Abstract
Background
We aimed to characterize the localization and prognostic significance of tumour-associated macrophages (tams) in pancreatic ductal adenocarcinoma (pdac).
Methods
Tumour specimens from 70 patients with pdac and inflammatory specimens from 13 patients with chronic pancreatitis were collected and analyzed for tam and M2 macrophage counts by immunohistochemistry. Correlations between tam distributions and clinicopathologic features were determined.
Results
Immunohistochemical analysis showed that tam and M2 macrophage counts were higher in tissues from pdac than from chronic pancreatitis. The tams and M2 macrophages both infiltrated more into peritumour. Both macrophage types were positively associated with lymph node metastasis (p = 0.041 for tams in peritumour, p = 0.013 for M2 macrophages in introtumour, p = 0.006 for M2 macrophage in peritumour). In addition, abdominal pain was significantly more frequent in pdac patients with a greater tams count. The survival rate was much lower in patients having high infiltration by M2 macrophages than in those having low infiltration.
Conclusions
The tam count might be associated with neural invasion in pdac, and M2 macrophages might play an important role in lymph node metastasis. Higher counts of either macrophage type were associated with increased risk of lymph node metastasis, and the M2 macrophage count could potentially be a marker for evaluating prognosis.
Keywords: Pancreatic cancer, tumour-associated macrophages, lymph node metastasis, neural invasion
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (pdac), one of the most lethal digestive system malignancies, has a very poor prognosis1,2. In China, pdac incidence and mortality have increasing since the early 1990s. The disease is the 8th leading cause of death from cancer in men and the 11th leading cause in women in China. Surgery is the only potentially curative treatment, but more than 80% of these carcinomas are unresectable at the time of diagnosis because of metastasis into adjacent or distant organs, including liver and lung3. Chronic pancreatitis (cp) is a clearly identified and strong risk factor for pdac, being associated with a pdac incidence that is up to 20 times that in the general population. Lowenfels et al.4 observed that risk increases during the course of cp: for example, about 5% of cp patients develop pdac over 20 years. The lesions in cp contain abundant stroma that shows a morphology identical to that of pdac5–7. As a solid tumour, pdac is composed of a mixed population of cancer cells, extracellular matrix, and other non-malignant cell types, including regulatory T cells, cancer-associated fibroblasts, and tumour-associated macrophages (tams)8. Being the cardinal component of pdac (comprising up to 50%–80% of malignant tissue), tams might link inflammation with cancer and play an important role in tumour growth and metastasis9,10.
Studies have shown a positive association between a high number of tams and poor prognosis in human cancers such as bladder cancer, colorectal cancer, uveal melanoma, Hodgkin lymphoma, lung cancer, oral carcinoma, renal cell carcinoma, and even pdac11–17. Mantovani et al.18 classified tams into two functionally distinct types: classically activated (M1) and alternatively activated (M2) macrophages19–21. In the tumour microenvironment, tams trend mainly toward the M2 phenotype, and some clinical investigations showed that high infiltration by M2 macrophages indicated a poor prognosis for patients with pdac11,12,17. However, the most widely accepted marker for tams was CD6822, which could not distinguish between the phenotypes. Recently, mannose receptor (CD163), one of several receptors that are highly expressed by M2 macrophages, was recognized as a valuable specific marker23. Based on that report, we chose CD163 positivity as our means of identifying M2 macrophages.
The studies showing that high infiltration by M2 macrophages in tumour tissue is associated with poor prognosis in pdac patients11,12,17 were conducted in Japan. Being that no study had yet been conducted in China, we set out to determine any correlations of tam and M2 macrophage counts with prognosis in Chinese patients with pdac. Using the CD68 and CD163 markers, we evaluated the distribution and clinical significance of macrophages in tumour and peritumoural tissues and analyzed correlations between the distribution of tams and clinicopathologic features of the tumours.
2. METHODS
2.1. Patients and Specimens
We studied tissue specimens from 107 pdac and 13 cp patients who had been diagnosed at Sun Yat-sen Memorial Hospital of Sun Yat-sen University between September 2004 and December 2011. We excluded 37 pdac patients who had been lost to follow-up. Of the 70 remaining pdac patients, 44 were men (62.9%) and 26 were women (37.1%). Median age in the overall group was 58.5 years (range: 28.0–83.0 years). Clinical data—including age, sex, location and size of the tumour, and TNM staging—were collected using a predesigned template. Staging was performed according to the recommendations of the American Joint Committee on Cancer (7th edition). All patients had been followed for at least 2 years. Clinical materials were used for research purposes only after patient consent and approval from the hospital’s Research Ethics Committee had been obtained.
