibana K, Trudeau JB, Holguin F, Wenzel SE. Nitric oxide and related enzymes in asthma: relation to severity, enzyme function and inflammation. Clin Exp Allergy. 2012;42:760–8. doi: 10.1111/j.1365-2222.2011.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, et al. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175:2191–200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- 6.Li JJ, Wang W, Baines KJ, Bowden NA, Hansbro PM, Gibson PG, et al. IL-27/IFN-gamma induce MyD88-dependent steroid-resistant airway hyperresponsiveness by inhibiting glucocorticoid signaling in macrophages. J Immunol. 2010;185:4401–9. doi: 10.4049/jimmunol.1001039. [DOI] [PubMed] [Google Scholar]
- 7.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 8.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 9.Nieminen K, Valovirta E, Savolainen J. Clinical outcome and IL-17, IL-23, IL-27 and FOXP3 expression in peripheral blood mononuclear cells of pollen-allergic children during sublingual immunotherapy. Pediatr Allergy Immunol. 2010;21:e174–84. doi: 10.1111/j.1399-3038.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 10.Wittmann M, Zeitvogel J, Wang D, Werfel T. IL-27 is expressed in chronic human eczematous skin lesions and stimulates human keratinocytes. J Allergy Clin Immunol. 2009;124:81–9. doi: 10.1016/j.jaci.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Chae S-C, Li C-S, Kim KM, Yang JY, Zhang Q, Lee Y-C, et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. Journal of Human Genetics. 2007;52:355–61. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180:922–30. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 13.Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev. 2004;202:106–14. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- 14.Larousserie F, Pflanz S, Coulomb-L’Hermine A, Brousse N, Kastelein R, Devergne O. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202:164–71. doi: 10.1002/path.1508. [DOI] [PubMed] [Google Scholar]
- 15.Summers SA, Phoon RK, Ooi JD, Holdsworth SR, Kitching AR. The IL-27 receptor has biphasic effects in crescentic glomerulonephritis mediated through Th1 responses. Am J Pathol. 2011;178:580–90. doi: 10.1016/j.ajpath.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–23. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 17.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–16. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 18.Cardinale F, Giordano P, Chinellato I, Tesse R. Respiratory epithelial imbalances in asthma pathophysiology. Allergy Asthma Proc. 2013;34:143–9. doi: 10.2500/aap.2013.34.3631. [DOI] [PubMed] [Google Scholar]
- 19.Holgate ST, Davies DE, Puddicombe S, Richter A, Lackie P, Lordan J, et al. Mechanisms of airway epithelial damage: epithelial-mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl. 2003;44:24s–9s. doi: 10.1183/09031936.03.00000803. [DOI] [PubMed] [Google Scholar]
- 20.Cao J, Wong CK, Yin Y, Lam CW. Activation of human bronchial epithelial cells by inflammatory cytokines IL-27 and TNF-alpha: implications for immunopathophysiology of airway inflammation. J Cell Physiol. 2010;223:788–97. doi: 10.1002/jcp.22094. [DOI] [PubMed] [Google Scholar]
- 21.Diegelmann J, Olszak T, Goke B, Blumberg RS, Brand S. A Novel Role for Interleukin-27 (IL-27) as Mediator of Intestinal Epithelial Barrier Protection Mediated via Differential Signal Transducer and Activator of Transcription (STAT) Protein Signaling and Induction of Antibacterial and Anti-inflammatory Proteins. Journal of Biological Chemistry. 2011;287:286–98. doi: 10.1074/jbc.M111.294355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281:14111–8. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- 23.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors. November 1986. Am Rev Respir Dis. 1987;136:225–44. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 24.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162:2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 25.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D2 pathway upregulation: Relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol. 2013;131:1504–12. e12. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chibana K, Trudeau JB, Mustovich AT, Hu H, Zhao J, Balzar S, et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38:936–46. doi: 10.1111/j.1365-2222.2008.02969.x. [DOI] [PubMed] [Google Scholar]
- 28.Trudeau J, Hu H, Chibana K, Chu HW, Westcott JY, Wenzel SE. Selective downregulation of prostaglandin E2-related pathways by the Th2 cytokine IL-13. J Allergy Clin Immunol. 2006;117:1446–54. doi: 10.1016/j.jaci.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Maskrey B, Balzar S, Chibana K, Mustovich A, Hu H, et al. Interleukin-13-induced MUC5AC is regulated by 15-lipoxygenase 1 pathway in human bronchial epithelial cells. Am J Respir Crit Care Med. 2009;179:782–90. doi: 10.1164/rccm.200811-1744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravensberg AJ, Ricciardolo FL, van Schadewijk A, Rabe KF, Sterk PJ, Hiemstra PS, et al. Eotaxin-2 and eotaxin-3 expression is associated with persistent eosinophilic bronchial inflammation in patients with asthma after allergen challenge. J Allergy Clin Immunol. 2005;115:779–85. doi: 10.1016/j.jaci.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Shibata S, Tada Y, Asano Y, Yanaba K, Sugaya M, Kadono T, et al. IL-27 activates Th1-mediated responses in imiquimod-induced psoriasis-like skin lesions. J Invest Dermatol. 2013;133:479–88. doi: 10.1038/jid.2012.313. [DOI] [PubMed] [Google Scholar]
