INTRODUCTION
Approximately 9200 people in Canada die from colorectal cancer each year1, and about 20% of those tumours involve the rectum2. Surgery is the first-line treatment for nonmetastatic rectal cancer; the goal is complete removal of the tumour with no residual cancer cells left behind.
Rectal cancer care has become increasingly multidisciplinary. Most treatment guidelines suggest that neoadjuvant therapy, including chemotherapy and radiation, be considered in the treatment of stage ii and iii rectal cancer, both to shrink the tumour and to improve surgical and overall outcomes3.
The circumferential resection margin (crm), also called the radial margin, is very important in rectal cancer surgery, being a prognostic factor in patients who undergo such surgery. As recorded in the pathology report, a positive crm has been defined as a margin of normal tissue that is 1 mm or less from the edge of the original tumour, a measurement that comes from evidence-based guidelines4. A crm of less than 1 mm has been shown to be a strongly negative prognostic factor: specifically, it predicts for subsequent locoregional recurrence and poorer overall survival4.
Guidelines recommend negative margins (>1 mm) for all rectal cancer patients who undergo resection5,6, and the rate of crm positivity is widely used as a quality indicator in rectal cancer care.
Two major changes in rectal cancer treatment since the early 1990s are recognition of the importance of surgical technique (and specifically the adoption of total mesorectal excision) and increased use of neoadjuvant therapies. Those changes have contributed both to a reduction in positive crm rates and in local and regional recurrences. However, despite best efforts, concern remains about potential variations in rectal cancer treatment and outcomes.
The System Performance Initiative at the Canadian Partnership Against Cancer regularly reports on standardized indicators at a national level to help identify opportunities for pan-Canadian system improvements. Recognizing that surgical resection is generally recommended as first-line treatment for nonmetastatic disease, the first indicator presented in this rectal cancer surgery–focused report is the rectal cancer resection rate. The second indicator is the rate of crm positivity among patients undergoing rectal cancer resection. A better understanding of the data can help to identify best practices, which in turn can inform quality improvements.
METHODS
Patients with stage ii or iii rectal cancer who were diagnosed in 2009 and 2010 were identified in each of the provincial cancer registries using the relevant codes from the International Classification of Diseases for Oncology (3rd edition) and the recorded stage at diagnosis as outlined by the American Joint Committee on Cancer.
Patients undergoing rectal resection were identified either by codes for the relevant surgical procedures in the registries or by linkage to hospital records (depending on the province). Patients were included only if they had undergone resection (identified using relevant procedure codes from the Canadian Classification of Health Interventions) within 1 year of diagnosis. The detailed calculation methodology is provided in the Technical Appendix to the 2014 Cancer System Performance Report (http://www.cancerview.ca/idc/groups/public/documents/webcontent/sp_report_2014_tech_app.pdf).
Aggregated counts of rectal cancer cases, with the site-specific factor for crm status, were retrieved from Canadian Cancer Registry using real-time remote access (managed by Statistics Canada). Data for rectal cancer patients 20 years of age and older who were diagnosed in 2010 were included in the analysis. To prevent the possibility that statistical data would be associated with any identifiable individual, the counts were randomly rounded either up or down to a multiple of 5 before release. The impact of this random rounding is typically greater for smaller provinces because of smaller case counts. Data for some provinces are omitted because of deviations from the indicator specifications that affected data comparability with other provinces.
RESULTS
Rates of Rectal Cancer Resection
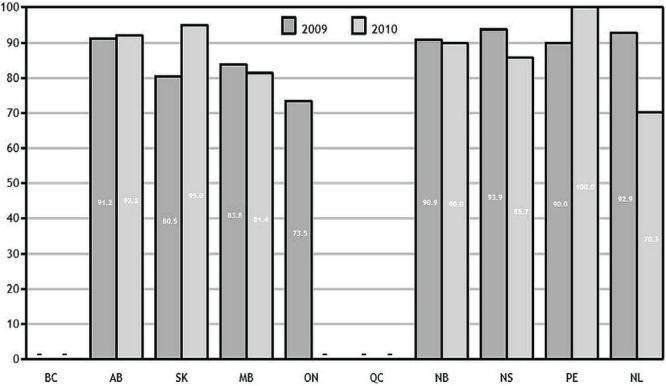
Interprovincial variation, ranging from 70.3% to 100% in 2010 (Figure 1), was observed for the percentage of patients who underwent surgical resection for rectal cancer over the two study years. Small case volumes are likely causing year-to-year fluctuations in the smaller provinces (Prince Edward Island and Newfoundland and Labrador). British Columbia data included only cases referred to provincial cancer centres (through the 2010 diagnosis year).