2.2. Immunohistochemistry for CD68 and CD163
The 70 pdac and 13 cp tissue specimens had been formalin-fixed and paraffin-embedded. Paraffin-embedded tissues were cut to a thickness of 3 μm. After deparaffinization, slides were racked and washed as follows: 100% ethanol (2×1 minute), 95% ethanol (2×1 minute), 80% ethanol (1×1 minute), and a rinse of running cold water (1 minute). After being incubated with deionized water for 20 minutes at room temperature, the slides were washed for 1 minute in distilled water and 5 times for 2 minutes each in phosphate-buffered saline. The sections were then drained and incubated with primary mouse anti-human CD68 antibody [clone PG-M1: Dako, Glostrup, Denmark (1:50 dilution)] or with mouse anti-human CD163 antibody [clone 10D6: Abcam, Cambridge, U.K. (prediluted)] at 37°C. After rinsing 5 times for 2 minutes each in phosphate-buffered saline, the specimens were blocked for 10 minutes using 3% H2O2. After rinsing 3 times for 5 minutes each in phosphate-buffered saline, the sections were incubated with EnVision (Dako) at room temperature. After a wash with tap water, the sections were dehydrated in a graded alcohol series and then mounted. Paraffin slides of tonsil and placenta were used as positive controls.
Histology sections from all 83 samples were independently reviewed by two pathologists blinded to the clinicopathologic data. Counting of CD68 and CD163 immunoreactivity-positive membrane or cytoplasm and nonreactive nuclei was performed under light microscopy (Olympus CX-40: Olympus, Tokyo, Japan). Macrophages were counted separately in the peripheral and central areas of lesions as described in an earlier study17. The means of the counts from 3 random high-power fields (400× magnification) were used in the analyses.
2.3. Statistical Analysis
Statistical analyses of the data were performed using IBM SPSS Statistics (version 19.0: IBM, Armonk, NY, U.S.A.). For statistical purposes, most of the clinicopathologic factors were divided into two groups at the median value. Circulating tumour markers (including carcinoembryonic antigen, cancer antigen 125, and carbohydrate antigen 19-9) were divided into groups according at the upper limit of normal. Comparative analyses involving just two groups used two-tailed Mann–Whitney tests. Analyses involving multiple groups used one-way nonparametric analysis of variance (Kruskal–Wallis test). Univariate analyses of overall survival by prognostic factor were performed using the log-rank test. Survival curves were estimated by the Kaplan-Meier method and compared using the two-sided log-rank test. A p value of 0.05 was the criterion for statistical significance. All reported p values are 2-tailed.
3. RESULTS
3.1. Distribution of CD68- and CD163-Positive Macrophages in PDAC and CP
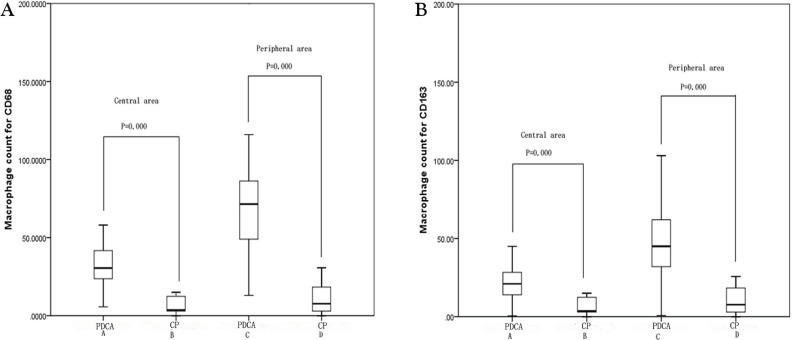
Figures 1 and 2 show expression of CD68 and CD163 in the 70 pdac and 13 cp specimens analyzed by immunohistochemistry. Macrophages positive for CD68 and CD163 were present not only in the central and peripheral areas of cp lesions, but also strongly in pdac lesions. In Figure 3, median CD68-positive macrophage counts per high-power field were 30.5 (range: 5.7–93.0) in group A, 3.7 (range: 0.0–33.3) in group B, 71.5 (range: 13.0–198.0) in group C, and 7.7 (range: 0.0–71.7) in group D. Median CD163-positive macrophage counts were 21.0 (range: 0.3–88.3) in group A, 3.7 (range: 0.0–31.7) in group B, 45.0 (range: 0.7–186.0) in group C, and 7.7 (range: 0.0–71.7) in group D.