- 32.Cao J, Zhang L, Li D, Xu F, Huang S, Xiang Y, et al. IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10. Chest. 2012;141:121–30. doi: 10.1378/chest.10-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008;180:6325–33. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- 34.van Wetering S, Zuyderduyn S, Ninaber DK, van Sterkenburg MA, Rabe KF, Hiemstra PS. Epithelial differentiation is a determinant in the production of eotaxin-2 and -3 by bronchial epithelial cells in response to IL-4 and IL-13. Mol Immunol. 2007;44:803–11. doi: 10.1016/j.molimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, et al. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133:1280–1288. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujita H, Teng A, Nozawa R, Takamoto-Matsui Y, Katagiri-Matsumura H, Ikezawa Z, Ishii Y. Production of both IL-27 and IFN-gamma after the treatment with a ligand for invariant NK T cells is responsible for the suppression of Th2 response and allergic inflammation in a mouse experimental asthma model. J Immunol. 2009;183:254–260. doi: 10.4049/jimmunol.0800520. [DOI] [PubMed] [Google Scholar]
- 39.Freitas do Rosario AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012;188:1178–90. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Wang S, Erekosima N, Li Y, Hong J, Qi X, et al. IL-4 confers resistance to IL-27-mediated suppression on CD4+ T cells by impairing signal transducer and activator of transcription 1 signaling. J Allergy Clin Immunol. 2013;132:912–21. e1–5. doi: 10.1016/j.jaci.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shahabuddin S, Ji R, Wang P, Brailoiu E, Dun N, Yang Y, et al. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol. 2006;291:C34–9. doi: 10.1152/ajpcell.00441.2005. [DOI] [PubMed] [Google Scholar]
- 42.Lai ST, Hung CH, Hua YM, Hsu SH, Jong YJ, Suen JL. T-helper 1-related chemokines in the exacerbation of childhood asthma. Pediatr Int. 2008;50:99–102. doi: 10.1111/j.1442-200X.2007.02533.x. [DOI] [PubMed] [Google Scholar]
- 43.Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005;11:35–42. doi: 10.1097/01.mcp.0000144502.50149.e0. [DOI] [PubMed] [Google Scholar]
- 44.Liu L, Jarjour NN, Busse WW, Kelly EA. Enhanced generation of helper T type 1 and 2 chemokines in allergen-induced asthma. Am J Respir Crit Care Med. 2004;169:1118–24. doi: 10.1164/rccm.200312-1659OC. [DOI] [PubMed] [Google Scholar]
- 45.Dong S, Zhang X, He Y, Xu F, Li D, Xu W, Wang H, Yin Y, Cao J. Synergy of IL-27 and TNF-alpha in regulating CXCL10 expression in lung fibroblasts. Am J Resp cell mol. 2013;48:518–530. doi: 10.1165/rcmb.2012-0340OC. [DOI] [PubMed] [Google Scholar]
- 46.Wong CK, Chen da P, Tam LS, Li EK, Yin YB, Lam CW. Effects of inflammatory cytokine IL-27 on the activation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther. 2010;12:R129. doi: 10.1186/ar3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saha SK, Berry MA, Parker D, Siddiqui S, Morgan A, May R, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–91. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berry MA, Parker D, Neale N, Woodman L, Morgan A, Monk P, et al. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114:1106–9. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 49.Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2013;41:330–8. doi: 10.1183/09031936.00223411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interf Cytok Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 51.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, et al. Opposing roles of STAT1 and STAT3 in T cell–mediated hepatitis: regulation by SOCS. Journal of Clinical Investigation. 2002;110:1503–13. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem. 2004;279:41679–85. doi: 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]
- 53.Costa-Pereira AP, Tininini S, Strobl B, Alonzi T, Schlaak JF, Is’harc H, et al. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc Natl Acad Sci U S A. 2002;99:8043–7. doi: 10.1073/pnas.122236099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1:STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol. 2008;19:351–9. doi: 10.1016/j.semcdb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Xu B, Bhattacharjee A, Roy B, Xu HM, Anthony D, Frank DA, et al. Interleukin-13 induction of 15-lipoxygenase gene expression requires p38 mitogen-activated protein kinase-mediated serine 727 phosphorylation of Stat1 and Stat3. Mol Cell Biol. 2003;23:3918–28. doi: 10.1128/MCB.23.11.3918-3928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umeshita-Suyama R, Sugimoto R, Akaiwa M, Arima K, Yu B, Wada M, et al. Characterization of IL-4 and IL-13 signals dependent on the human IL-13 receptor α chain 1: redundancy of requirement of tyrosine residue for STAT3 activation. International immunology. 2000;12:1499–1509. doi: 10.1093/intimm/12.11.1499. [DOI] [PubMed] [Google Scholar]
- 57.Wery-Zennaro S, Letourneur M, David M, Bertoglio J, Pierre J. Binding of IL-4 to the IL-13Ralpha(1)/IL-4Ralpha receptor complex leads to STAT3 phosphorylation but not to its nuclear translocation. FEBS letters. 1999;464:91–96. doi: 10.1016/s0014-5793(99)01680-4. [DOI] [PubMed] [Google Scholar]
- 58.Newcomb DC, Boswell MG, Zhou W, Huckabee MM, Goleniewska K, Sevin CM, et al. Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J Allergy Clin Immunol. 2011;127:1006–13. e1–4. doi: 10.1016/j.jaci.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peters AT, Kato A, Zhang N, Conley DB, Suh L, Tancowny B, et al. Evidence for altered activity of the IL-6 pathway in chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology. 2010;125:397–403. e10. doi: 10.1016/j.jaci.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 61.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391–6. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.