FIGURE 1.
Percentage of stage ii or iii rectal cancer patients diagnosed in 2009 and 2010 who had a surgical resection within 1 year of diagnosis, by province. Data for Quebec and British Columbia and 2010 data for Ontario not available. Data source: provincial cancer agencies.
| Diagnosis year |
Patients by province
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | |
| 2009 | — | 308 | 123 | 173 | 1283 | — | 121 | 131 | 20 | 84 |
| 2010 | — | 320 | 119 | 156 | — | — | 110 | 147 | 9 | 91 |
Examining resection rates by age and sex helps to identify demographic variations in the use of, or access to, surgery. Older patients diagnosed in 2010 were less likely than younger patients to receive rectal surgery. The disparity was especially true for women undergoing surgery for rectal cancer: the difference in the resection rate for women younger and older than 70 years was about 15 percentage points (data not shown).
Rates of Positive CRM
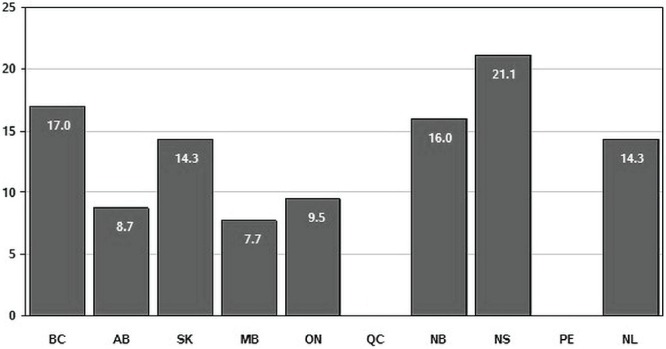
The percentage of rectal cancer cases with a positive crm ranged from a low of 7.7% in Manitoba to a high of 21.1% in Nova Scotia (Figure 2). The wide range persisted even when the comparison was limited to larger provinces with higher volumes of rectal surgery. For example, the positive margin rate in British Columbia was nearly twice the rate reported in Alberta (17.0% and 8.7% respectively).
FIGURE 2.
Percentage of invasive rectal cancer resections (2010 diagnosis year, patients 20 years of age and older) with a positive circumferential margin, by province. Data for Quebec not available, and data for Prince Edward Island supressed because of statistical unreliability owing to small numbers. Data source: Statistics Canada, Canadian Cancer Registry.
| Diagnosis year |
Resections by province
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC | AB | SK | MB | ON | QC | NB | NS | PE | NL | |
| 2010 | 500 | 345 | 105 | 130 | 1050 | — | 125 | 95 | — | 105 |
DISCUSSION AND CONCLUSIONS
Reporting on rates of rectal resection and crm positivity is intended to prompt review of how rectal cancers are treated across Canada and to quantify the extent of positive crm findings. Interprovincial variation can reflect differences in measurement, but the scale of the variations observed here probably reflects actual differences in practice between provinces.
Rate of Resection
Data concerning surgical resection for stage ii or iii rectal cancer are comparable in Canada and the United States, being approximately 80% in both countries7. Consistent with the findings reported here, a population-based study in the United States reported lower rates of resection for stage ii or iii rectal cancer patients who were 75 and older than for those who were younger8–10. The methods used by the provinces to extract and collect the relevant data (that is, linking registry data with discharge abstract databases) could be contributing to the observed interprovincial variation in resection rates.
It is important to note that patterns of care might reflect combinations of patient preferences, care appropriateness, and judgment of the attending physicians. The indicator calculations do not take into account factors such as comorbidities and complications. Further work is needed to evaluate the patterns observed—for example, by examining regional variations within provinces and investigating resection rates by type of surgery performed.
CRM Involvement
Our analysis also showed interprovincial variation in the incidence of crm involvement. Once additional years of collaborative staging data are available, it will be possible to determine whether those variations reflect either or both of differences in surgery and pathology practices (that is, whether pathologists use accepted techniques, including multiple sectioning of specimens to ensure that the whole tumour is assessed) and differences in data capture and reporting. The variability in crm rates between provinces is reflected in the results of other population-based studies that found positive crm rates ranging from 8% to 22%3.
The observed interprovincial variation could reflect the use of different treatment regimens for rectal cancer. Regimens might include a course of radiotherapy (long or short) or use of neoadjuvant radiation or chemotherapy, adjuvant chemotherapy, or a temporary diverting ostomy. Variations in crm involvement could also be related to the surgical technique in use (that is, total mesorectal excision).