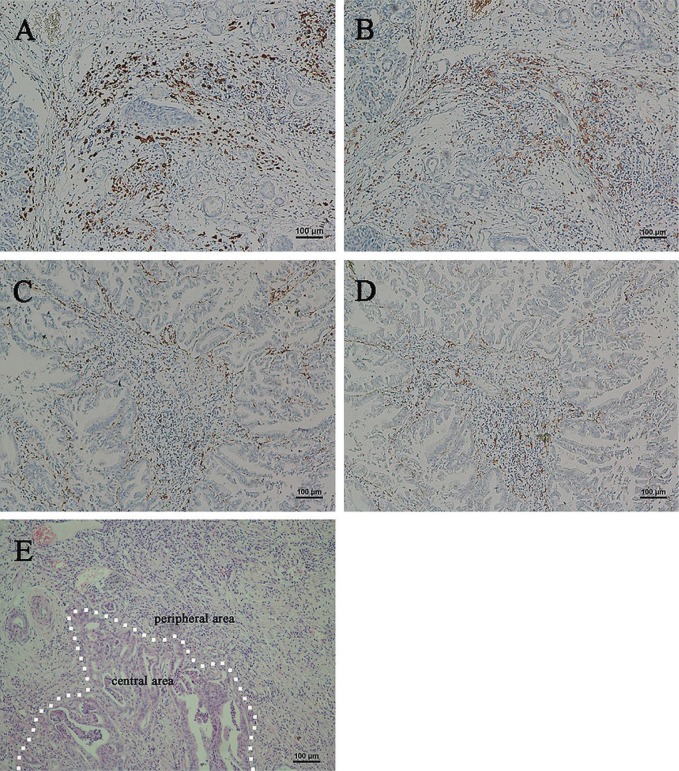
FIGURE 1.
Immunohistochemical analysis of CD68- and CD163-positive macrophages in specimens of pancreatic ductal adenocarcinoma. (A) High expression of CD68 and (B) high expression of CD163 in a single section. (C) Low expression of CD68 and (D) low expression of CD163 in a single section. (E) The dotted line shows the boundary between the adenocarcinoma and adjacent noncancerous tissue (hematoxylin and eosin stain). All images, 100× original magnification.
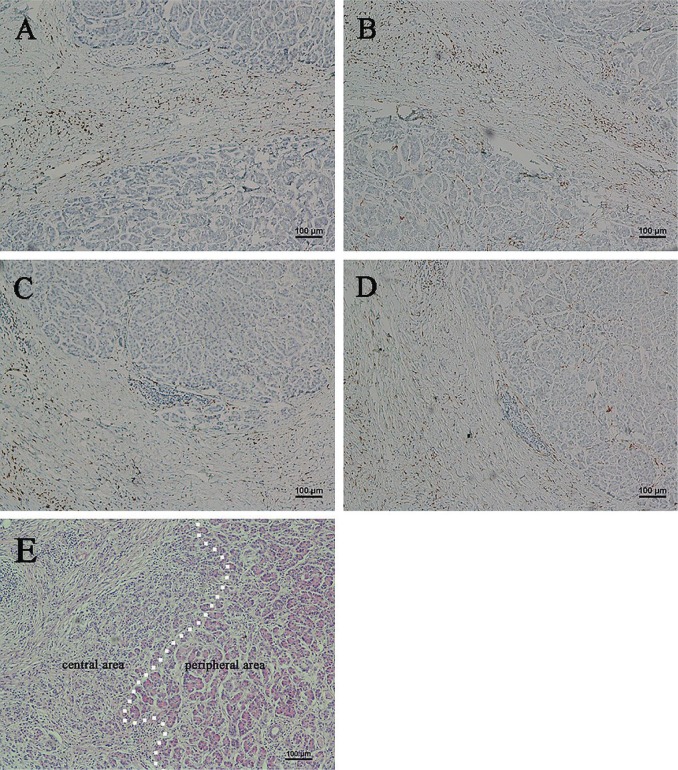
FIGURE 2.
Immunohistochemical analysis of CD68- and CD163-positive macrophages in specimens of chronic pancreatitis. (A) High expression of CD68 and (B) high expression of CD163 in a single section. (C) Low expression of CD68 and (D) low expression of CD163 in a single section. (E) The dotted line roughly shows the boundary between dense and relatively less-dense fibrotic lesion (hematoxylin and eosin stain). All images, 100× original magnification.
FIGURE 3.
Statistical analysis of the immunohistochemistry results for CD68 and CD163. Immunopositivity for (A) CD68 and (B) CD163 was significantly higher in pancreatic ductal adenocarcinoma (pdca) than in chronic pancreatitis (cp).
Results of staining showed that, in the central area of lesions, expression levels of CD68 and CD163 were higher in pdac samples than in cp samples (p < 0.001). Results at peripheral areas of the lesions were consistent with those in the central areas, and the difference between the pdac and cp samples was significant (p < 0.001). Furthermore, more CD68- and CD163-positive macrophages were found in peripheral areas than in central areas in pdac (p < 0.001). In contrast, expression of both CD68 and CD163 was low in cp, and the difference between the central and peripheral areas of lesions was nonsignificant (p = 0.504 for CD68, p = 0.472 for CD163).
