Worldwide, more than 1 million men are diagnosed with prostate cancer each year and more than 300,000 die of the disease1. Current U.S. statistics show that either 1 in 5 or 1 in 6 men will be diagnosed with prostate cancer during their lifetime. With such a high incidence, should we be alarmed? What is a reasonable response to a risk of cancer as high as 1:5?
Although the number of men diagnosed with prostate cancer has been on the rise, the number of men dying from the disease has declined in recent years. Indeed, prostate cancer cases increased by 54% between 1975 and 2010, but mortality declined by 30% over the same period2. Prostate cancer is on the rise partly because of its correlation with age. Since the early 1800s, antiseptics, antibiotics, and vaccines—better health care all around—have more than doubled mean life expectancy for men, and prostate cancer risk is linked to age. No one doubts, however, that the rise in the number of newly diagnosed prostate cancer patients is also linked to improved diagnostics. Before the emergence of the prostate-specific antigen (psa) test in 1986, far more men who were found to have prostate cancer were diagnosed with incurable and advanced disease. In more financially developed regions of the world, the convenience and relatively low cost of the psa test has led physicians to encourage regular psa screening for their older patients, with subsequent biopsy. Prostate cancer incidence rates are highest in Australia, New Zealand, North America, and western and northern Europe1; in those places, men are now often diagnosed when the disease is asymptomatic. However, long-term data from two large randomized trials revealed either no or only a modest benefit from psa screening3,4. Indeed, analyses in the latter study of the effects of screening on prostate cancer mortality and on quality of life indicate that the benefit of psa screening is diminished because of overdiagnosis, overtreatment, and loss of life-years free of prostate cancer—that is, lead-time years5.
We now realize that with early detection comes early treatment, which requires balancing the risk of death from cancer with the risk of adverse effects from treatment. After radical prostatectomy, the percentage of patients experiencing moderate-to-severe urinary incontinence is about the same as the percentage of men who die from the disease, and more than half of all treated men can expect some persistent erectile dysfunction for a year or more after surgery6. The incidence of sexual side effects is about the same for patients who elect radiotherapy over surgery, although the effects develop more slowly.
So, how should physicians and patients react to a disease with a lifetime risk of 1:5?
When prostate cancer patients were asked to interpret various ways in which risk was presented, they favoured simple statistics such as absolute risk over other measures such as odds ratios and relative risk, which they found more difficult to understand7. However, simple risk ratios say nothing about the impact of the disease on either survival or quality of life. They also provide no information that could help a patient assess the benefits of treatment against the risks.
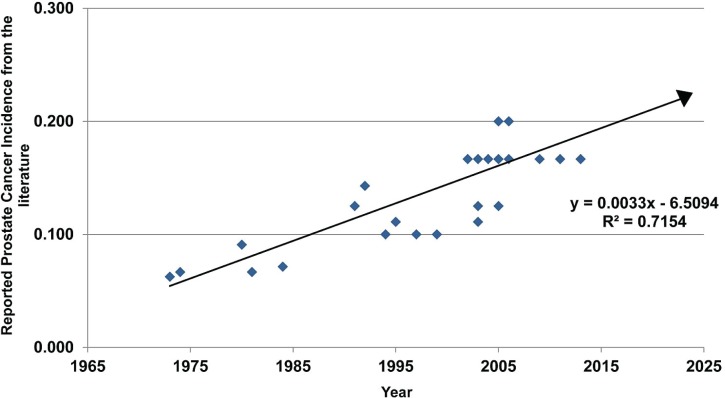
All of which brings us to the title of this essay. Figure 1 uses data pulled from the U.S. literature to plot the lifetime risk of a prostate cancer diagnosis. Our earliest data point comes from 1973. Back then, 1 man in 16 could expect to be diagnosed with the disease. Between 1973 and 2013, 38 available data points document the increase in the reported incidence of prostate cancer over 40 years.
FIGURE 1.
A plot of the likelihood of a man being diagnosed with prostate cancer, as reported in the literature during the 40 years between 1973 and 2013. A simple linear regression of the data (n = 38), when extrapolated into the future, suggests that by 2124 (in 110 years) half of all men can expect to diagnosed with prostate cancer in their lifetime. Extending the line further, by 2275 (in 261 years) every man can expect to be diagnosed with the disease at some time during his life. See the text for a discussion of how incidence data in the form 1:5, 1:2, and 1:1 (that is, every man having the disease) might influence fear of prostate cancer, compared with a willingness to accept the disease as a chronic condition.
Looking at the positive slope of the line in Figure 1, a question arises: At what future date can half of all men be expected to receive a diagnosis of prostate cancer during their lifetime? By extrapolation, a ratio of 1:2 is reached in just 110 years. Although that approximation is a rough one, we do not think it premature to ask how much more distressing a ratio of 1:2 will be if a ratio of 1:5 already seems distressingly high.