Involvement of the crm is known to be a predictor of survival. Many studies have analyzed the difference in survival rates between patients with a positive and a negative crm, and crm involvement has invariably been associated with worse outcomes. For example, a national population-based study in Norway examined rates of 5-year local recurrence, distant metastasis, and overall survival for patients undergoing resection for rectal cancer. In patients with a crm of 0–2 mm, the corresponding rates were 23.7%, 43.9%, and 44.5%; for those with wider margins, the rates were 8.9%, 21.7%, and 66.7%11. The overall survival rate for all rectal cancer patients in the study was 64.6%. Another study reported 5-year relative survival rates of 40% for crm-positive cases and 79% for crm-negative cases12. In Canada, the 5-year relative survival ratio for rectal cancer is 64%13, and survival can be assumed to depend to some degree on crm status. Because pan-Canadian data are available starting only from 2010, follow-up is not yet sufficient to calculate 5-year survival estimates.
In future, it will be essential to track and assess the association between crm involvement and survival after rectal cancer as an indicator of patient outcomes. Although the relationship between crm status and the local recurrence rate was not examined here, it would be a relevant indicator to investigate by province. Such an analysis would help to identify real potential for improvement and might suggest a potential need for quality improvement initiatives targeting areas with high rates of crm positivity.
The indicators presented in this snapshot report are intended to identify potential opportunities for improvements in practice. Clinical outcomes such as locoregional and distant relapse and survival must be considered before recommendations about quality of care are made.
More information on the System Performance Initiative indicators and data can be explored at the Canadian Partnership Against Cancer (http://systemperformance.ca/).
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest and declare that we have none.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. [Google Scholar]
- 2.Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N substage on survival and disease relapse in adjuvant rectal cancer: a pooled analysis. Int J Radiat Oncol Biol Phys. 2002;54:386–96. doi: 10.1016/S0360-3016(02)02945-0. [DOI] [PubMed] [Google Scholar]
- 3.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–12. doi: 10.1200/JCO.2007.12.7027. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Fort Washington PA: NCCN; 2011. Ver 12.015. [Current version available online at: http://www.nccn.com/files/cancer-guidelines/breast/index.html (free registration required); cited December 11, 2012] [DOI] [PubMed] [Google Scholar]
- 5.Smith AJ, Driman DK, Spithoff K, et al. on behalf of the Expert Panel on Colon and Rectal Cancer Surgery and Pathology . Optimization of Surgical and Pathological Quality Performance in Radical Surgery for Colon and Rectal Cancer: Margins and Lymph Nodes. Toronto, ON: Cancer Care Ontario; 2008. [Google Scholar]
- 6.Hermanek P, Junginger T. The circumferential resection margin in rectal carcinoma surgery. Tech Coloproctol. 2005;9:193–9. doi: 10.1007/s10151-005-0226-1. [DOI] [PubMed] [Google Scholar]
- 7.Butler EN, Chawla N, Lund J, Harlan LC, Warren JL, Yabroff KR. Patterns of colorectal cancer care in the United States and Canada: a systematic review. J Natl Cancer Inst Monogr. 2013;2013:13–35. doi: 10.1093/jncimonographs/lgt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant chemotherapy for stage iii colon cancer: implications of race/ ethnicity, age, and differentiation. JAMA. 2005;294:2703–11. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 9.Moore M, Gibbs P. Adjuvant chemotherapy use among older patients with stage iii colon cancer. JAMA. 2010;303:2353. doi: 10.1001/jama.2010.775. [DOI] [PubMed] [Google Scholar]
- 10.Esnaola NF, Stewart AK, Feig BW, Skibber JM, Rodriguez–Bigas MA. Age-, race-, and ethnicity-related differences in the treatment of nonmetastatic rectal cancer: a patterns of care study from the National Cancer Data Base. Ann Surg Oncol. 2008;15:3036–47. doi: 10.1245/s10434-008-0106-9. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein TE, Endreseth BH, Romundstad P, Wibe A, on behalf of the Norwegian Colorectal Cancer Group Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg. 2009;96:1348–57. doi: 10.1002/bjs.6739. [DOI] [PubMed] [Google Scholar]
- 12.Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235:449–57. doi: 10.1097/00000658-200204000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison LF, Bryant H, Lockwood G, Shack L. Conditional survival analyses across cancer sites. Health Rep. 2011;22:21–5. [PubMed] [Google Scholar]