3.2. Correlation of CD68 and CD163 Expression with Clinicopathologic Characteristics of PDAC Patients
Tables i and ii summarize, respectively, the associations of CD68 and CD163 expression with the clinicopathologic characteristics of the pdac tumours. Of the tumours examined, 47 (67.1%) were in the pancreatic head and 23 (32.9%) were in the body or tail. The size of the tumour was 3 cm or smaller in 22 cases (31.4%) and larger than 3 cm in 48 cases (68.6%). Differentiation was high in 32.9% of the tumours (grade 1) and moderate or poor (grade 2 or 3) in 67.1%. Of the 70 pdacs, invasion had reached the pancreatic stroma or parenchyma in 1 patient with a tumour 2 cm or less in size (T1) and in 12 patients with a tumour larger than 2 cm (T2). Direct invasion of the extrapancreatic tissue, common bile duct, and duodenum (T3) had occurred in 50 patients and of the stomach, colon, and major arteries or veins near the pancreas (T4) in 7 patients. Lymph node metastasis was found in 39 patients (55.7%). Furthermore, 58 patients were categorized as M0, and 12 were categorized as M1.
TABLE I.
Correlations of CD68-positive macrophage counts and clinicopathologic factors in chronic pancreatitis
| Characteristic | Pts (n) | CD68-positive macrophages | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Centrally | Peripherally | ||||||
|
|
|
||||||
| Median | Range | p Valuea | Median | Range | p Valuea | ||
| Abdominal pain | |||||||
| Yes | 41 | 26.7 | 5.7–93.0 | 0.025 | 62.3 | 13.0–97.7 | |
| No | 29 | 34.3 | 9.3–58.0 | 76.0 | 30.7–198.0 | 0.009 | |
| Tumour site | |||||||
| Head | 47 | 31.3 | 5.7–58.0 | 0.035 | 73.0 | 13.0–198.0 | 0.211 |
| Body or tail | 23 | 24.7 | 8.0–93.0 | 56.0 | 19.0–144.0 | ||
| Tumour size | |||||||
| ≤3.0 cm | 22 | 35.2 | 9.3–58.0 | 0.188 | 73.0 | 40.3–99.0 | 0.121 |
| >3.0 cm | 48 | 29.5 | 5.7–93.0 | 67.0 | 13.0–198.0 | ||
| Histopathologic grade | |||||||
| Grade 1 | 23 | 29.3 | 15.0–48.7 | 0.957 | 73.0 | 35.0–116.0 | 0.234 |
| Grade 2 | 25 | 31.0 | 5.7–54.7 | 72.0 | 13.0–198.0 | ||
| Grade 3 | 22 | 30.5 | 7.0–93.0 | 66.0 | 13.0–144.0 | ||
| T Stage | |||||||
| T1 | 1 | 54.7 | 0.110 | 73.0 | 0.601 | ||
| T2 | 12 | 32.8 | 18.0–47.3 | 70.0 | 45.0–99.0 | ||
| T3 | 50 | 29.8 | 8.0–93.0 | 72.5 | 13.0–198.0 | ||
| T4 | 7 | 24.7 | 5.7–43.0 | 48.3 | 13.0–94.0 | ||
| N Stage | |||||||
| N0 | 31 | 25.3 | 5.7–93.0 | 0.054 | 57.0 | 13.0–99.0 | 0.041 |
| N1 | 39 | 33.7 | 7.0–58.0 | 75.7 | 13.0–198.0 | ||
| M Stage | |||||||
| M0 | 58 | 30.5 | 5.7–93.0 | 0.585 | 72.07 | 13.0–198.0 | 0.265 |
| M1 | 12 | 29.3 | 7.0–58.0 | 58.2 | 13.0–93.7 | ||
Significant values appear in boldface type.
TABLE II.