Our extrapolation is, of course, far enough into the future that dramatic changes in life expectancy (perhaps from better cures for prostate cancer) could make it of little consequence or interest. But working from a contemporary mindset, we believe that a prevalence of 1:2 will likely scare many men even more than a risk ratio of 1:5 does. The fear of having prostate cancer—or, for that matter, any cancer—seems to trump the suggestion that we are overusing diagnostics such as the psa test.
Epidemiologic data show only a modest overall benefit from psa testing. That observation led the U.S. Preventive Services Task Force to make their controversial 2012 recommendation to reduce testing. In October 2014, the Canadian Task Force on Preventive Health Care made a similar recommendation9. There is some evidence that the U.S. recommendation led to lesser psa testing by primary care physicians in that country, but patients under the care of urologists weren’t taken into consideration8.
Of men who are tested and found to have early prostate cancer, more and more are being offered active surveillance rather than radical treatments; however, factors inf luencing acceptance of and adherence to active surveillance have not been well explored10. Despite a strong push to have men view prostate cancer as a chronic illness like diabetes or hypertension, numbers such as 1:5—or worse, 1:2—are just too scary for many men to tolerate.
What, then, would it take to have men accept prostate cancer as a chronic disease to be lived with, rather than a life-threatening condition to be aggressively treated? If we ask the question “How far must the line in Figure 1 be extrapolated to arrive at a time when all men can expect to be diagnosed with prostate cancer,” the answer is 261 years. Such an extrapolation opens the way to speculation about how the attitude toward a prostate cancer diagnosis and its treatment might shift if men are informed that all of them will develop the disease.
We acknowledge that the linear regression in Figure 1 is overly simplistic; some curvilinear fit would be more realistic. Extrapolating as far forward as 261 years on just 40 years of data (and without any confidence intervals around those data) imparts little faith that the forecast year is particularly reliable. But the precise year when all men will be diagnosed with prostate cancer is not the issue. The fact that the line has a positive and significant slope affirms that the risk of a man developing prostate cancer is increasing and that all men, if they live long enough, can expect to get the disease.
More than 200 years might seem far off, particularly given that prostate cancer wasn’t even a definable disease 200 years ago. George Langstaff’s 1817 report of an abnormal prostate gland in a 68-year-old man with urinary obstruction was the first publication congruent with the modern understanding of adenocarcinoma of the prostate11. However, it wasn’t until 1853 that John Adams described the histopathology that formally characterizes the disease. What is relevant now is Adams’s assertion that the disease was “a great rarity”12. Notably, Langstaff’s and Adams’s patients were both quite old for men in the 19th century.
So, what was “a great rarity” in 1853—and was ostensibly unknown 200 years ago—is now the most common neoplasm in men. But men can respond quite differently to a condition that is common than to a condition that everyone has. Our guess is that, compared with a 1:5 or 1:2 ratio, a 1:1 ratio would lead to a very different perspective on prostate cancer.
The increasing incidence of prostate cancer is first and foremost a testimony to the overall improvement in health care since prostate cancer was first identified in the early 1800s. If prostate cancer isn’t yet viewed as a chronic disease that rarely warrants radical treatment, it is certainly heading in that direction. There is no need, though, to wait some 261 years to view it that way. Humanity could immediately benefit from accepting prostate cancer as a chronic illness that rarely needs treatment.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest and declare that we have none.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. globocan 2012: Estimated Cancer Incidence and Mortality Worldwide in 2012 [Web resource] Lyon, France: International Agency for Research on Cancer; 2013. Ver 1.0. [Google Scholar]
- 2.Esserman LJ, Thompson IM, Jr, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA. 2013;310:797–8. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijnsdijk EA, Wever EM, Auvinen A, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med. 2012;367:595–605. doi: 10.1056/NEJMoa1201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 7.Ilic D, Murphy K, Green S. Risk communication and prostate cancer: identifying which summary statistics are best understood by men. Am J Mens Health. 2012;6:497–504. doi: 10.1177/1557988312453616. [DOI] [PubMed] [Google Scholar]
- 8.Cohn JA, Wang CE, Lakeman JC, et al. Primary care physician psa screening practices before and after the final U.S. Preventive Services Task Force recommendation. Urol Oncol. 2014;32:41.e23–30. doi: 10.1016/j.urolonc.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Bell N, Gorber SC, Shane A, et al. on behalf of the Canadian Task Force on Preventive Health Care Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ. 2014;186:1225–34. doi: 10.1503/cmaj.140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penson DF. Factors influencing patients’ acceptance and adherence to active surveillance. J Natl Cancer Inst Monogr. 2012;45:207–12. doi: 10.1093/jncimonographs/lgs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langstaff G. Cases of fungus haematodes, with observations. Med Chir Trans. 1817;8:272–314. doi: 10.1177/095952871700800114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams J. A case of scirrhus of the prostate gland, with a corresponding affection of the lymphatic glands in the lumbar region and in the pelvis. Lancet. 1853;61:393–4. [Google Scholar]