Correlations of CD163-positive macrophage counts and clinicopathologic factors in chronic pancreatitis
| Characteristic | Pts (n) | CD163-positive macrophages | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Centrally | Peripherally | ||||||
|
|
|
||||||
| Median | Range | p Valuea | Median | Range | p Valuea | ||
| Abdominal pain | |||||||
| Yes | 41 | 21.0 | 0.3–88.3 | 0.725 | 46.0 | 0.7–186.0 | 0.621 |
| No | 29 | 20.7 | 4.0–45.0 | 45.0 | 13.0–170.0 | ||
| Tumour site | |||||||
| Head | 47 | 21.0 | 0.3–65.0 | 0.726 | 45.0 | 0.7–186.0 | 0.604 |
| Body or tail | 23 | 21.0 | 10.0–88.3 | 45.0 | 11.0–95.0 | ||
| Tumour size | |||||||
| ≤3.0 cm | 22 | 23.2 | 0.3–65.0 | 0.096 | 63.5 | 0.7–186.0 | 0.025 |
| >3.0 cm | 48 | 20.9 | 4.0–88.3 | 43.0 | 11.0–158.0 | ||
| Histopathologic grade | |||||||
| Grade 1 | 23 | 16.7 | 0.3–40.3 | 0.035 | 36.0 | 0.7–186.0 | 0.386 |
| Grade 2 | 25 | 22.0 | 4.0–65.0 | 48.0 | 13.0–158.0 | ||
| Grade 3 | 22 | 24.7 | 8.3–88.3 | 48.5 | 11.0–103.0 | ||
| T Stage | |||||||
| T1 | 1 | 65.0 | 0.097 | 73.0 | 0.357 | ||
| T2 | 12 | 18.5 | 4.0–31.3 | 40.0 | 11.0–73.0 | ||
| T3 | 50 | 22.9 | 0.3–88.3 | 48.0 | 0.7–186.0 | ||
| T4 | 7 | 36.0 | 24.0–50.0 | 36.0 | 24.0–50.0 | ||
| N Stage | |||||||
| N0 | 31 | 18.0 | 0.3–88.3 | 0.013 | 37.0 | 0.7–59.0 | 0.006 |
| N1 | 39 | 24.0 | 4.0–65.0 | 50.0 | 13.0–186.0 | ||
| M Stage | |||||||
| M0 | 58 | 21.0 | 0.3–88.3 | 0.161 | 45.5 | 0.7–170.0 | 0.697 |
| M1 | 12 | 24.2 | 14.0–45.0 | 44.0 | 11.0–186.0 | ||
Significant values appear in boldface type.
Analysis of the data revealed that abdominal pain was significantly more frequent in pdac patients with high CD68 counts in tumour and peritumour. Furthermore, infiltration by a greater number of CD68-positive macrophages into the central area of the lesion was significantly associated with tumour site (head of the pancreas: p = 0.035), and high peritumour infiltration by CD68 was significantly associated with lymph node metastasis (p = 0.041). In addition, expression of CD163 in the central area of the lesion was positively correlated with grade of differentiation (p = 0.035). Expression of CD163 in the peritumoural area was negatively associated with tumour size (p = 0.025). Only lymph node metastasis significantly associated with infiltration by CD163-positive macrophages in both the intratumoural and peritumoural areas (p = 0.013 intratumoural, p = 0.006 peritumoural). We observed no significant differences between the expression of CD68 or CD163 and other clinicopathologic factors such as age, sex, smoking, diabetes mellitus, serum carcinoembryonic antigen, cancer antigen 199, cancer antigen 125, albumin, glutamyl transferase, cholesterol, alkaline phosphatase, uric acid, and calcium (data not shown).
3.3. Survival and Prognostic Factors
Among our patients with pdac, only 24 (34.3%) received systemic chemotherapy. Surgery, including radical and palliative procedures, was offered to 62 patients (88.6%). The 1-, 3-, and 5-year overall survival probabilities were 44.3% (n = 31), 7.1% (n = 5), and 4.3% (n = 3) respectively. Based on univariate analysis, 5 variables—tumour site (p = 0.018), tumour 3 cm or less in size (p = 0.009), M0 status (p = 0.006), low CD163 centrally (p = 0.004), and low CD163 peripherally (p = 0.001)—were significantly associated with better overall survival (Table iii).
TABLE III.
Univariate analysis of variables predicting overall survival in patients with chronic pancreatitis
| Characteristic | Pts (n) | Overall survival | ||
|---|---|---|---|---|
|
| ||||
| Median | Range | p Valuea | ||
| Tumour site | ||||
| Head | 47 | 12.0 | 1.0–69.0 | 0.018 |
| Body or tail | 23 | 5.0 | 1.0–65.0 | |
| Tumour size | ||||
| ≤3.0 cm | 22 | 12.0 | 4.0–69.0 | 0.009 |
| >3.0 cm | 48 | 6.0 | 1.0–50.0 | |
| M Stage | ||||
| M0 | 58 | 11.0 | 1.0–69.0 | 0.006 |
| M1 | 12 | 4.0 | 2.0–23.0 | |
| CD68 centrally | ||||
| ≤30.5 | 35 | 11.0 | 1.0–65.0 | 0.975 |
| >30.5 | 35 | 11.0 | 3.0–69.0 | |
| CD68 peripherally | ||||
| ≤71.5 | 35 | 10.0 | 1.0–65.0 | 0.456 |
| >71.5 | 35 | 11.0 | 2.0–69.0 | |
| CD163 centrally | ||||
| ≤21.0 | 36 | 13.0 | 1.0–69.0 | 0.004 |
| >21.0 | 34 | 6.0 | 1.0–55.0 | |
| CD163 peripherally | ||||
| ≤45.0 | 33 | 13.0 | 3.0–69.0 | 0.001 |
| >45.0 | 37 | 6.0 | 1.0–55.0 | |
Significant values appear in boldface type.
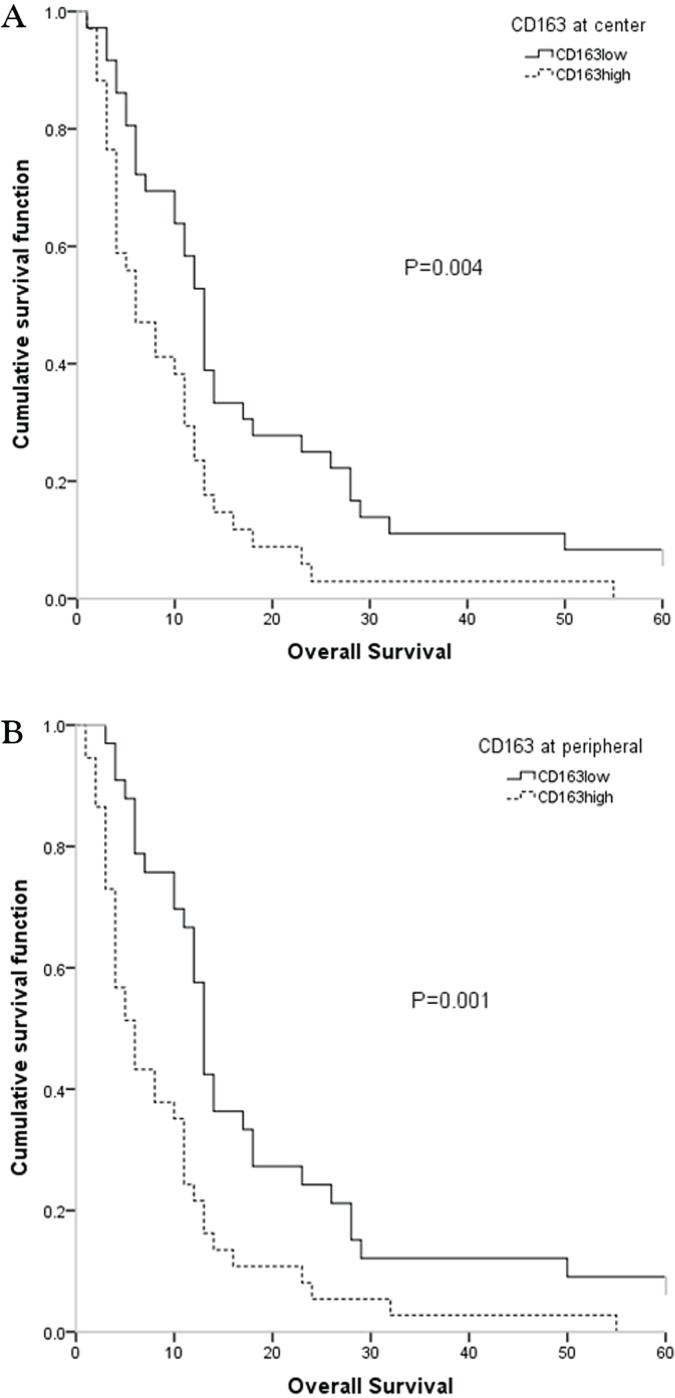
As Figure 4 shows, median overall survival was higher when infiltration by CD163-positive macrophages was lower (below the median value), a difference [compared with higher infiltration (above the median value)] that was statistically significant (p = 0.004 for the central area, p = 0.001 for the peripheral area).
FIGURE 4.
Kaplan–Meier survival curves classified by the number of CD163-positive macrophages found (A) centrally and (B) peripherally in the studied lesions. The count of positive macrophages in the peripheral area was better than the count in the central area for dividing patients into prognostic groups.
4. DISCUSSION
Because the pancreas lies deep in the belly in front of the spine and because early symptoms can be absent or quite subtle, pdac is usually widespread and unresectable when diagnosed. Even with sophisticated imaging, demonstration of pdacs less than 3 cm in size remains difficult. In most of our 70 pdac patients, tumours were 3 cm or larger at the time of diagnosis. Despite recent progress in pdac diagnosis and treatment, patient prognosis remains unsatisfactory and unpredictable because of extensive local tumour invasion, early systemic dissemination, and profound resistance to existing chemoradiation therapies. No curative treatment is available for most patients, and outcomes for untreated patients are dismal, with a median survival of 6 months after diagnosis.
Pancreatic inflammation has been correlated with an increased risk of pdac. Consistent with those reports, we observed a higher number of CD68- and CD163-positive macrophages in pdac tissues than in cp tissues. A variety of chemokines cause macrophages to differentiate from circulating monocytes recruited at the lesion. Those chemokines—for example, monocyte chemotactic protein 124,25—might be more frequent in tumour tissue than in inflammatory tissue. We also observed that the density of macrophages was significantly greater in the peritumoural stroma of pdac. Lower infiltration by tams in the centre of the tumour might be a result of central necrosis. In that microenvironment, the vascular system is damaged, and hence it is difficult for inflammatory cells to reach the centre of the tumour.
High numbers of intratumoural tams are often correlated with poor prognosis, and recent studies have also highlighted that the increased presence of macrophages correlates with tumour metastasis to distant organs26–30. Lymph node metastasis is one of the main routes for tumour metastasis. In pdac, lymph node metastasis occurs early and is known to be a strong prognostic factor in pdac patients31–34. In the present study, 39 of our 70 pdac patients (55.7%) already had lymph node metastasis. Interestingly, tams infiltration (tams in the peripheral area, and M2 macrophages both centrally and peripherally) was strongly associated with the incidence of lymph node metastasis. A 2002 report noted that tams express vascular endothelial growth factor C and affect tumour lymphangiogenesis in the peritumoural inflammatory microenvironment35. A positive correlation between lymphatic microvessel density and peritumoural tams infiltration has been found in lung adenocarcinoma, and lymph node metastasis was found to be significantly associated with lymphatic microvessel density36. Those findings indicate that tams might have the ability to release cytokines and chemokines that affect the tumour cell microenvironment, enabling lymph node metastasis.
In the present study, 41 patients with pdac (58.6%) experienced abdominal pain, and that pain was significantly associated with a higher level of infiltrating tams, but not of M2 macrophages. As a common symptom of pdac, abdominal pain is thought to be associated with organ invasion or infiltration of cancer cells into pancreatic nerves37. We hypothesize that tams facilitate tumour invasion and metastasis, but an alternative possibility is that tams are involved the process of neural invasion. Earlier studies showed that the nerve growth factor–TrkA system is associated with the generation of pain38–40. To confirm our hypothesis, future analyses of the relationships between tams infiltration and neural invasion in pdac are needed.
In contrast with earlier studies, we found no associations of other clinicopathologic characteristics such as tumour site and histopathologic grade with the number of infiltrating tams. Those differences might be a result of different tams cut-off values and a small sample size. In the present study, tumours of the body or tail of the pancreas were associated with a dismal prognosis because early distant metastasis, without specific symptoms, often occurs with tumours of the body and tail41,42. Tumour size greater than 3 cm and distant metastasis were also significant indicators of poorer survival rates. Interestingly, a high number of M2 macrophages was negatively associated with survival, but the number of tams showed no such association. The M2 macrophages are a subgroup of tams and might play the most important role in tumour progression. As the data demonstrate, our 70 pdac patients could be separated into various prognostic subgroups according to the median number of M2 macrophages detected by CD163 immunostaining. We therefore propose that M2 macrophages are a strong indicator of pdac prognosis.
5. CONCLUSIONS
In pdac, most infiltrating tams are found at the edges of the tumour. And tams, especially M2 macrophages, seem to be associated with lymph node metastasis and to play an important role in tumour progression. More detailed studies are necessary to clarify which subpopulation of tams is more important in lymph node metastasis and neural invasion in pdac. The tams assist tumour cells in invasion and metastasis because they produce a large array of growth factors and communicate with the tumour cells. Their clear molecular mechanism in the tumour microenvironment therefore requires further investigation.
6. ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (nos. 81072045 and 81302140), the Medical Science Foundation of Guangdong Province (no. A2014262), the Medical Science Foundation of Sun Yat-sen University (no. 14ykpy29). Grant KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes, Sun Yat-sen University and grant [2013]163 from the Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology are acknowledged.
7. CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest and declare that we have none.
8. REFERENCES
- 1.Raraty MG, Magee CJ, Ghaneh P, Neoptolemos JP. New techniques and agents in the adjuvant therapy of pancreatic cancer. Acta Oncol. 2002;41:582–95. doi: 10.1080/028418602321028184. [DOI] [PubMed] [Google Scholar]
- 2.Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer: challenge of the facts. World J Surg. 2003;27:1075–84. doi: 10.1007/s00268-003-7165-7. [DOI] [PubMed] [Google Scholar]
- 3.Niederhuber JE, Brennan MF, Menck HR. The national cancer data base report on pancreatic cancer. Cancer. 1995;76:1671–7. doi: 10.1002/1097-0142(19951101)76:9<1671::AID-CNCR2820760926>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 5.Gress TM, Wallrapp C, Frohme M, et al. Identification of genes with specific expression in pancreatic cancer by cdna representational difference analysis. Genes Chromosomes Cancer. 1997;19:97–103. doi: 10.1002/(SICI)1098-2264(199706)19:2<97::AID-GCC5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Gress TM, Muller–Pillasch F, Geng M, et al. A pancreatic cancer–specific expression profile. Oncogene. 1996;13:1819–30. [PubMed] [Google Scholar]
- 7.Geng M, Wallrapp C, Müller–Pillasch F, Frohme M, Hoheisel JD, Gress TM. Isolation of differentially expressed genes by combining representational difference analysis (rda) and cdna library arrays. Biotechniques. 1998;25:434–8. doi: 10.2144/98253st05. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Allavena P, Sica A, Garlanda C, Mantovani A. The yin–yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 10.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (tam) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 11.Kurahara H, Shinchi H, Mataki Y, et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res. 2011;167:e211–19. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Kurahara H, Takao S, Maemura K, et al. M2-Polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas. 2013;42:155–9. doi: 10.1097/MPA.0b013e318254f2d1. [DOI] [PubMed] [Google Scholar]
- 13.Ayari C, LaRue H, Hovington H, et al. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette–Guérin immunotherapy. Eur Urol. 2009;55:1386–95. doi: 10.1016/j.eururo.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Cui YL, Li HK, Zhou HY, Zhang T, Li Q. Correlations of tumor-associated macrophage subtypes with liver metastases of colorectal cancer. Asian Pac J Cancer Prev. 2013;14:1003–7. doi: 10.7314/APJCP.2013.14.2.1003. [DOI] [PubMed] [Google Scholar]
- 15.Herwig MC, Bergstrom C, Wells JR, Holler T, Grossniklaus HE. M2/M1 Ratio of tumor associated macrophages and ppar-gamma expression in uveal melanomas with class 1 and class 2 molecular profiles. Exp Eye Res. 2013;107:52–8. doi: 10.1016/j.exer.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshikawa K, Mitsunaga S, Kinoshita T, et al. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci. 2012;103:2012–20. doi: 10.1111/j.1349-7006.2012.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–70. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 19.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 Macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–13. [PubMed] [Google Scholar]
- 23.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 24.Bottazzi B, Polentarutti N, Acero R, et al. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–12. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 25.Negus RP, Stamp GW, Relf MG, et al. The detection and localization of monocyte chemoattractant protein-1 (mcp-1) in human ovarian cancer. J Clin Invest. 1995;95:2391–6. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An T, Sood U, Pietruk T, Cummings G, Hashimoto K, Crissman JD. In situ quantitation of inflammatory mononuclear cells in ductal infiltrating breast carcinoma. Relation to prognostic parameters. Am J Pathol. 1987;128:52–60. [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type ii polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40:1660–7. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with vegf expression and microvessel density. Oncol Rep. 2005;14:425–31. [PubMed] [Google Scholar]
- 29.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–30. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–72. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 33.Demeure MJ, Doffek KM, Komorowski RA, Wilson SD. Adenocarcinoma of the pancreas: detection of occult metastases in regional lymph nodes by a polymerase chain reaction-based assay. Cancer. 1998;83:1328–34. doi: 10.1002/(SICI)1097-0142(19981001)83:7<1328::AID-CNCR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Kurahara H, Takao S, Maemura K, Shinchi H, Natsugoe S, Aikou T. Impact of lymph node micrometastasis in patients with pancreatic head cancer. World J Surg. 2007;31:483–90. doi: 10.1007/s00268-006-0463-0. [DOI] [PubMed] [Google Scholar]
- 35.Schoppmann SF, Birner P, Stockl J, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–56. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol. 2011;28:1447–52. doi: 10.1007/s12032-010-9638-5. [DOI] [PubMed] [Google Scholar]
- 37.Caraceni A, Portenoy RK. Pain management in patients with pancreatic carcinoma. Cancer. 1996;78(suppl):639–53. doi: 10.1002/(SICI)1097-0142(19960801)78:3<639::AID-CNCR45>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 38.McMahon SB. ngf as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:431–40. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- 39.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of ngf-induced hyperalgesia. Eur J Neurosci. 1994;6:1903–12. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 40.Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441–8. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- 41.Johnson CD, Schwall G, Flechtenmacher J, Trede M. Resection for adenocarcinoma of the body and tail of the pancreas. Br J Surg. 1993;80:1177–9. doi: 10.1002/bjs.1800800937. [DOI] [PubMed] [Google Scholar]
- 42.Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg. 1996;223:506–11. doi: 10.1097/00000658-199605000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]